Abstract
Purpose
Investigate the mechanisms of regulation and role associated with EZH2 expression in lung cancer cells.
Experimental Design
We investigated the mechanisms of EZH2 expression associated with the vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR-2) pathway. Furthermore, we sought to determine the role of EZH2 in response of lung adenocarcinoma to platinum-based chemotherapy, as well as the effect of EZH2 depletion on VEGFR-2–targeted therapy in lung adenocarcinoma cell lines. Additionally, we characterized EZH2 expression in lung adenocarcinoma specimens and correlated it with patients’ clinical characteristics.
Results
In this study, we demonstrate that VEGF/VEGFR-2 activation induces expression of EZH2 through the upregulation of E2F3 and HIF-1α, and downregulated expression of miR-101. EZH2 depletion by treatment with 3-deazaneplanocin A and knockdown by siRNA decreased the expression of EZH2 and H3K27me3, increased PARP-C level, reduced cell proliferation and migration, and increased sensitivity of the cells to treatment with cisplatin and carboplatin. Additionally, high EZH2 expression was associated with poor overall survival in patients who received platinum-based adjuvant therapy, but not in patients who did not receive this therapy. Furthermore, we demonstrated for the first time that the inhibition of EZH2 greatly increased the sensitivity of lung adenocarcinoma cells to the anti-VEGFR-2 drug AZD2171.
Conclusion
Our results suggest that VEGF/VEGFR-2 pathway plays a role in regulation of EZH2 expression via E2F3, HIF-1α and miR-101. EZH2 depletion decreases the malignant potential of lung adenocarcinoma and sensitivity of the cells to both platinum-based and VEGFR-2–targeted therapy.
Keywords: EZH2, NSCLC, VEGF/VEGFR-2 pathway, DZNep
Introduction
Enhancer of zeste homolog 2 (EZH2) is the catalytic subunit of PRC2, which catalyses the addition of methyl groups to lysine 27 on histone H3 (H3K27) in the promoters of target genes, leading to repression of gene transcription (1–3). EZH2 is involved in the methylation of DNA, and its combined action on DNA and histones leads to downregulation of expression of tumor suppressor genes (2, 4, 5). Researchers have demonstrated EZH2 overexpression in a wide variety of human cancers, including NSCLC (3, 5–7), and implicated roles for it in cell proliferation, cell-colony formation, tumor progression, and angiogenesis activation (2, 3, 8). Thus, it is acting with oncogenic properties. Although EZH2 overexpression has occurred in a wide variety of cancers, regulation of its expression and its role in the response of NSCLC to therapy are poorly understood. Authors have reported on posttranscriptional regulation of EZH2 expression by miR-101 in different cancer cell lines; specifically, overexpression of an miR-101 mimic downregulates expression of EZH2 (9–11). Although upregulation of EZH2 expression in endothelial cells may be regulated by VEGF/VEGFR-2 pathway via E2F and miR-101, the mechanisms of regulation of EZH2 expression in malignant epithelial cells are unknown. In certain tumors, genomic loss of miR-101 leads to overexpression of EZH2, resulting in cancer progression (3, 12).
In addition to its role in tumor cells, upregulation of EZH2 gene expression in endothelial cells is regulated by VEGF/VEGFR-2 pathway at both the transcriptional and posttranscriptional level (3, 8–10). At the transcriptional level, VEGF increases the expression of the transcription factor E2F, which directly enhances EZH2 expression (8, 9); this effect can be blocked by treatment with an anti-VEGF receptor 2 (VEGFR-2) antibody (8). In endothelial cells, VEGF/VEGFR-2 activity downregulates expression of miR-101 and thus indirectly increases expression of EZH2 (9). In breast cancer cells, a hypoxic tumor microenvironment increases EZH2 expression via the action of hypoxia-inducible factor (HIF)-1α (11). In this context, we recently observed that VEGF regulates HIF-1α expression levels in NSCLC cell lines overexpressing VEGFR-2 independently of hypoxia (13). This suggests the possibility that VEGF/VEGFR-2 pathway may regulate tumor expression of EZH2 via HIF-1α expression.
We investigated the ability of the VEGF/VEGFR-2 pathway to regulate the expression of EZH2 in lung adenocarcinoma cell lines and the biologic impact of EZH2 abrogation by pharmacologically induced and small interfering RNA (siRNA)-mediated depletion of EZH2 on tumor cell proliferation, migration, and chemoresistance in response to both standard platinum-based chemotherapy and VEGFR-2–targeted therapy in lung adenocarcinoma cell lines. To further explore the role and function of EZH2 in lung cancer pathogenesis we characterized EZH2 and miR-101 expression in lung adenocarcinoma specimens and correlated it with clinical characteristics of patients. Our studies provide evidence of how EZH2 expression is deregulated, its important role of EZH2 in lung cancer pathogenesis, and the possibility of making it a therapeutic target and the clinicopathologic consequences for patients of its deregulation in lung adenocarcinoma.
Materials and Methods
Cell lines and tumor specimens
Lung adenocarcinoma cell lines were provided by Drs. Adi Gazdar and John Minna (The University of Texas Southwestern Medical Center) and authenticated using DNA fingerprinting (14). The cell lines were cultured in RPMI 1640 (Cellgro; Mediatech, Inc.) containing 10% fetal bovine serum (FBS) and antibiotics (Sigma-Aldrich) at 37°C in 5% CO2 in a cell culture incubator.
Archived frozen and formalin-fixed, paraffin-embedded tumor specimens obtained from NSCLC patients who underwent surgical resection with curative intent were collected from the Lung Cancer Specialized Program of Research Excellence tissue bank at The University of Texas MD Anderson Cancer Center. One hundred forty-nine specimens were selected randomly: 56 were obtained from patients given adjuvant platinum-based chemotherapy, and 93 were obtained from patients who did not receive this therapy. Detailed clinical and pathologic information on the patients is presented in Supplementary Table 1. The study protocol was approved by the MD Anderson Institutional Review Board.
mRNA and microRNA analyses
Total RNA was extracted from cell lines and frozen tumor specimens using TRI Reagent (Life Technologies). Spectrophotometric analysis using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) was performed to determine the RNA quantity in cell lines and tumor specimens, and the quality of RNA was assessed using Agilent BioAnalyzer RNA Nanochips (Agilent Technologies). RNA extracted from lung adenocarcinoma cell lines was subjected to quantitative reverse transcriptase (qRT)-polymerase chain reaction (PCR) analysis using a High Capacity RNA-to-cDNA Kit and TaqMan Gene Expression PCR assays (Applied Biosystems) to detect their EZH2 message levels using GAPDH as an endogenous control. Also, TaqMan microRNA assay (Applied Biosystems) was employed to detect the levels of miR-101 expression using U6 as an endogenous control. An ABI PRISM 7300 Sequence Detection System (Applied Biosystems) under standard PCR assay cycling conditions with triplicate specimens was used to determine relative levels of expression miR-101 in cell lines with the 2-ΔΔCt method and ABI 7300 SDS software program (version 1.4; Applied Biosystems). Total RNA extracted from tumor specimens was used to determine EZH2 and miR-101 expression levels in the specimens using Illumina WG-6 v.3 mRNA and Agilent Technologies V3 human microRNA arrays, respectively. Illumina v.3 datasets of 275 primary lung adenocarcinomas and squamous cell carcinomas (SPORE dataset) (GSE41271) have been deposited in the GEO repository (15).
VEGF stimulation
Cells lines were serum-starved for 24 hours and stimulated in fresh medium with 50 ng/mL VEGF-A (Cell Signaling Technology). Cells were then incubated under normoxic conditions, and protein lysates were collected 18 hours later. Western blot analysis was carried out using specific antibodies against EZH2 (AC22), H3K27me3 (C36B11), HIF-1α (HIF-1α antibody), VEGFR-2 (55B11) (Cell Signaling Technology), and E2F3 (ab54945; Abcam).
Transfection of lung adenocarcinoma cells with siRNAs and human KDR cDNA and cell migration/proliferation assays
Lung adenocarcinoma cell lines were transfected with three gene-specific siRNA (siRNA-3) duplexes for the EZH2, KDR, and HIF-1α genes, respectively, and a control siRNA (OriGene Technologies) at a final concentration of 10 nmol/L using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions. To verify the knockdown efficiency of each gene what was knocked down, mRNA and proteins were collected from transfected cells for qRT-PCR and Western blot analysis. VEGFR-2 (KDR) full-length human cDNA and control plasmid constructs were purchased from OriGene Technologies, and the lung adenocarcinoma cell line A549 was transfected with KDR plasmid cDNA (pKDR) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. A cell migration assay was carried out using Boyden chambers as described previously (13). Also, cell proliferation assays were carried out using the MTS assay according to the assay manufacturer’s protocols.
Treatment with 3-deazaneplanocin A
The day after placement of NSCLC cell lines in tissue-culture dishes, the media in the dishes were replaced with fresh complete media or complete media containing 3-deazaneplanocin A (DZNep; Cayman Chemical) at increasing concentrations. To determine the effect of treatment with DZNep on EZH2 expression and assess apoptosis, cell lines were treated with DZNep at different concentrations (0, 2.5, and 5.0 μM) for 72 hours. Also, protein lysates were collected from subconfluent cultures after 72 hours of growth in media with 5% FBS and in the presence or absence of DZNep and subjected to Western blot analysis with specific antibodies against EZH2 (AC22), trimethylated lysine 27 on histone H3 (H3K27me3; C36B11), and cleaved poly(ADP-ribose) polymerase (PARP-C; Asp214) (Cell Signaling Technology).
MTS assay and treatment of lung adenocarcinoma cells with platinum agents and a VEGFR-2 inhibitor
Cisplatin, carboplatin, and the VEGFR-2 inhibitor AZD2171 (cediranib) were purchased from Selleck Chemicals. To determine the median half-maximal inhibitory concentrations (IC50s) of these drugs, lung adenocarcinoma cells were seeded in octuplicate at a density of 2000 cells per well in 96-well plates. The following day, cells were treated with the drugs at increasing concentrations, and an endpoint viability assay was performed after 72 hours of treatment using MTS assays (Promega). The dual drug studies (cisplatin+DZNep, carboplatin+DZNep and AZD2171+DZNep) were carried out in a similar manner.
Xenograft studies
Female athymic nude mice, 6 to 8 weeks old, were injected subcutaneously in the flank with 1 × 105 of HCC4006 cell lines. Tumors were allowed to grow for two week; once tumors were palpable mice were randomized into treatment groups of eight mice per group for the tumor growth experiments. The following treatments were administered in cohorts of 8 mice for each treatment: vehicle alone, DZNep PBS 1mg/kg (50ul, PI), AZD2171 (Cediranib) was suspended in 1% (w/v) aqueous polysorbate 80, 1.5 mg/kg (50ul, oral gavage), or the combination at the indicated doses (DZNep 1 mg/kg+ AZD2171 1.5 mg/kg). DZNep was administered thrice per week treatment for three week and AZD2171 was administered daily. Tumors were measured twice a week with calipers. Tumor volumes were calculated according to (L×W2)/2. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at The University of Texas MD Anderson Cancer Center.
Statistical analysis
Data obtained from cell culture assays and qRT-PCR and Western blot analyses were summarized using descriptive and inferential statistics accompanied by graphs of relative expression with the Prism software program (version 5.0; GraphPad Software). Western blot analyses were carried out multiples times and normalized to β-Actin protein by densitometric scanning and quantified using ImageJ software National institutes of health (NIH’s). The patients’ demographic and clinical information was compared using the chi-square, Fisher exact, Wilcoxon rank-sum, and Kruskal-Wallis tests. Overall survival (OS) and recurrence-free survival (RFS) distributions were estimated using the Kaplan-Meier method, and the distributions in the groups of patients were compared using the log-rank test. Cox proportional hazard models were used for regression analysis of survival data and conducted for OS duration, defined as the time from surgery to the patient’s death or last contact and RFS duration, defined as the time from surgery to tumor recurrence or the patient’s last contact. The follow-up duration was censored at 5 years.
Results
EZH2 and miR-101 expression in lung adenocarcinoma cell lines
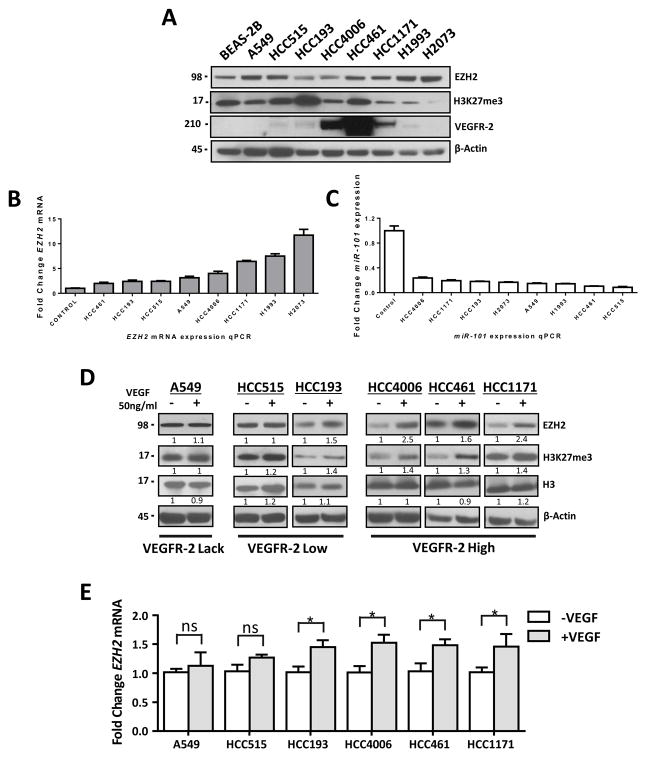
To explore the mechanisms of regulation and role of EZH2 in lung cancer pathogenesis in vitro, we first characterized EZH2, miR-101and VEGFR-2 expression in lung adenocarcinoma cell lines. We screened a panel of eight lung adenocarcinoma cell lines for expression of EZH2 mRNA and protein using qRT-PCR and Western blotting, respectively. In addition, we examined the global levels of H3K27me3 and VEGFR-2 expression in these cells using Western blotting. We found that all eight lung adenocarcinoma cell lines had detectable expression of EZH2 mRNA and protein and of H3K27me3 and high, low, and lack of VEGFR-2 protein expression (Figs. 1A, and 1B). Because miR-101 is known to posttranscriptionally downregulate the expression of EZH2, we examined miR-101 expression in the same set of cell lines using qRT-PCR. Consistent with the expression of EZH2, we observed low levels of miR-101 expression in all eight cell lines (.Fig. 1C).
Figure 1.
VEGF stimulation leads to significantly increased expression of EZH2 and increased methylation of H3K27 in lung adenocarcinoma cell lines overexpressing VEGFR-2. A, Western blot of EZH2, H3K27me3 and VEGFR-2 expression in lung adenocarcinoma cell lines showing detectable expression of EZH2 and of H3K27me3 and high, low, and lack of VEGFR-2 protein expression. B, EZH2 mRNA expression using qRT-PCR analysis. C, miR-101 expression using a TaqMan microRNA assay and detected low miR-101 expression levels. D, Western blot of EZH2, H3K27me3 and Histone H3 expression in lung adenocarcinoma cell lines stimulated with VEGF.. E, EZH2 mRNA expression in lung adenocarcinoma cell lines stimulated with VEGF as determined using qRT-PCR. *P < 0.05.
Activation of the VEGF/VEGFR-2 pathway upregulates tumor expression of EZH2 in a VEGFR dependent manner
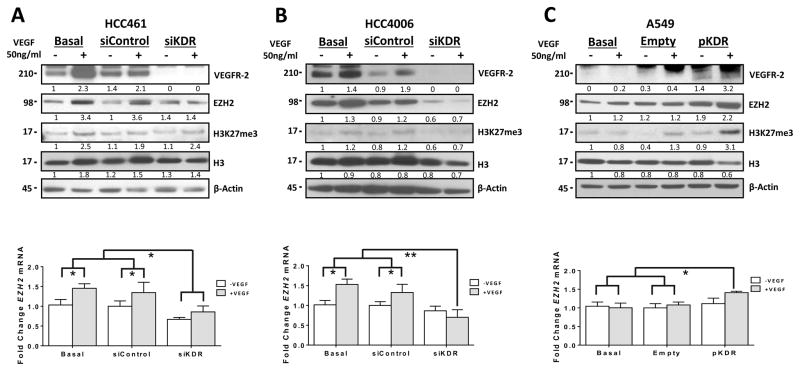
Based on previous reports describing an association between increased activity of VEGF/VEGFR-2 pathway and EZH2 in tumor endothelial cells (8, 9), we sought to determine whether VEGF/VEGFR-2 pathway can regulate the expression of EZH2 in an larger panel of lung adenocarcinoma cells. We found that upon VEGF stimulation, serum-starved cell lines had increased expression of EZH2 protein and mRNA (Figs. 1D and 1E). We also observed increased H3K27me3 levels in cells treated with VEGF (Fig. 1D). Interestingly, these changes in EZH2 and H3K27 were more common in lung adenocarcinoma cell lines with high expression of VEGFR-2 (HCC4006, HCC461, HCC1171, H2085 and CALU-1) than in cell lines with low expression of it (HCC515, HCC193 and H1993), and the changes did not occur at all in a cell line lacking VEGFR-2 expression (A549, H2073, HCC827 and H23) (Figs. 1D and E and Supplementary Figs. 1A and B). To determine whether VEGFR-2 expression is required for induction of EZH2 expression in the presence of VEGF, we knocked down VEGFR-2 expression using treatment with a VEGFR-2–specific siRNA (siKDR) in HCC461 and HCC4006 cells, which have high levels of VEGFR-2 protein expression. In both cell lines, treatment with siKDR resulted in decreased VEGFR-2 expression than that in nontransfected cells or cells transfected with scrambled siRNA (control) (Figs. 2A and 2B). Moreover, EZH2 mRNA and protein and H3K27me3 expression levels in HCC461 cells remained unchanged in response to exposure to VEGF, whereas the expression levels decreased in HCC4006 cells after knockdown of VEGFR-2 expression (Figs. 2A and 2B,). Additionally, we investigated overexpression of VEGFR-2 induced by transfection of a VEGFR-2 cDNA (pKDR) construct to determine whether it can induce expression of EZH2 in the presence of VEGF. To that end, we transfected pKDR into the A549 cell line, which lacks expression of VEGFR-2, and observed significantly increased expression of EZH2 mRNA and protein and H3K27me3 level in response to exposure to VEGF than that in control cells transfected with an empty vector (Figs. 2C).
Figure 2.
Knockdown of VEGFR-2 expression by treatment with siRNA decreased the expression of EZH2 and H3K27me3 in lung adenocarcinoma cell lines stimulated by VEGF. A, and B, Western blots of VEGFR-2 (siKDR), EZH2, H3K27me3, and H3 expression in the lung adenocarcinoma cell lines HCC461 and HCC4006 upon knockdown of VEGFR-2 expression by treatment with siRNA-3 following VEGF stimulation. A, and B,, bottom panel, EZH2 mRNA expression in lung adenocarcinoma cells as determined using qRT-PCR. *P < 0.05; **P < 0.03. C, Western blot of VEGFR-2, EZH2, H3K27me3, and H3 expression in A549 cells transfected with VEGFR-2 (pKDR). C, bottom panel, EZH2 mRNA expression in lung adenocarcinoma cells as determined using qRT-PCR. *P < 0.05. Overexpression of VEGFR-2 (pKDR) increased the expression of EZH2 and H3K27 following VEGF stimulation in A549 cells.
EZH2 expression is regulated by E2F3 via the VEGF/VEGFR-2 pathway
VEGF is known to transcriptionally regulate the expression of EZH2 in tumor endothelial cells via the E2F transcription factors, mainly E2F1 and E2F3 (8, 16). To determine whether this regulatory mechanism is also present in tumor cells, we analyzed the E2F1 and E2F3 mRNA expression in lung adenocarcinoma cell lines stimulated with VEGF. We found that VEGF induced expression of E2F3 transcripts and protein but not of E2F1 in cell lines expressing VEGFR-2 (HCC515, HCC193, HCC4006, HCC461, and HCC1171) (Supplementary Figs. 2A and 2B). We did not observe this effect in the A549 cell line, which lacks VEGFR-2 expression (Supplementary Figs. 2A and B) Additional experiments demonstrated that increased expression of E2F3 in response to VEGF stimulation was dependent on the presence of VEGFR-2, as knockdown of VEGFR-2 expression abrogated the response of HCC461 and HCC4006 cells to VEGF stimulation (Supplementary Figs. 2C and D)). Moreover, we found increased expression of E2F3 in response to VEGF stimulation in A549 cells transfected with VEGFR-2 cDNA (Supplementary Fig. 2E). These findings suggest that the VEGF/VEGFR-2 pathway play key role in regulation of EZH2 expression via E2F3 in lung adenocarcinoma cell lines.
HIF-1α also increases EZH2 via the VEGF/VEGFR-2 pathway in a hypoxia independent manner
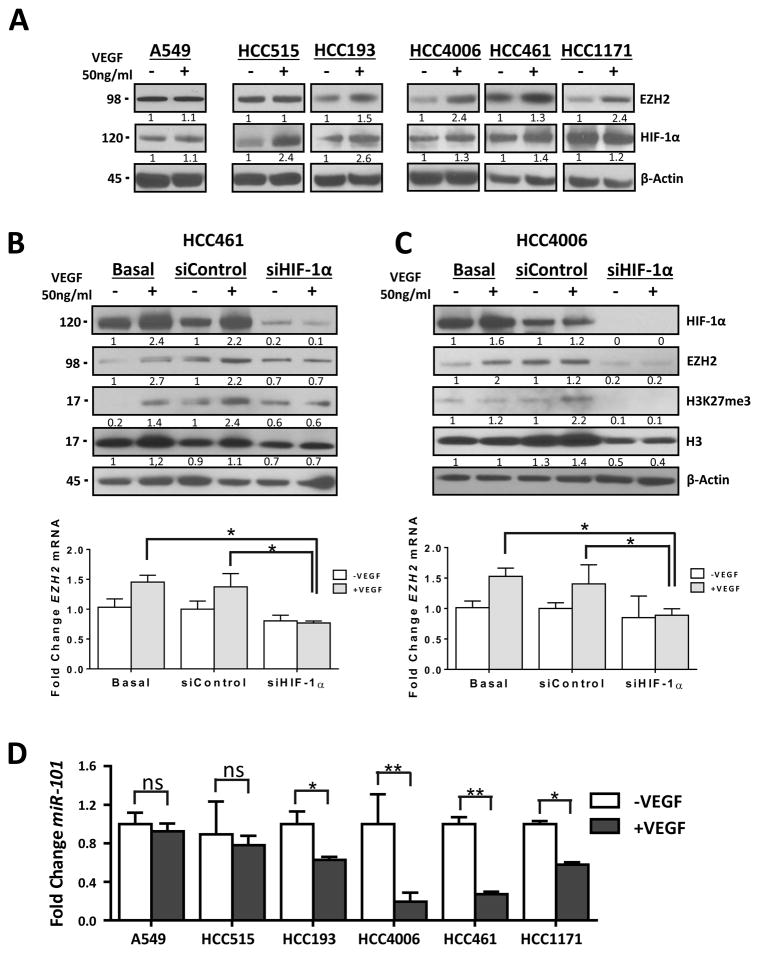
In addition to regulation of EZH2 expression via VEGF/VEGFR-2/E2F3, a recent report on breast tumors stated that a hypoxic tumor microenvironment increases EZH2 expression via induction of HIF-1α expression (11). Additionally, we recently observed that VEGF regulates the expression of HIF-1α independently of hypoxia in NSCLC cell lines that overexpress VEGFR-2 (13). We confirmed these previous results in the present study and observed responses of HIF-1α and EZH2 expression to VEGF stimulation. Specifically we detected a pronounced increase in co-expression of EZH2 and HIF-1α in response to VEGF stimulation in lung adenocarcinoma cell lines with high expression of VEGFR-2 (HCC4006, HCC461, and HCC1171) (Fig. 3A ). The increase in co-expression was less pronounced in cell lines with low VEGFR-2 expression (HCC515 and HCC193) and absent from a cell line lacking VEGFR-2 expression (A549) (Fig. 3A). The increased expression of HIF-1α in response to VEGF stimulation was reduced by knocking down expression of VEGFR-2 in HCC461 and HCC4006 cells (Supplementary Figs. 2C and D)). Moreover, we found increased expression of HIF-1α in response to VEGF stimulation in A549 cells transfected with VEGFR-2 cDNA (Supplementary Fig. 2E). To determine whether HIF-1α expression is required for induction of EZH2 expression in the presence of VEGF, we knocked down HIF-1α expression using an HIF-1α–specific siRNA (siHIF-1α) in HCC461 and HCC4006 cells, which exhibited strong responses to stimulation with VEGF. siHIF-1α produced lower HIF-1α expression in both cell lines than in control cells transfected with scrambled siRNA and in nontransfected cells (Figs. 3B and 3C). We also found that increased expression of EZH2 and H3K27me3 in response to VEGF stimulation was reduced by knocking down expression of HIF-1α, which was more evident in HCC4006 than HCC461 cells (Figs. 3B and 3C). These results suggested that increased expression of HIF-1α mediated by stimulation with VEGF can upregulate expression of EZH2 in lung adenocarcinoma cell lines expressing VEGFR-2. Additionally we evaluate whether a hypoxic microenvironment induces the expression of EZH2 in lung cancer. HCC4006 and HCC461 cells were plated in incubated in normoxia (20% oxygen) or hypoxia (1% oxygen). Hypoxia increase in co-expression of EZH2 and HIF-1α in lung adenocarcinoma cell lines (Supplementary Figs. 3A and B)
Figure 3.
Knockdown of HIF-1α expression by treatment with siRNA induces decreased EZH2 and H3K27me3 expression and EZH2 inhibitor increased the sensitivity of lung adenocarcinoma cells to VEGFR-2–targeted therapy. A, Western blot of EZH2 and HIF-1α expression in lung adenocarcinoma cell lines stimulated with VEGF. B and C, Western blots of HIF-1α, EZH2, and H3K27me3 expression in HCC461 and HCC4006 cells upon knockdown of HIF-1α expression by treatment with siRNA-3 following VEGF stimulation. B and C, bottom panel, EZH2 mRNA expression in lung adenocarcinoma cells as determined using qRT-PCR. *P < 0.05. D, miR-101 expression in lung adenocarcinoma cell lines stimulated with VEGF as determinate using qRT-PCR. *P < 0.05; **P < 0.03.
miR-101 regulates EZH2 via the VEGF/VEGFR-2 pathway
Recently, investigators showed that EZH2 expression is regulated at the posttranscriptional level by miR-101 (10, 12). Of note, in endothelial cells, VEGF downregulates miR-101 expression, resulting in upregulation of EZH2 expression (9). To determine the effects of VEGF stimulation on miR-101 expression and whether decreased miR-101 expression correlates with EZH2 expression, we stimulated lung adenocarcinoma cell lines with VEGF and analyzed miR-101 expression in the cell lines using qRT-PCR. We found that VEGF stimulation led to downregulation of miR-101 expression and increased EZH2 expression. This phenomenon was more pronounced in cell lines with high expression of VEGFR-2 (HCC4006, HCC461, and HCC1171), less pronounced in those with low expression of VEGFR-2 (HCC515 and HCC193), and absent from a cell line lacking expression of VEGFR-2 (A549) (Fig. 3D). Downregulation of miR-101 expression in response to VEGF stimulation was reduced by knocking down VEGFR-2 expression in HCC461 and HCC4006 cells (Supplementary Figs. 2C and D, bottom panel)). This finding was more evident in HCC4006 cells than in HCC461 cells. Furthermore, we observed downregulation of miR-101 expression together with an increase in EZH2 expression in response to VEGF stimulation in A549 cells transfected with VEGFR-2 cDNA (Supplementary Fig. 2E, bottom panel). These results suggested that VEGF/VEGFR-2 pathway regulates EZH2 expression via miR-101 in lung adenocarcinoma cell lines. Additionally, we evaluated the expression of miR-101 in hypoxia condition in lung adenocarcinoma cell lines. Hypoxia condition decreased miR-101 expression correlates with increase of EZH2 expression in lung adenocarcinoma cell lines (Supplementary Figs. 3C)
Moreover, to determine whether increased EZH2 is the result of decreased miR-101 by stimulation with VEGF, we overexpressed miR-101 by transfection of a miR-101-3p mimic in HCC461 cells and stimulated in the presence or absence of VEGF. Transfection of miR-101 inhibits the expression of EZH2 mRNA and protein and remained unchanged in response to exposure to VEGF in HCC461 cells (Supplementary Figs. 4A-C). These results confirm that miR-101 regulates EZH2 expression in lung adenocarcinoma cell lines.
Effect of inhibition of EZH2 activity and expression by treatment with a small molecule inhibitor EZH2 activity and siRNA
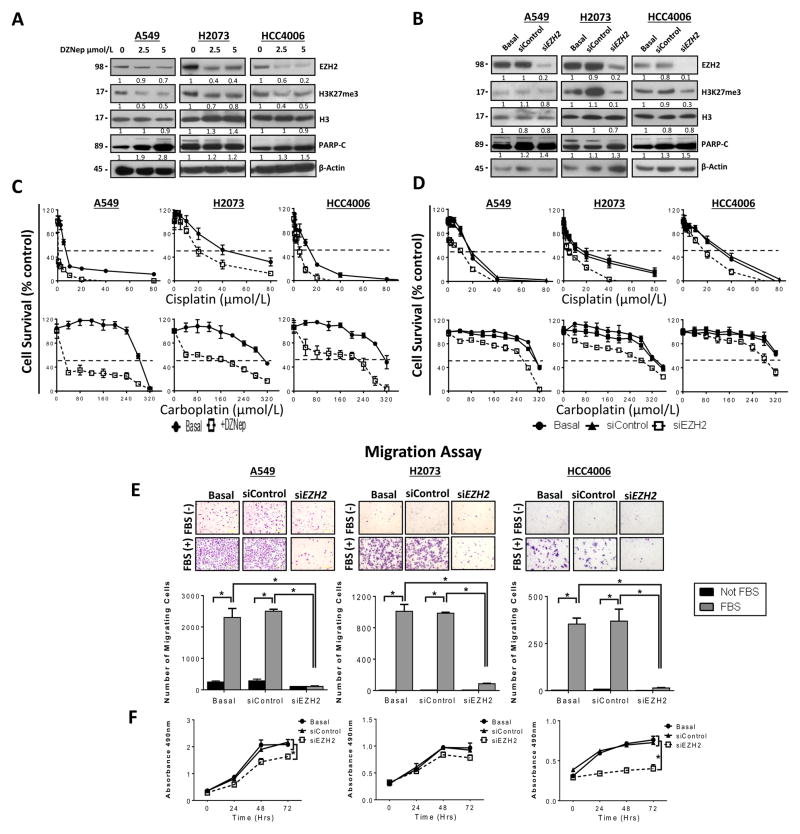
To determine the effects of abrogation of EZH2 activity in lung adenocarcinoma cell lines, we selected a subset of cell lines based on their EZH2 protein expression profiles (HCC4006 low expression; A549, intermediate expression; and H2073, high expression). In these three cell lines, EZH2 activity was inhibited pharmacologically by treatment with DZNep, and EZH2 gene expression was knocked down by treatment with siRNA. Researchers recently identified DZNep, a small molecule that efficiently inhibits EZH2 activity, by depleting EZH2 expression levels and inhibiting trimethylation of H3K27 in cancer cells via treatment with this molecule in a dose-dependent manner (17, 18). To determine the effect of DZNep on cell viability, we treated the cell lines with it at increasing doses (range, 0–10 μM) and observed that DZNep only slightly decreased cell viability by 15% in H2073 and A549 cells, and 30% in HCC4006 cells (Supplementary Fig. 5). This response plateaued at higher concentrations, suggesting that DZNep had a cytostatic effect on these cell lines. Additionally, we determined whether DZNep decreased the expression of EZH2 and induced apoptosis in these cell lines by treating them with the molecule at different doses (0, 2.5, and 5.0 μM) for 72 hours. We found DZNep treatment decreased the expression of EZH2 in a dose-dependent manner (Fig. 4A), and reduced levels of H3K27 and increased the expression of cleaved poly(ADP-ribose) polymerase (PARP-C) in all of the cell lines (Fig. 4A). DZNep is also known to mediate the depletion of other components of PRC2, such as embryonic ectoderm development (17). We validated the results of treatment with DZNep by knocking down EZH2 expression in these cell lines using treatment with siRNA. Similar to our results with DZNep, Western blot analysis of protein lysates of cell lines treated with EZH2-specific siRNA demonstrated lower EZH2 expression (Fig. 4B) and H3K27me3 expression concomitant with higher PARP-C level (Fig. 4B) than that in nontransfected cells and cells transfected with scrambled siRNA (control). Thus, we found targeting EZH2 pharmacologicaly or genetically resulted in decreased EZH2 levels, reduced H3K27 methylation and increased markers of apoptosis.
Figure 4.
Pharmacologic inhibition of EZH2 activity by treatment with DZNep and knockdown of EZH2 expression by treatment with siRNA decreased the expression of EZH2 and H3K27me3, induced apoptosis, increased sensitivity to treatment with cisplatin and carboplatin, and reduced migration and proliferation in lung adenocarcinoma cells. A and B, Western blot analysis of EZH2, H3K27me3, and PARP-C expression in the lung adenocarcinoma cell lines A549, H2073, and HCC4006 upon inhibition of EZH2 by treatment with different doses of DZNep (0, 2.5, and 5.0 μM) (A) and knockdown of EZH2 expression by treatment with siRNA-3 (B). Both approaches decreased the expression of EZH2 and H3K27me3 and increased the expression of PARP-C. C and D, pharmacologic inhibition of EZH2 by treatment with DZNep (C) and knockdown of EZH2 expression by treatment with siRNA-3 (D) decreased the viability of the lung adenocarcinoma cell lines A549, H2073, and HCC4006 exposed to cisplatin and carboplatin according to an MTS assay (data are graphed as the mean percent increase ± percent standard deviation). Treatment with DZNep caused a 26-fold (P < 0.03) decrease in the cisplatin IC50 in A549 cells, a 1.7-fold (P < 0.05) decrease in it in H2073 cells, and a 2.4-fold (P < 0.05) decrease in it in HCC4006 cells. Treatment with DZNep also caused 27.7 (P < 0.03) -, 3.7 (P < 0.05) -, and 2.3-fold (P < 0.05) decreases in the carboplatin IC50 in A549, H2073, and HCC4006 cells, respectively. Knockdown of EZH2 expression caused a 3.8-fold (P < 0.05) decrease in the cisplatin IC50 in A549 cells, a 1.5-fold (P < 0.05) decrease in it in H2073 cells, and a 1.7-fold (P < 0.05) decrease in it in HCC4006 cells. Additionally, knockdown of EZH2 expression caused 1.2-, 1.5-, and 1.2-fold (P < 0.05) decreases in the carboplatin IC50 in A549, H2073, and HCC4006 cells, respectively. E, inhibition of migration of A549, H2073, and HCC4006 cells by treatment with siEZH2 with and without stimulation with FBS 5% in a Boyden chamber assay. E, bottom panel, quantification of the results of a migration assay of lung adenocarcinoma cell lines before and after knockdown of EZH2 expression by treatment with siEZH2 with and without stimulation with FBS showed decreased cell migration (*P<0.01). F, knockdown of EZH2 expression resulted in significantly lower rates of proliferation of HCC4006 and A549 cells (*P < 0.05) and, to a lesser extent, lower rates of that of H2073 cells than of control siRNA-transfected and nontransfected cells.
We next investigated the effects of EZH2 inhibition on the sensitivity of the cell lines to treatment with cisplatin and carboplatin. We pretreated three lung adenocarcinoma cell lines (HCC4006, A549, and H2073) with 2.5 μM DZNep for 24 hours and then treated them with cisplatin or carboplatin in the presence or absence of DZNep at a fixed concentration (2.5 μM) for 72 hours. We found that the sensitivity of the three cell lines to cisplatin and carboplatin in vitro (Fig. 4C) was significantly higher (P < 0.05) with DZNep-based treatment than without it. This strongly suggested that DZNep-mediated inhibition of EZH2 expression and activity sensitizes lung adenocarcinoma cell lines to platinum-based drugs. We confirmed these results via knockdown of EZH2 expression by treatment with siRNA (siEZH2) and noted similarly greater sensitivity of the three cell lines to cisplatin and carboplatin (Fig. 4D). These findings suggest that depletion of EZH2 significantly increases the sensitivity of lung adenocarcinoma cell lines to platinum drugs. Additionally, as shown above the overexpression of VEGFR-2 increased expression of EZH2. We evaluated whether this increased EZH2, produced by the overexpression of VEGFR-2 into the A549 cell line which lacks expression of VEGFR-2, resulting in a decrease in sensitivity to cisplatin and if this can be reversed by knockdown of EZH2 or inhibition with DZNep. We observed that increased expression of VEGFR-2 induces a decrease in sensitivity to cisplatin. To evaluate whether this decrease in sensitivity to cisplatin is due to increased expression of EZH2. EZH2 activity was inhibited by knockdown using treatment with siRNA or treatment with DZNep. We observed that the decrease in sensitivities to cisplatin produced by the overexpression of VEGFR-2, was reversed by EZH2 knockdown and more strongly by inhibition with treatment with DZNep (Supplementary figure 6). These finding suggest a functional connection between VEGFR-2 and EZH2 to promote malignant phenotype and modulate the response to therapy. We evaluated the effect of EZH2 knockdown by siRNA on lung adenocarcinoma cell migration and proliferation. We found that knockdown of EZH2 expression resulted in significantly less migration (Figs. 4E) and proliferation (Fig. 4F) of the three cell lines than of nontransfected cells or cells transfected with scrambled siRNA. Overall, the results of this in vitro analysis of lung adenocarcinoma cell lines supported our hypothesis that EZH2 overexpression promotes the malignant phenotype in lung adenocarcinoma cells by increasing their proliferation, migration, and resistance to platinum drugs.
Association of EZH2 and miR-101 expression with clinicopathologic features and clinical outcome in lung adenocarcinoma patients
We analyzed EZH2 and miR-101 mRNA expression in lung adenocarcinoma specimens using our own Illumina WG-6 v.3 mRNA and Agilent V3 human microRNA array data sets, respectively. Specifically, we investigated the expression of EZH2 and/or miR-101 to determine whether it was associated with clinicopathologic features of the 149 surgically resected lung adenocarcinoma specimens. We found that high EZH2 expression (EZH2High) was significantly correlated with ever-smoker status (P = 0.001) and large tumors (P = 0.049) (Supplementary Table 2). Also, low miR-101 expression (miR-101Low) was significantly correlated with ever-smoker status (P = 0.012) and male sex (P = 0.001) but was not correlated with tumor size.
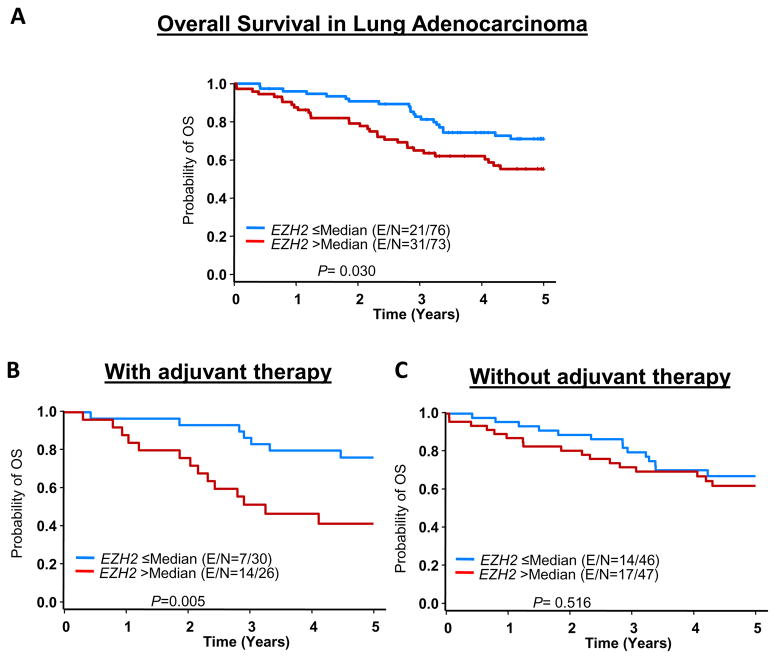
In univariate analysis, EZH2High was significantly associated with poor OS duration (hazard ratio [HR], 1.844 [95% confidence interval (CI), 1.059–3.210)]; P = 0.030) (Fig. 5A). These findings were confirmed in a multivariate analysis, after adjustment for age, tumor size, overall stage, and adjuvant therapy, EZH2High was significantly associated with poor OS duration (HR, 1.828 [95% CI, 1.041–3.209]; P = 0.036, data not show). In multivariate analysis EZH2High was marginally significant correlated with poor OS duration (HR, 2.33 [95% CI, 0.956–5.678]; P = 0.062) in lung adenocarcinoma patients who received adjuvant platinum-based therapy but not in patients who did not receive this therapy (Figs. 5B and 5C and Supplementary Table 3). We did not find a correlation between EZH2 expression and RFS duration or between miR-101 expression and patient outcome.
Figure 5.
Association between EZH2 expression and clinical outcome in patients with lung adenocarcinoma. Gene expression data on RNA extracted from frozen tumor specimens were examined using Illumina WG-6 v.3 mRNA and Agilent Technologies V3 human microRNA arrays. A, Kaplan-Meier OS curves by according to EZH2 expression in all patients. B, Kaplan-Meier OS curves in patients who received adjuvant platinum-based therapy. C, Kaplan-Meier OS curves in patients who did not receive adjuvant platinum-based therapy. E, event; N, total number of cases.
Inhibition of EZH2 activity by DZNep sensitizes lung adenocarcinoma cells to VEGRF-2-targeted therapy
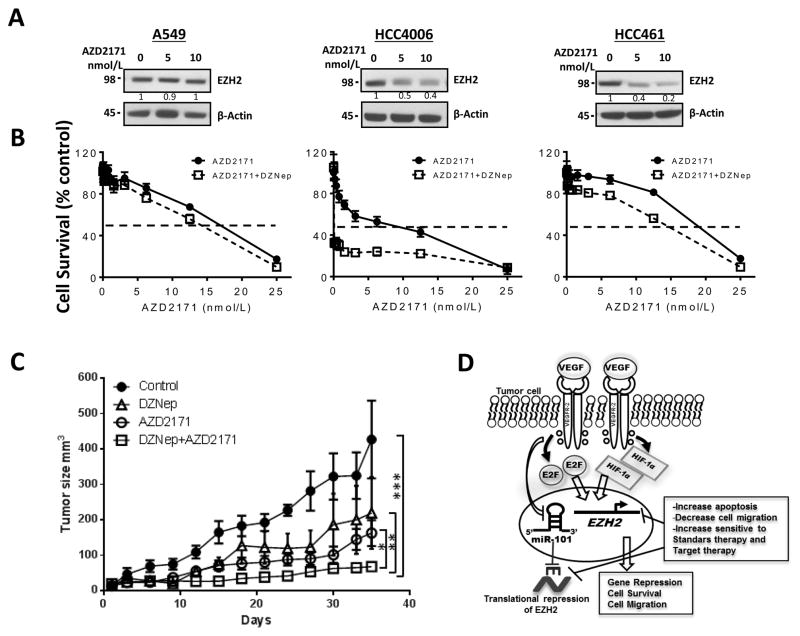
Our in vitro findings demonstrated that treatment with the EZH2 inhibitor DZNep increased the sensitivity of lung adenocarcinoma to treatment with cisplatin and carboplatin. We then asked whether treatment with the combination of an EZH2 inhibitor and VEGFR-2–targeting drugs provides additional therapeutic benefits in NSCLC patients. We first sought to determine whether treatment with AZD2171, a known inhibitor of VEGFR-2 activity, decreases EZH2 expression. We treated A549, HCC461, and HCC4006 cells with different doses of AZD2171 (0, 5, and 10 nM) for 48 hours. We found that this treatment decreased the expression of EZH2 in HCC4006 and HCC461 cells expressing VEGFR-2 but not in A549 cells lacking expression of VEGFR-2 in a dose-dependent manner (Fig. 6A).
Figure 6.
Inhibition of EZH2 activity by DZNep sensitizes in vitro and in vivo to lung adenocarcinoma cells to VEGR-2-targeted therapy. A, Western blots of EZH2 expression in A549, HCC461, and HCC4006 cells upon treatment with different doses of VEGFR-2–inhibitor AZD2171 (0, 5 and 10 nM). AZD2171 decreased the expression of EZH2 in HCC4006 and HCC461 cells expressing VEGFR-2 in a dose-dependent manner but did not do so in A549 cells lacking expression of VEGFR-2. B, pharmacologic inhibition of EZH2 activity by treatment with DZNep decreased the viability of A549, HCC4006, and HCC461 cells exposed to AZD2171 according to an MTS assay (data are graphed as the mean percent increase ± percent SD). DZNep increased sensitivity to AZD2171 treatment in HCC4006 (3.8-fold decrease IC50, P<0.01) and HCC461 (1.4-fold decrease IC50, P<0.05) and slight increase in the sensitivity of A549 cells (1.2-fold decrease in IC50) to this treatment. C, Athymic nude mice were inoculated with HCC4006 cell lines and then treated with vehicle, DZNep, AZD2171 or a combination of DZNep plus AZD2171. Tumor volume was determined for each treatment. The sensitivity in vivo to AZD2171 was significantly increased in the presence of DZNep, observing an inhibition of tumor growth with the combination of DZNep plus AZD2171 versus either treatment alone (*P < 0.05; **P < 0.03, ***P < 0.001)D, Proposed model of the tumor-cell VEGF/VEGFR-2 pathway that upregulates EZH2 expression via increased E2F3 and HIF-1α and downregulated miR-101 expression, promoting the malignant phenotype in lung adenocarcinoma cells. EZH2 depletion at the genetic or protein level promotes increased apoptosis, decreased cell migration, and increased sensitivity to standard platinum-based and VEGFR-2–targeted therapy.
Additionally, we pretreated A549, HCC461, and HCC4006 cells with 2.5 μM DZNep for 24 hours and subsequently exposed them to AZD2171 at various concentrations in the presence or absence of DZNep at a fixed concentration (2.5 μM) for 72 hours. We found that the in vitro sensitivity to AZD2171 was significantly higher in HCC4006 and HCC461 cells (P < 0.05) and slightly higher in A549 cells in the presence of DZNep than in cells treated with AZD2171 in the absence of DZNep (Fig. 6B). This response was more potent in HCC461 and HCC4006 cells, which have high VEGFR-2 expression, than in A549 cells, which lack this expression. Xenograft studies showed similar results, the sensitivity in vivo to AZD2171 in nude mice inoculated with HCC4006 cell lines was significantly increased in the presence of DZNep, observing a inhibition of tumor growth when combined EZH2 inhibition with DZNep and VEGFR-2–target therapy with AZD2171, compared to the result of mice treated with DZNep or AZD2171 alone (Fig. 6C). These results suggested that treatment with anti-VEGFR-2 drugs in combination with an EZH2 inhibitor greatly increases the sensitivity of lung adenocarcinoma to VEGFR-2–targeted therapy.
Discussion
Although EZH2 is widely overexpressed in aggressive tumors, the genetic mechanism of EZH2 upregulation in malignant epithelial cells is unknown. In the present study, we demonstrated that VEGF induced expression of EZH2 and concomitantly increased of H3K27me3 levels, in lung adenocarcinoma cells expressing VEGFR-2. This finding was more common in cells overexpressing VEGFR-2 than in cell lines with low expression or cell than lacking VEGFR-2 expression. In addition to increasing the expression of EZH2, we observed that VEGF stimulation increased the expression of E2F3 and HIF-1α and downregulated the expression of miR-101, three known regulators of EZH2 expression at the transcriptional and posttranscriptional levels. Increased expression of EZH2 together with that of E2F3 and HIF-1α and downregulation of expression of miR-101 in response to VEGF stimulation were reduced by knockdown of expression of VEGFR-2. This finding suggests that the VEGF/VEGFR-2 pathway plays an important role in the regulation of EZH2 expression via upregulation of E2F3 and HIF-1α expression and downregulation of miR-101 expression (Fig. 6D).
VEGF and its receptor VEGFR-2 act as master regulators of angiogenesis, stimulating endothelial cell functions and enhancement of vascular permeability (19–22). VEGF overexpression is associated with tumor progression and poor prognosis (23, 24). In tumor cells as in endothelial cells, overexpression of VEGFR-2 has been associated with cell migration, proliferation, and survival (13, 24–28). Additionally, stimulation with VEGF has caused overexpression of VEGFR-2 in a feed-forward loop (29). In endothelial cells, VEGF increases expression of E2F transcription factors, which bind directly to the promoter of EZH2 and thus activate its transcription (8, 9), and downregulates expression of miR-101, a negative regulator of EZH2, contributing to increased expression of EZH2 (9, 10, 12, 30). In addition, recent work by our group demonstrated that VEGFR-2 overexpression product of the increasing the gene copy number in NSCLC were highly associated with resistance to platinum-based chemotherapy (13). This finding suggests that activation of the VEGF/VEGFR-2 pathway in malignant cells overexpressing VEGFR-2 promotes the malignant phenotype by upregulating EZH2 expression. In malignant cells, EZH2 acts as an intermediary of the functions regulated by the VEGF/VEGFR-2 pathway promoting cell migration, proliferation, and survival.
The hypoxic microenvironment triggered by tumor growth induces expression of HIF-1α, a key regulator that mediates adaptation to hypoxia responsible for the induction of expression of genes that facilitate adaptation and survival of tumor cells to hypoxia microenvironments (31, 32). In a hypoxic microenvironment, HIF-1α binds to the VEGF promoter to stimulate increased VEGF production (11, 33–35). As we recently demonstrated, VEGF in turn regulates HIF-1α expression levels in NSCLC cell lines independently of hypoxia (13) This suggests the existence of a paracrine or autocrine loop between VEGF and HIF-1α that maintains high levels of expression of both of them and promotes angiogenesis. Of note, researchers have found that HIF-1α is associated with chemoresistance of many tumor types (36–38). Also, investigators recently showed that in breast tumors, a hypoxic state increases EZH2 expression via HIF-1α (11), as the EZH2 promoter region contains consensus sequences of with HIF-1α response elements (11). In the present study, we showed for the first time that in malignant cells, the VEGF/VEGFR-2 pathway directly regulates the expression of EZH2 via HIF-1α independently of hypoxia. This suggests that in malignant cells, the VEGF/VEGFR-2 pathway can regulate expression of EZH2 in various ways, increase the expression of E2F3 and HIF-1α, and downregulate the expression of miR-101. Authors have described overexpression of both VEGF and VEGFR-2 in NSCLC cells and that it was associated with poor prognosis (13, 24). Increased activation of this pathway produced by overexpression of VEGF and VEGFR-2 may account for the increased levels of EZH2 expression that we detected in lung adenocarcinoma specimens. However, we do not rule out other regulatory mechanisms associated with EZH2 expression. In addition, recently authors have described in breast cancer that MEK–ERK activation pathway leads to EZH2 overexpression(39), possibly intrinsic status of activation of this pathway may account for the different levels of response to stimulation with VEGF/VEGFR-2 pathways.
Our findings suggest that EZH2 overexpression promotes a more malignant phenotype in lung adenocarcinoma cells. In addition, we demonstrated that pharmacologic and genetic depletion of EZH2 increased the sensitivity of lung adenocarcinoma cell lines to cisplatin and carboplatin, increased apoptosis and reduced the cells’ migration and proliferation capabilities. Furthermore, our results demonstrate that high EZH2 expression in resected lung adenocarcinoma specimens was associated with poor OS durations in patients who received adjuvant platinum-based therapy but not in patients who did not receive this therapy. Interestingly, the association with clinicopathologic features revealed a significant correlation between EZH2 high expression and larger tumors. This correlation is consistent with our in vitro findings showing that the inhibition of EZH2 decreases cell proliferation. These findings suggest that EZH2 is a predictive marker of poor outcome in patients with lung adenocarcinoma treated with platinum agents and that targeting EZH2 with specific inhibitors of it improves the response of lung adenocarcinoma to platinum-based therapy and can reduce the metastatic potential and proliferation of this disease.
EZH2 has oncogenic functions and its overexpression has been associated with a malignant phenotype in tumor cells, and it is implicated to have a role in neoplastic transformation and progression in many tumors (3, 6, 11, 16). EZH2 overexpression has been linked with silencing of numerous tumor suppressor genes that control important cellular processes, such as p15 (INK4b) (40), p19 (ARF) (41), cyclin A (42), E-cadherin (2), Bim (43), and RUNX3 (5). Additionally, authors have extensively described EZH2 overexpression in tumor endothelial cells (3, 5, 7, 8, 44). In tumor endothelial cells, EZH2 overexpression contributes to angiogenesis by methylating and silencing vasohibin 1, an endothelial cell-specific antiangiogenic factor (8), identifying EZH2 as a key regulator of tumor angiogenesis (8, 9). The oncogenic characteristics of EZH2 and its ability to repress gene targets make it a potential therapeutic target in lung cancer cases that may regulate the epigenome to overcome therapy resistance and angiogenesis. In the present study, we demonstrated that treatment with platinum drugs in combination with depletion of EZH2 significantly improved the response of lung adenocarcinoma cells to this therapy. The mechanisms responsible for these results may be in part from induction of a change in the gene expression profile by EZH2 depletion, facilitating the re-expression of epigenetically silenced genes, favoring response to chemotherapy and inducing apoptosis in lung adenocarcinomas. Researchers recently found that EZH2 has a role in modulation of DNA damage response (45). Specifically, EZH2 depletion results in abrogation of both G1 and G2/M cell-cycle checkpoints and promotes DNA-damaging agent-induced apoptosis in both p53+/+ and p53−/− cancer cells via release of transcriptional repression of FBXO32, which binds to and directs p21 for proteasome-mediated degradation (45). Another possible explanation is that EZH2 depletion may cause structural changes in the state of compaction of chromatin that facilitates the accessibility of these genotoxic agents as cisplatin and carboplatin and their interaction with DNA, allowing DNA damage. These mechanisms support the notion that the depletion of EZH2 can overcome resistance to chemotherapy in patients receiving adjuvant platinum-based therapy and exhibit high EZH2 expression, facilitating access to DNA and inducing apoptosis by DNA-damaging agents.
In recent years, investigators have examined a number of approaches to inhibiting VEGF/VEGFR-2 signaling and blocking angiogenesis (46). However, all of the agents directed against VEGF/VEGFR-2 signaling in these studies exhibited low efficiency and high toxicity (47). This raises the need to design new, better strategies therapeutic that overcome these problems. In this context, EZH2 may be an attractive target because of its functionality in promoting angiogenesis, proliferation, migration, and survival of endothelial and tumor cells. Our present results not only demonstrate that the combination of inhibition of EZH2 and platinum-based chemotherapy improves the response of lung adenocarcinoma to this standard chemotherapy but also show for the first time that treatment with the combination of inhibition of EZH2 and targeting of VEGFR-2 with the agent AZD2171 results in a significant increase in sensitivity of tumors to these agents. Additionally, these results can be translated into significant clinical benefits such as reducing the doses used, which in turn can reduce the severity of side effects of these therapies. In summary, our findings indicate that activation of the VEGF/VEGFR-2 pathway upregulates EZH2 expression via increased E2F3 and HIF-1α and downregulated miR-101 expression, indicating the malignant phenotype in lung adenocarcinoma cell (Fig. 6D). EZH2 depletion decreases the malignant potential of lung adenocarcinoma, which decreases the migratory and proliferative capacity of tumor cells, while increasing apoptosis in the cells and their sensitivity to both standard and VEGFR-2–targeted therapy. Additionally, our findings indicate that high EZH2 expression is associated with poor OS durations in patients with lung adenocarcinoma who receive adjuvant platinum-based chemotherapy. The data presented herein identify the VEGF/VEGFR-2 pathway as a regulator of EZH2 expression in malignant cells. Furthermore, the data reveal the role of EZH2 in lung adenocarcinoma progression and identify it as a potential target for overcoming the resistance of tumors to therapy.
Supplementary Material
Translational relevance.
Enhancer of zeste homolog 2 (EZH2) overexpression occurs in a wide variety of cancers. However, the mechanisms of regulation and role associated with EZH2 expression in lung cancer cells are unknown. In this study, we demonstrate for the first time that VEGF/VEGFR-2 pathway is a novel regulator of EZH2 expression in malignant epithelial lung cancer cells through E2F3, HIF-1α, and miR-101. Our work elucidates the importance of EZH2 in lung adenocarcinoma pathogenesis, and identifies it as a potential target for overcoming resistance of tumors to platinum-based chemotherapy and VEGFR-2 targeted therapy. Our finding can be translated into significant clinical benefits such as reducing the doses used, which in turn can reduce the severity of side effects of these therapies.
Acknowledgments
Financial Support: This study was supported part by a Department of Defense PROSPECT grant (W81XWH-07-1-0306), the UT Lung Specialized Programs of Research Excellence grant (P50CA70907; to I.I.W.), and the MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Yu J, Cao Q, Mehra R, Laxman B, Tomlins SA, Creighton CJ, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–31. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 4.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 5.Fujii S, Ochiai A. Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells. Cancer Sci. 2008;99:738–46. doi: 10.1111/j.1349-7006.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huqun, Ishikawa R, Zhang J, Miyazawa H, Goto Y, Shimizu Y, et al. Enhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancer. Cancer. 2011 doi: 10.1002/cncr.26441. [DOI] [PubMed] [Google Scholar]
- 8.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kottakis F, Polytarchou C, Foltopoulou P, Sanidas I, Kampranis SC, Tsichlis PN. FGF-2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B-miR-101-EZH2 pathway. Mol Cell. 2011;43:285–98. doi: 10.1016/j.molcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, et al. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CJ, Yang JY, Xia W, Chen CT, Xie X, Chao CH, et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell. 2011;19:86–100. doi: 10.1016/j.ccr.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JG, Guo JF, Liu DL, Liu Q, Wang JJ. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thorac Oncol. 2011;6:671–8. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Tang X, Riquelme E, Behrens C, Nilsson MB, Giri U, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71:5512–21. doi: 10.1158/0008-5472.CAN-10-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–48. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M, Larsen JE, Lee W, Sun H, Shames DS, Dalvi MP, et al. Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-12-0634-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–35. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp CD, Rao M, Xi S, Inchauste S, Mani H, Fetsch P, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res. 2012;18:77–90. doi: 10.1158/1078-0432.CCR-11-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crea F, Hurt EM, Mathews LA, Cabarcas SM, Sun L, Marquez VE, et al. Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumorigenicity and tumor progression in prostate cancer. Mol Cancer. 2011;10:40. doi: 10.1186/1476-4598-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–94. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–30. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 21.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–68. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson A, Cao R, Roy J, Tritsaris K, Wahlestedt C, Dissing S, et al. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation. 2003;107:1532–8. doi: 10.1161/01.cir.0000055324.34758.32. [DOI] [PubMed] [Google Scholar]
- 23.Fontanini G, Faviana P, Lucchi M, Boldrini L, Mussi A, Camacci T, et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br J Cancer. 2002;86:558–63. doi: 10.1038/sj.bjc.6600130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontanini G, Boldrini L, Chine S, Pisaturo F, Basolo F, Calcinai A, et al. Expression of vascular endothelial growth factor mRNA in non-small-cell lung carcinomas. Br J Cancer. 1999;79:363–9. doi: 10.1038/sj.bjc.6690058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii H, Yazawa T, Sato H, Suzuki T, Ikeda M, Hayashi Y, et al. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs) Lung Cancer. 2004;45:325–37. doi: 10.1016/j.lungcan.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Ludovini V, Gregorc V, Pistola L, Mihaylova Z, Floriani I, Darwish S, et al. Vascular endothelial growth factor, p53, Rb, Bcl-2 expression and response to chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2004;46:77–85. doi: 10.1016/j.lungcan.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Seto T, Higashiyama M, Funai H, Imamura F, Uematsu K, Seki N, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53:91–6. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Spannuth WA, Nick AM, Jennings NB, Armaiz-Pena GN, Mangala LS, Danes CG, et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124:1045–53. doi: 10.1002/ijc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, et al. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–93. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, Niers JM, et al. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710–20. doi: 10.18632/oncotarget.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 32.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–7. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 33.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–12. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 34.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 35.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jubb AM, Buffa FM, Harris AL. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med. 2010;14:18–29. doi: 10.1111/j.1582-4934.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minn H, Jaakkola P. Tumor hypoxia:advantage or disadvantage? Duodecim. 2005;121:1601–4. [PubMed] [Google Scholar]
- 38.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Fujii S, Tokita K, Wada N, Ito K, Yamauchi C, Ito Y, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30:4118–28. doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 40.Paul TA, Bies J, Small D, Wolff L. Signatures of polycomb repression and reduced H3K4 trimethylation are associated with p15INK4b DNA methylation in AML. Blood. 2010;115:3098–108. doi: 10.1182/blood-2009-07-233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi J, Takashina T, Kinoshita I, Kikuchi E, Shimizu Y, Sakakibara-Konishi J, et al. Epigenetic therapy with 3-deazaneplanocin A, an inhibitor of the histone methyltransferase EZH2, inhibits growth of non-small cell lung cancer cells. Lung Cancer. 2012;78:138–43. doi: 10.1016/j.lungcan.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY, Yu Q. Polycomb protein EZH2 regulates E2F1-dependent apoptosis through epigenetically modulating Bim expression. Cell Death Differ. 2010;17:801–10. doi: 10.1038/cdd.2009.162. [DOI] [PubMed] [Google Scholar]
- 44.Smits M, Mir SE, Nilsson RJ, van der Stoop PM, Niers JM, Marquez VE, et al. Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS One. 2011;6:e16282. doi: 10.1371/journal.pone.0016282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Lee ST, Qiao Y, Li Z, Lee PL, Lee YJ, et al. Polycomb protein EZH2 regulates cancer cell fate decision in response to DNA damage. Cell Death Differ. 2011;18:1771–9. doi: 10.1038/cdd.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone RL, Sood AK, Coleman RL. Collateral damage: toxic effects of targeted antiangiogenic therapies in ovarian cancer. Lancet Oncol. 2010;11:465–75. doi: 10.1016/S1470-2045(09)70362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.