Abstract
Recent advances in the understanding of the complex biology of non-small cell lung carcinoma (NSCLC), particularly activation of oncogenes by mutation, translocation and amplification, have provided new treatment targets for this disease, and allowed the identification of subsets of NSCLC tumors, mostly with adenocarcinoma histology, having unique molecular profiles that can predict response to targeted therapy. The identification of a specific genetic and molecular targetable abnormalities using tumor tissue and cytology specimens followed by the administration of a specific inhibitor to the target, are the basis of personalized lung cancer treatment. In this new paradigm, the role of a precise pathology diagnosis of lung cancer and the proper handling of tissue and cytology samples for molecular testing is becoming increasingly important. These changes have posed multiple new challenges for pathologists to adequately integrate routine histopathology analysis and molecular testing into the clinical pathology practice for tumor diagnosis and subsequent selection of the most appropriate therapy.
Keywords: adenocarcinoma, squamous cell carcinoma, targeted therapy, molecular testing, next-generation of sequencing
Introduction
Lung cancer is the leading cause of deaths in the United States and worldwide.1 The high mortality associated with lung cancer is in part due to late diagnosis after regional or distant spread of the disease.2 From biological and clinical perspectives, lung cancer is a heterogeneous disease with multiple histological subtypes, being the most frequent non-small cell lung carcinoma (NSCLC). Traditionally, NSCLC has been used to designate tumors that exhibit histological and cytological features different than small cell carcinoma (SCLC). Most NSCLCs can be grouped into three main categories: squamous cell carcinoma, adenocarcinoma and large cell carcinoma; however, there are other less frequently diagnosed histologic types.3 Nowadays, due to the utilization of new therapeutic strategies and molecular diagnostic testing in NSCLC, particularly adenocarcinomas,4 it is imperative that pathologists are more specific in the diagnosis of subtypes of NSCLC and they make sure that there is sufficient tissue or cytology sample for molecular testing.
In this review, we described the most frequently described targetable genetic abnormalities in NSCLC and discuss the current status and challenges of molecular testing in this disease, including the implementation of new molecular methodologies to better predict the outcome of the disease and select the appropriate therapy.
Clinically relevant molecular abnormalities of NSCLC
During the last decade, multiple molecular abnormalities affecting oncogenes and tumor suppressor genes have been described in NSCLC.5,6 Of those, several gene mutations, amplifications and rearrangements have been identified as potential molecular targets. Here we review the characteristics of key cancer-related genes that have been emerged as potential targets in NSCLC using either tyrosine kinase inhibitors (TKIs) or monoclonal antibodies.
EGFR (epidermal growth factor receptor gene)
Mutations of EGFR in lung cancer are mostly limited to the first four exons of the tyrosine kinase domain (exons 18–21). The most frequent mutations are in-frame deletions in exon 19 (44% of all mutations) and missense mutations in exon 21 (41% of all mutations). These mutations are frequently diagnosed in lung adenocarcinomas (~20%–48%, vs. other NSCLC histologies ~2%), and strongly correlate with never-smoking status (50–60%), female gender (40–60%), and East Asian ethnicity (30–50%).7 There are some reports suggesting that EGFR mutations are encountered most frequently in lung adenocarcinomas with non-mucinous differentiation and with a lepidic or papillary predominant pattern.8,9 Activating EGFR mutations are biologically important because most of them have enhanced tyrosine kinase activity in response to epidermal growth factor stimulation.2,10 EGFR mutations are diagnosed mostly using gene sequencing methodologies, although quantitative (q)PCR-based assays are also available. (Figure 1) There are some antibodies that identify mutant EGFR proteins, but they have not shown to be clinically useful.
Figure 1. Histology section-based molecular tests for NSCLC.
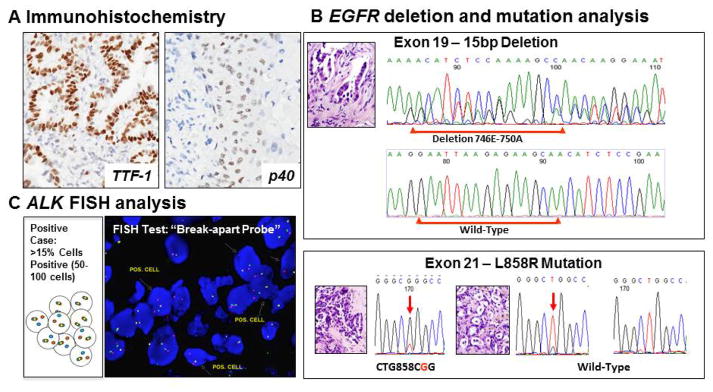
A. Immunohistochemistry panel: Thyroid transcription factor (TTF-1) is a marker of adenocarcinoma, and p40 (p63) is a marler of squamous cell carcinoma. B. EGFR mutation analysis. C. EML4-ALK fusion fluorescent in situ hybridyzation (FISH) analysis.
The presence these EGFR mutations are clinically relevant because they have been associated with sensitivity to small molecule TKIs (gefitinib and erlotinib).11–13 Unfortunately, some patients with activating EGFR mutations that respond initially EGFR TKIs subsequently relapse.14 This resistance appears to occur through a range of different mechanisms, including most frequently, a second EGFR mutation (50%) in exon 20 (T790M and D761Y),15 as well as other molecular mechanisms that include amplification of the MET oncogene (21%), 16,17,18 mutations of PI3KCA, 19,20,21 22 and epithelial-to mesenchymal transition (EMT) phenomenon.
ALK (anaplastic lymphoma kinase gene)
In lung cancer, aberrant ALK expression has been identified in a subset of NSCLC, mostly adenocarcinomas. This abnormality consists in the formation of a fusion transcript with cell transforming activity and that is the product of a translocation of EML4 (echinoderm microtubule associated protein like-4 gene) gene located at chromosome 2p21 and the ALK gene located at 2p23.23 The encoded fusion protein with increased catalytic activity contains the N-terminal part of EML4 and the intracellular catalytic domain of ALK.23 EML4-ALK rearrangements have multiple distinct isoforms with demonstrated transforming activity and that can be detected by multiplex reverse transcription-PCR methodologies.24,25 The EML4-ALK fusion positive tumors are detected in 2–7% of NSCLC,26,27 mostly adenocarcinomas arising usually in young never- or light-smokers patients.28,29,30,31 Tumors with EML4-ALK translocation usually lack EGFR and KRAS mutations. 32,33,34 ALK rearrangement has been mostly associated with an acinar pattern including a cribriform morphology and with signet ring cell features.35
It has been demonstrated that crizotinib, an oral inhibitor of the ALK and MET tyrosine kinases, showed that this drug is effective against advanced NSCLC carrying activated EML4-ALK translocation assessed by a fluorescence in situ hybridization (FISH) utilizing an ALK “break-apart” probe.36 The cut-off criteria for positive ALK “break-apart” FISH test is the presence of >15% tumor cells having split ALK 5′ and 3′ probe signals, or had isolated 3′ signals.30 (Figure 1) The overall partial and complete tumor response rate observed in patients with NSCLC tumors with positive FISH test and treated with crizotinib was shown to be of 57%, and the rate of stable disease was 33%.26 It has been shown that patients with NSCLC EML4-ALK rearrangement treated with ALK inhibitors developed resistance.37 The genetic alterations associated with documented acquired resistance to crizotinib are ALK amplification or secondary mutations within the kinase domain of gene (L1196M, C1156Y and F1174L).38
ROS1 (c-ros 1 gene)
This gene encodes for a tyrosine-kinase receptor of the insulin receptor family.39 Gene rearrangements affecting ROS1 with the development of oncogenic fusion protein have been identified in approximately 1% of NSCLC, and more frequently in younger, nonsmoking patients with adenocarcinoma.40,41 A phase I clinical trial has demonstrated that crizotinib has dramatic antitumor activity in patients with ROS1-rearranged NSCLC, with a high objective response rate of 57.1%.42 Therefore, the identification of ROS1 fusion variants is important for personalized therapy in lung cancer, particularly in patients adenocarcinoma histology.43,44,40,41 Up to now, a couple of fusion gene partners of ROS1 have been identified in lung tumors.45,46
RET
The tyrosine kinase receptor RET is involved in cell proliferation, migration and differentiation.47,48 RET mutations are known to incline to multiple endocrine neoplasia type 2 and sporadic medullary thyroid cancer.48 A novel fusion oncogene between RET and KIF5B (kinesin family member 5B gene) was reported recently in lung cancer affecting approximately 1% of patients with lung adenocarcinoma, mostly young never smokers.49,50
KRAS (Kirsten rat sarcoma viral oncogene homolog gene) and BRAF (v-raf murine sarcoma viral oncogene homolog B gene)
KRAS is RAS family gene most frequently activated in lung cancer by point mutations detected in approximately 20% of lung adenocarcinomas, and more frequently found in patients with smoking history.51 Most KRAS mutations are single amino acid substitutions in codon 12,13 and 61.2,52 Ras signaling pathways are also activated in tumors in which growth-factor-receptor tyrosine kinases have been overexpressed.53,54 Of great interest, EGFR and KRAS mutations in lung adenocarcinoma are mutually exclusive, suggesting different pathways to lung cancer in smokers and never smokers. KRAS mutations a have been associated to low response rates to EGFR-TKI therapies.55 RAS is considered a not targetable molecule, therefore recent studies have evaluated the activation of the Ras downstream pathway, RAS/RAF/MEK, as a potential target for therapy in lung cancer.56,57
BRAF is a serine/threonine kinase that lies downstream of RAS in the RAS-RAF-MEK-ERK-MAP pathway.58 The V600E BRAF mutation is frequently identified in melanomas.58 BRAF mutations occur in 2 to 4% of with lung adenocarcinoma.58,59,60,61 Most BRAF mutations detected in lung cancer are non-V600E mutations affecting exons 11 and 15, and they are mutually exclusive to EGFR and KRAS mutations.62,63,64
HER2 (human epidermal growth factor receptor 2 gene)
The incidence of HER2 mutations ranges from 1 to 6 % of lung adenocarcinomas. In lung cancer, HER2 kinase domain mutations (in-frame insertion in exon 20) and EGFR kinase domain mutations have similar associations with female gender, non-smoker and Asian ethnicity.65,66,67,68 HER2 amplification has been reported in 4%–5% of NSCLCs and is also more frequent in the adenocarcinoma histology (8%).69,70
MET
This gene encodes for a receptor tyrosine kinase that activates multiple signaling pathways involved in cell proliferation, survival motility, and invasion.71 MET amplification occurs in up to 7 % of NSCLC72,16, and has been associated to resistance to targeted therapy in patients whose tumors harbor EGFR mutation treated with EGFR TKIs. 16,17,18
PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha gene)
Phosphatidylinocitol 3-kinases (PI3Ks) are family of lipid kinase that play an important role in regulating cell growth, proliferation and survival.2,73,74 PIK3CA mutations are found in approximately 1 to 3% of NSCLCs.19,20,21,22 PIK3CA copy number gain (>3 copies per cell) is a common abnormality in NSCLC, predominantly in squamous cell carcinomas (33%–35 %) compared to adenocarcinomas (2%–6%).21,75 PI3K, and its downstream effectors, PTEN, mTOR and AKT, are potential therapeutic targets for NSCLC therapy and are evaluated in clinical trials for lung cancer.76 PTEN is a lipid phosphatase that inhibits PI3K-dependent signaling with tumor suppressor gene activity.77 PTEN mutations are common in squamous cell carcinomas of the lung.6,78,79,80 PTEN inactivation has been related decreasing EGFR TKIs sensitivity of EGFR-mutant lung tumors. 6,7
FGFR1 (fibroblast growth factor receptor type 1 gene)
This gene encodes a cell surface tyrosine kinase receptor of the FGFR tyrosine kinase family that includes four kinases (FGFR1 to 4). FGFRs play a critical role in cell proliferation and survival.74,81 It has been reported that FGFR1 is somatically amplified in ~20% of squamous cell carcinomas and in 1–3% of adenocarcinomas of the lung.82,83,84 Currently, the preferred method to assess FGFR1 copy number is FISH, but the definitions of copy number gain and gene amplification still need to be determined.
DDR2 (discoidin domain receptor 2 gene)
This receptor tyrosine kinase has been reported to be mutated in ~4% of squamous cell carcinoma of the lung.85,86 Mutations were found both in the kinase domain and in other regions of the protein sequence without hot-spots, which makes the analysis of mutations of this gene challenging. DDR2-mutant tumors have been suggested to respond to dasatinib therapy in patients with squamous cell carcinoma of the lung.
Molecular testing of lung cancer
The recent advances in NSCLC targeted therapy require the analysis of a panel of molecular abnormalities in tumor specimens, including gene mutations, amplifications and rearrangements, by applying different methodologies to tumor tissue specimens.87,5 However, the diagnostic biopsy or cytology specimens available for molecular testing in advanced metastatic lung tumors are likely to be small specimens, including core needle biopsies (CNB) and/or fine needle aspiration (FNA), which may significantly limit molecular testing with currently available methodologies and technologies. It is known that both formalin fixation and paraffin embedding compromise the integrity of proteins and nuclei acids for molecular testing, particularly when non-buffered formalin is utilized and the specimens are fixed in formalin for greater than 24 hours. The cytology specimens are usually fixed in alcohol which is optimal for preservation of DNA. When the cytology specimen has abundant material, the sample can be fixed in formalin and processed as a cell block to obtain histology sections; both smears and cell block sections with abundant malignant cells can be successfully used for molecular testing in lung cancer. Few studies showed that the sensitivity of cell block specimens for molecular testing in lung cancer showing that, although slightly lower, are compatible to smears and ThinPreps.88,89,90,91 92
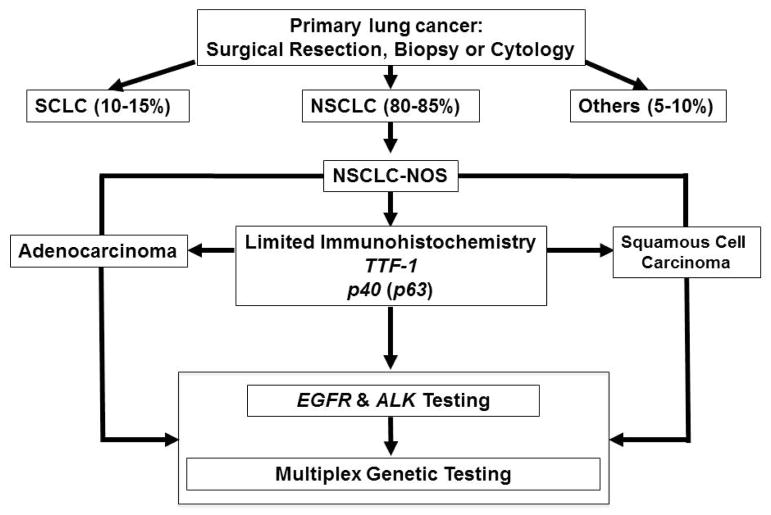
Currently, the surgical pathologist plays a crucial role on determining the appropriate therapy for patients with NSCLC. The handling of the biopsy and cytology specimens for pathological diagnosis and subsequent molecular testing requires thoughtful prioritization of the utilization of the sample to prevent the loss of tissue in less important analysis that the molecular testing requires for selection of therapy. (Figure 2) Also, the pathologist should determine if the amount of malignant cells available in the specimen is adequate for DNA extraction and also for histology section-based molecular tests (e.g., fluorescent in situ hybridization and immunohistochemistry).
Figure 2. Histology subtyping based on treatment algorithm for lung cancer.
The utilization of multiplex platforms to test mutations in tumor samples allows testing all NSCLC histologies for panel of mutations and other gene abnormalities regardless of their histology. SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; NOS, not otherwise specified
On the other hand, our growing understanding of cancer biology of NSCLC, particularly the molecular evolution of tumors during local progression and metastasis, and the identification of molecular abnormalities developed after resistance to targeted therapies, emphasizes the importance of characterize the molecular abnormalities of the disease at every stage of its evolution. For molecular testing of advanced metastatic NSCLC is important to sample and analyze the tumors’ specimen at each time point of clinical decision-making.93,76 In lung cancer, the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO) and the International Association for the Study of Lung Cancer (IASLC)/College of American Pathologists (CAP)/Association of Molecular Pathology (AMP) recommended to use testing for EGFR mutations and ALK fusions to guide patient selection for appropriate TKI therapy in all patients with advanced stage adenocarcinoma, regardless of sex, race, smoking history, or other clinical risk factors.94,95,96
New techniques for molecular testing
The most used technique for DNA mutation analysis is direct sequencing previous PCR amplification of extracted DNA. There are several of these methods available for mutation analysis of DNA extracted from FFPE tumor tissue specimens, including lung cancer. Sanger sequencing is one of the preferred sequencing methods to detect mutations of clinically relevant genes, such as the EGFR hot-spot mutations for selection of EGFR TKI therapy. It can detect essentially all base substitutions, small insertions and deletions. However, the main disadvantage is the relatively low sensitivity of mutant alleles, estimated to be ~20% of mutant vs. wild-type alleles,97 and most importantly, its inability to examine multiple gene hot-spots simultaneously. The need for analysis of multiple genetic changes in small, clinically relevant biopsy and cytology specimens, has prompted to the development of multiplexed approaches for molecular testing, particularly for gene mutation analysis.
Multiplex genotyping methodologies
Multiplex PCR is defined as the simultaneous amplification of two or more DNA or cDNA targets in one reaction.98 There are two major highly sensitive multiplex genotyping methodologies widely used for mutation analysis in lung cancer, the primer extension (SNaPshot®) assay (Life Technologies, Grans Island, NY) and the matrix-assisted laser desorption ionization time-off light (MALDI-TOF) mass spectrometry (Sequenom®, San Diego, CA). SNaPshot involves multiplexed PCRs, multiplexed single-base primer extension, and capillary electrophoresis. 99,100 Sequenom® involves multiplexed PCR and a mass spectrometry detection system. Both systems are able to detect multiple hot-spot mutation simultaneously using small amounts of DNA obtained from small FFPE biopsy specimens.101,102 These multiplex genotyping platforms are not designed for discovery purposes.
Next generation sequencing (NGS)
This technology has been available since 2004.103 NGS has been applied to studies of DNA and RNA to examine the whole genome, exome, transcriptome and epigenome, and is rapidly changing the paradigm of lung cancer research and patient care.103 Currently, commercially available NGS platforms include, among others, Illumina® HiSeq 2500 (Illumina Inc, San Diego CA), Personal Genome Machine (PGM™) and Ion Torrent™ systems (Life Technologies Grand Island, NY).6 NGS technologies have been rapidly applied to clinical setting almost all tumor types. NGS can detect mutations, chromosomal rearrangements and copy number alterations at high resolution.5,104,76 Currently, clinical application of NGS is hampered by the large amount of data generated and the resultant and computational bioinformatics challenges needed for secondary verification, and the relatively high cost.76
Future directions
In lung cancer, molecular testing of tumors is usually performed using samples obtained for histological diagnostic intent and often using residual tissue specimens obtained from surgical resection procedures for treatment. However, based on our growing understanding of the molecular events associated to tumor progression and the mechanisms of resistance to targeted therapy, it is becoming clearer that molecular analysis should be applied directly to clinically relevant tumor specimens. This is important to consider in recurrences of surgically resected stages I–III tumors or refractory advanced metastatic chemotherapy-treated tumors obtained at treatment or diagnosis of the disease, respectively. These “old” samples may not reflect the current state of biomarkers after tumor progression or treatment with chemotherapy. Therefore, to ensure the most accurate assessment of a lung cancer patient’s disease and treatment responsiveness, their tumors should be molecularly characterized at multiple time points during the clinical decision-making process.
On the other hand, there are increasing concerns that intra-tumor heterogeneity of lung cancer can lead to underestimation of tumor genomics landscape portrayed from a single tumor biopsy and may present major challenges to personalized-treatment and biomarker development.105,106 In lung adenocarcinoma, mixed populations of EGFR-mutant and wild-type cells have been reported and associated to reduced response to EGFR TKI.107 Recently, it has been demonstrated in renal cell carcinoma that 73–75% of the driver genetic aberrations detected using NGS were sub-clonal, confounding the estimation of driver mutation prevalence. The presence of sub-clonal driver events in tumors, including lung, may provide an explanation for the inevitable acquisition of resistance to targeted therapeutics in advanced disease.108,105
Abbreviations
- ALK
anaplastic lymphoma kinase gene
- AMP
Association of Molecular Pathology
- ASCO
American Society of Clinical Oncology
- BRAF
v-raf murine sarcoma viral oncogene homolog B gene
- CAP
College of American Pathologists
- CNB
core needle biopsies
- DDR2
discoidin domain receptor 2 gene
- EGFR
epidermal growth factor receptor gene
- FFPE
formalin-fixed and paraffin-embedded
- FGFR1
fibroblast growth factor receptor type 1gene
- FNA
fine needle aspirations
- HER2
human epidermal growth factor receptor 2 gene
- IASLC
International Association for the Study of Lung Cancer
- IHC
immunohistochemistry
- KIF5B
kinesin family member 5B gene
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- MALDI-TOF MS
matrix assisted laser desorption/ionization-time of flight mass spectrometry
- NCCN
National Comprehensive Cancer Network
- NGS
next-generation sequencing
- NSCLC
non-small cell lung carcinoma
- PCR
polymerase chain reaction
- PI3K
phosphatidylinocitol 3-kinase gene
- PIK3CA
Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha, gene
- PTEN
phosphatase and tensin homolog gene
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- ROS1
c-ros 1 gene
- SCLC
small cell lung carcinoma
- TKI
tyrosine kinase inhibitor
- VIPR1
Vasoactive Intestinal Peptide Receptor 1 gene
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. The New England journal of medicine. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis W Organization., W. H., Cancer., I. A. f. R. o., Cancer., I. A. f. t. S. o. L. & Pathology., I. A. o. Pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC Press Oxford University Press (distributor); 2004. [Google Scholar]
- 4.Travis WD, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shames DS, Wistuba II. The evolving genomic classification of lung cancer. J Pathol. 2014;232:121–133. doi: 10.1002/path.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sos ML, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer research. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan X, et al. Immunostaining with EGFR mutation-specific antibodies: a reliable screening method for lung adenocarcinomas harboring EGFR mutation in biopsy and resection samples. Human pathology. 2013;44:1499–1507. doi: 10.1016/j.humpath.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Soma S, et al. Intratumoral distribution of EGFR-amplified and EGFR-mutated cells in pulmonary adenocarcinoma. Pathology, research and practice. 2014;210:155–160. doi: 10.1016/j.prp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The lancet oncology. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 13.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 15.Balak MN, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 16.Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 18.Yano S, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer research. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 19.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer research. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 20.Wislez M, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer research. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer research. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawano O, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54:209–215. doi: 10.1016/j.lungcan.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi K, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 25.Soda M, et al. A prospective PCR-based screening for the EML4-ALK oncogene in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5682–5689. doi: 10.1158/1078-0432.CCR-11-2947. [DOI] [PubMed] [Google Scholar]
- 26.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koivunen JP, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw AT, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw AT, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The lancet oncology. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodig SJ, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–1780. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inamura K, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 33.Wong DW, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 34.Gainor JF, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida A, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. The American journal of surgical pathology. 2011;35:1226–1234. doi: 10.1097/PAS.0b013e3182233e06. [DOI] [PubMed] [Google Scholar]
- 36.Paik JH, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:466–472. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 37.Choi YL, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. The New England journal of medicine. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chemical biology & drug design. 2011;78:999–1005. doi: 10.1111/j.1747-0285.2011.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushime H, Wang LH, Shibuya M. Human c-ros-1 gene homologous to the v-ros sequence of UR2 sarcoma virus encodes for a transmembrane receptor like molecule. Molecular and cellular biology. 1986;6:3000–3004. doi: 10.1128/mcb.6.8.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergethon K, et al. ROS1 rearrangements define a unique molecular class of lung cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rimkunas VM, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 42.Rothschild SI, Gautschi O. Crizotinib in the treatment of non--small-cell lung cancer. Clinical lung cancer. 2013;14:473–480. doi: 10.1016/j.cllc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Charest A, et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21) Genes Chromosomes Cancer. 2003;37:58–71. doi: 10.1002/gcc.10207. [DOI] [PubMed] [Google Scholar]
- 44.Gu TL, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6:e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto I, et al. Multiplex genomic profiling of non-small cell lung cancers from the LETS phase III trial of first-line S-1/carboplatin versus paclitaxel/carboplatin: results of a West Japan Oncology Group study. Oncotarget. 2014;5:2293–2304. doi: 10.18632/oncotarget.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai W, et al. Coexistence of Three Variants Involving Two Different Fusion Partners of ROS1 Including a Novel Variant of ROS1 Fusions in Lung Adenocarcinoma: A Case Report. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:e43–46. doi: 10.1097/JTO.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 47.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 48.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nature reviews. Cancer. 2014;14:173–186. doi: 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- 49.Ju YS, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohno T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudin CM, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5646–5661. doi: 10.1158/1078-0432.CCR-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kranenburg O. The KRAS oncogene: past, present, and future. Biochim Biophys Acta. 2005;1756:81–82. doi: 10.1016/j.bbcan.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Downward J. Targeting RAS signalling pathways in cancer therapy. Nature reviews. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 54.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nature reviews. Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 55.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4769–4777. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 56.Repasky GA, Chenette EJ, Der CJ. Renewing the conspiracy theory debate: does Raf function alone to mediate Ras oncogenesis? Trends in cell biology. 2004;14:639–647. doi: 10.1016/j.tcb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 58.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 59.Marchetti A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 60.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. The lancet oncology. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 61.Paik PK, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shigematsu H, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. Journal of the National Cancer Institute. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 63.Tam IY, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 64.Kosaka T, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer research. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 65.Shigematsu H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer research. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 66.Wang SE, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 67.Stephens P, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 68.Landi L, Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther. 2013;13:1219–1228. doi: 10.1586/14737140.2013.846830. [DOI] [PubMed] [Google Scholar]
- 69.Hirsch FR, et al. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. British journal of cancer. 2002;86:1449–1456. doi: 10.1038/sj.bjc.6600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heinmoller P, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5238–5243. [PubMed] [Google Scholar]
- 71.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 72.Cappuzzo F, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1667–1674. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 74.Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1097–1104. doi: 10.1200/JCO.2012.42.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Massion PP, et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer research. 2002;62:3636–3640. [PubMed] [Google Scholar]
- 76.Thomas A, Rajan A, Lopez-Chavez A, Wang Y, Giaccone G. From targets to targeted therapies and molecular profiling in non-small cell lung carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2013;24:577–585. doi: 10.1093/annonc/mds478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davidson L, et al. Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene. 2010;29:687–697. doi: 10.1038/onc.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsit CJ, et al. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Human pathology. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 79.Jin G, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69:279–283. doi: 10.1016/j.lungcan.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Network., T. C. G. A. R. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantripragada K, Khurshid H. Targeting genomic alterations in squamous cell lung cancer. Front Oncol. 2013;3:195. doi: 10.3389/fonc.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiss J, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dutt A, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS One. 2011;6:e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malchers F, et al. Cell-autonomous and non-cell-autonomous mechanisms of transformation by amplified FGFR1 in lung cancer. Cancer Discov. 2014;4:246–257. doi: 10.1158/2159-8290.CD-13-0323. [DOI] [PubMed] [Google Scholar]
- 85.Ford CE, et al. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. British journal of cancer. 2007;96:808–814. doi: 10.1038/sj.bjc.6603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammerman PS, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:983–994. doi: 10.1200/JCO.2007.12.9858. [DOI] [PubMed] [Google Scholar]
- 88.Nigro K, Tynski Z, Wasman J, Abdul-Karim F, Wang N. Comparison of cell block preparation methods for nongynecologic ThinPrep specimens. Diagnostic cytopathology. 2007;35:640–643. doi: 10.1002/dc.20713. [DOI] [PubMed] [Google Scholar]
- 89.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. American journal of clinical pathology. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 90.Richardson HL, Koss LG, Simon TR. An evaluation of the concomitant use of cytological and histocytological techniques in the recognition of cancer in exfoliated material from various sources. Cancer. 1955;8:948–950. doi: 10.1002/1097-0142(1955)8:5<948::aid-cncr2820080515>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 91.Liu K, Dodge R, Glasgow BJ, Layfield LJ. Fine-needle aspiration: comparison of smear, cytospin, and cell block preparations in diagnostic and cost effectiveness. Diagnostic cytopathology. 1998;19:70–74. doi: 10.1002/(sici)1097-0339(199807)19:1<70::aid-dc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 92.Kalhor N, Wistuba Perfecting the fine-needle aspirate cell block. Cancer cytopathology. 2013;121:109–110. doi: 10.1002/cncy.21284. [DOI] [PubMed] [Google Scholar]
- 93.Kim ES, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keedy VL, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 95.Lindeman NI, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–860. doi: 10.5858/arpa.2012-0720-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Travis WD, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137:685–705. doi: 10.5858/arpa.2012-0264-RA. [DOI] [PubMed] [Google Scholar]
- 97.Tsiatis AC, et al. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. The Journal of molecular diagnostics : JMD. 2010;12:425–432. doi: 10.2353/jmoldx.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Persson K, Hamby K, Ugozzoli LA. Four-color multiplex reverse transcription polymerase chain reaction--overcoming its limitations. Analytical biochemistry. 2005;344:33–42. doi: 10.1016/j.ab.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 99.Su Z, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. The Journal of molecular diagnostics : JMD. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sequist LV, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2011;22:2616–2624. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kris M, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011:Abstract CRA7506. [Google Scholar]
- 102.Johnson B, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013:Abstract 8019. [Google Scholar]
- 103.Daniels M, et al. Whole genome sequencing for lung cancer. J Thorac Dis. 2012;4:155–163. doi: 10.3978/j.issn.2072-1439.2012.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glenn TC. Field guide to next-generation DNA sequencers. Mol Ecol Resour. 2011;11:759–769. doi: 10.1111/j.1755-0998.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 105.Gerlinger M, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med. 2012;4:127ps110. doi: 10.1126/scitranslmed.3003854. [DOI] [PubMed] [Google Scholar]
- 107.Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008;99:929–935. doi: 10.1111/j.1349-7006.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]