Abstract
The super-relaxed state of myosin (SRX), in which the myosin ATPase activity is strongly inhibited, has been observed in a variety of muscle types. It has been proposed that myosin heads in this state are inhibited by binding to the core of the thick filament in a structure known as the interacting-heads motif. The myosin inhibitor blebbistatin has been shown in structural studies to stabilize the binding of myosin heads to the thick filament, and here we have utilized measurements of single ATP turnovers to show that blebbistatin also stabilizes the SRX in both fast and slow skeletal muscle, providing further support for the proposal that myosin heads in the SRX are also in the interacting-heads motif. We find that the SRX is stabilized using blebbistatin even in conditions that normally destabilize it, e.g., rigor ADP. Using blebbistatin we show that spin-labeled nucleotides bound to myosin have an oriented spectrum in the SRX in both slow and fast skeletal muscle. This is to our knowledge the first observation of oriented spin probes on the myosin motor domain in relaxed skeletal muscle fibers. The spectra for skeletal muscle with blebbistatin are similar to those observed in relaxed tarantula fibers in the absence of blebbistatin, demonstrating that the structure of the SRX is similar in different muscle types and in the presence and absence of blebbistatin. The mobility of spin probes attached to nucleotides bound to myosin shows that the conformation of the nucleotide site is closed in the SRX.
Introduction
The thick filaments, which form the central part of the sarcomere in striated muscle fibers, are composed primarily of the protein myosin. The tail regions of myosin aggregate together, along with a number of accessory proteins, to form the core of the filament. The myosin heads can project out from the filament in a disordered state, where they interact with the actin thin filaments to produce force. The myosin heads can bind to the core of the thick filament in an ordered helical array, as observed using small-angle x-ray diffraction and electron microscopy (1–4). Cryo-electron microscopy has provided images of the structure of the myosin heads on the surface of the thick filament, with the greatest resolution in tarantula filaments (5,6). The two myosin heads of one molecule were shown to interact with one another in a structural arrangement called the interacting-heads motif (IHM). A similar structural arrangement had been seen earlier in two-dimensional crystals of a fragment of myosin from smooth muscle (7). Because the evolutionary distance between tarantula exoskeletal fibers and vertebrate smooth muscle cells is so great, this observation suggested that the IHM is likely widespread among diverse muscle types (2,4,8). The motif has now been observed by electron microscopy in a number of invertebrate muscle fibers, including scorpion, Limulus, and scallop, as well as in thick filaments from vertebrate cardiac muscle and isolated proteins from vertebrate skeletal muscle (2,4–7,9–12).
Blebbistatin is a small, recently discovered molecule that inhibits the ATPase activity of myosin 2 (13). It binds inside the prominent 50 K cleft that bisects the head region of myosin (14). It is thought that by preventing the cleft from closing, blebbistatin stabilizes a conformation of the nucleotide pocket, inhibiting the rapid hydrolysis and release of nucleotides. It inhibits the ATPase activity of skeletal myosin by 10- to 20-fold (15,16). Blebbistatin also stabilizes the binding of myosin heads to the core of the thick filament in the helical array (17,18). X-ray diffraction of vertebrate fast skeletal muscle fibers shows that blebbistatin induces a roughly three times greater affinity of myosin heads for the core of the thick filament (17). Blebbistatin has also been used to stabilize this helical array for electron microscopy of cardiac filaments (11) and to stabilize the IHM in purified preparations of fast skeletal muscle myosin (2). It is hypothesized that by stabilizing a conformation of the myosin head with a closed nucleotide pocket, blebbistatin favors the IHM (18,19).
Our own lab has been studying single fluorescent nucleotide turnovers by myosin in a variety of muscle types. This has led to the identification of a highly inhibited state of myosin in which ATP turnover is ∼10 times slower than the activity observed for purified myosin in a test tube (20–22). We have called this new state the super-relaxed state (SRX). A correlation between structural studies and measurements of ATP turnover have suggested that the inhibition of myosin seen in the SRX is due to the binding of myosin heads to the core of the thick filament in the IHM (22). Supporting this hypothesis, previous observations showed that myosin in the IHM has an extremely slow ATP turnover rate in scallop filaments and also for purified smooth muscle myosin in the absence of a thick filament (23,24). In both of these systems, the primary control of muscle function is exerted by myosin modification, either phosphorylation or calcium binding (23–25). Our observations suggest that a similar but less extreme inhibition of myosin also occurs in vertebrate skeletal and cardiac muscles when myosin heads are in conditions that favor the IHM. A second relaxed state also exists, in which myosin heads are detached from the core of the thick filaments and are disordered and capable of binding weakly to actin. Disordered myosin heads in relaxed fibers have been demonstrated by spectroscopic probes and by loss of myosin-based layer lines in the x-ray diffraction patterns (26–28). We term this state the disordered relaxed state (DRX). In tarantula filaments, there is evidence for a third state with an intermediate lifetime, which may correspond to a structural state in which one of the two myosin heads is immobilized but its partner head has rotational flexibility (25,29). Because of the large difference in myosin ATPase activity between the SRX and the DRX, the relative populations of these states play a major role in whole-body metabolism.
To explore further the properties of the SRX, correlations with myosin structure, and ATPase, we extended our studies by observing the effect of blebbistatin on myosin turnover rates. We found that blebbistatin inhibited nucleotide turnover in both fast and slow skeletal muscle with a lifetime increase to 1 h or more. This stability of the SRX allowed us to observe structural features using electron paramagnetic resonance (EPR) spectroscopy of spin-labeled nucleotides. In contrast to previous studies, in which only disordered probes on the motor domain could be observed (30–32), we were able to identify oriented spin probes attached to nucleotides in the relaxation state. In addition, we were able to monitor the conformation of the nucleotide pocket in the SRX.
Materials and Methods
Chemicals and solutions
Chemicals were purchased from Sigma (St. Louis, MO), except blebbistatin ((±)-1-Phenyl-1,2,3,4-tetrahydro-4-hydroxypyrrolo(2.3-b)-7-methylquinolin-4-one), which was obtained from Toronto Research Chemicals (North York, Ontario, Canada). Blebbistatin was dissolved in dimethylformamide and stored at −20°C in the dark. It was diluted with buffer before use. Exposure to light was minimized, as ultraviolet light can lead to photooxidation of blebbistatin. Rigor buffer consisted of 120 mM Kacetate, 5 mM MgCl2, 5 mM EGTA, 5 mM Kphosphate, 50 mM 3-(N-morpholino)propanesulfonic acid, pH 6.8. Relaxing buffers were obtained by addition of 4 mM ATP, 250 μM mantATP, or 100 μM spin-labeled ATP EPR probe (SLATP) to the rigor buffer.
Preparation of muscle fibers
Rabbits were sacrificed according to protocols approved by the Institutional Animal Care and Use Committee. Muscle skeletal fibers were dissected, permeabilized by immersion in rigor buffer containing 50% glycerol and stored at −20°C, as described in Karatzaferi et al. (33). Fast fibers were from psoas and slow fibers from soleus or semimembranosus muscles. Tarantula fibers were prepared as described previously and also stored in a solution containing 50% glycerol at −20°C (25).
Measurement of fluorescence
For measurements of fluorescence, fibers were mounted in a simple flow cell as described previously (22). They were secured at either end with grease or, for some experiments, by stainless steel pins. They were observed with a TE2000 microscope (Nikon, Melville, NY), using a DAPI filter set (no. 89000, Chroma, Bellows Falls, VT). Sarcomere length varied from 2.2 to 2.6 μm. Stewart and co-workers reported no statistically significant difference in the SRX fractions with sarcomere length (22), and none were found here in the presence of blebbistatin. The decay of intensity in this experiment was not due to photobleaching. This is because the fiber is only exposed to the exciting light for a brief period during data acquisition, typically 30 ms, and data were acquired at intervals of 20 s. Fluorescence decreased by only 12% during constant illumination for 36 s (25), which is much more than the total exposure of ∼11 s during a 2-h epifluorescence microscopy run.
EPR spectroscopy
EPR measurements were performed with an EMX EPR spectrometer (Bruker, Billerica, MA). First-derivative, X-band spectra were recorded in a high-sensitivity microwave cavity using 50-s, 10-mT-wide magnetic-field sweeps. The instrument settings were as follows: microwave power, 25 mW; time constant, 164 ms; frequency, 9.83 GHz; and modulation, 0.1 mT at a frequency of 100 kHz. Each spectrum used in data analysis was an average of 10–50 sweeps from an individual experimental preparation. Temperature was 24 ± 2°C and was monitored using a thermistor placed close to the experimental sample.
Labeling fibers for EPR experiments
Small bundles of 8–10 fibers 10 mm long were dissected and cleaned from fat and connective tissues. The fibers were washed for 15 min with rigor buffer, then incubated for 15 min to 2 h in 40 μM blebbistatin solution in rigor buffer, to which was added 100 μM of the adenylate kinase inhibitor P1,P5-diadenosinepentaphosphate (AP5A). The longer incubation times were controls to insure adequate perfusion. No difference was noted between fiber bundles incubated for 15 min and those incubated for 2 h. Incubations of <15 min were not tried. Bundles were then labeled with 100 μM 2′3′-SLATP (34) in rigor buffer with or without 40 μM blebbistatin and AP5A. The EPR-probe moiety is attached to the 2′- and 3′-hydroxyls as an enantiomer species. 2′3′-SLATP is hydrolyzed by myosin, so our observations are from the diphosphate species. For EPR measurement, the fibers were aligned on the flat cell, covered with a glass coverslip that was sealed with vacuum grease to prevent dehydration, and placed in the cavity. EPR spectra of the fibers were obtained with the fiber axis aligned either parallel or perpendicular to the magnetic field. Random spectra were obtained using minced fibers that were treated the same way.
ATP chase experiments
The amount of nucleotide trapped at the ATP-binding site of myosin in the presence of blebbistatin was measured using EPR spectroscopy. Rabbit muscle fibers were labeled with 2′3′-SLATP in the presence and absence of blebbistatin, and the EPR spectra were recorded. Two protocols were employed to measure spectra during the chase with 4 mM ATP plus 100 μM AP5A. In the first protocol, which was used to obtain parallel and perpendicular spectra of the specifically bound SLADP, the coverslip was removed from the top of the fibers and the fibers were left on the flat cell. A 100 μL solution of 4 mM ATP in rigor buffer containing blebbistatin and AP5A was added to the fibers and the cell was agitated for ∼5–10 min. The extra solution was removed and the fibers were then aligned on the flat cell and covered with the glass slip as described above. The EPR spectra were monitored with time. Spectra of one to five scans were recorded for up to 1 h. The second protocol was used to measure nucleotide release during the chase. In this protocol, a small aliquot of concentrated ATP solution was added to the labeled fibers on the flat cell to produce a final concentration of 4 mM ATP. The EPR spectra were monitored with time. In this experiment, all nucleotides released remain in the observed volume and contribute to the spectrum.
Labeling myosin and subfragment 1
Rabbit skeletal myosin was prepared as described previously (35), and subfragment 1 was made according to the method of Weeds and Taylor (36). For trapping spin-labeled nucleotides with blebbistatin, a 60 μM myosin sample was incubated in rigor buffer containing 120 μM blebbistatin, followed by addition of 50 μM of 2′3′-SLATP. For the ATP competition experiment, 1–2 μL of a concentrated ATP solution was added to the above labeled myosin sample to generate a final 4 mM ATP concentration. EPR spectra were recorded at ∼2 min intervals for 1 h. Subfragment 1 (100 μM) was labeled with 80 μM 2′3′-SLATP according to the same procedure used to label myosin.
Calculations of the concentration of trapped nucleotide at the ATP binding site
The EPR spectrum of 2′3′-SLADP bound at the nucleotide-binding site of myosin shows two sets of peaks. The height of the high-field free peak was used to represent the concentration of free probe, whereas the height of the low-field bound peak was chosen to represent the bound probe. The heights of the free and bound peaks were measured for each spectrum and were plotted versus time after the addition of ATP. A plot of the height of the free probe versus that of the bound probe gave a straight line. The slope provided a measure of the relative number of spins that were contributing to each peak, with the free probes having a height per probe that was 25–30 times greater than that of bound probes.
Results
Measurements of nucleotide turnover in the SRX using fluorescent nucleotides
Skinned fibers are complex preparations containing a number of enzymes that rapidly turn over ATP. In addition, there is a small fraction of myosin heads that are not regulated and thus have high ATPase activity. These effects have dominated traditional ATPase measurements on relaxed skinned fibers. To measure the very slow ATP turnover of myosin in the SRX, it is necessary to measure single nucleotide turnovers. We use quantitative epifluorescence microscopy of fluorescent mant-nucleotides bound to myosin to observe the SRX (22). MantATP functions similarly to ATP and has an enhanced fluorescence when bound to myosin (37,38).
Single fibers in rigor solution were mounted in a flow cell, incubated in a relaxing solution containing mantATP (250 μM), then chased with a relaxing solution containing 4 mM ATP. The fiber fluorescence decayed as the mant-nucleotides were released from myosin, replaced by ATP, and subsequently diffused out of the fiber. Fig. 1 A shows the fluorescence signal during the chase phase of an experiment using a slow muscle fiber (control). The signal decays in multiple components. The initial rapid decay is compatible with the presence of rapid ATPases within the bundle (>0.05 s−1), the release of nonspecifically bound nucleotides (presumably fast), and the diffusion of these released nucleotides out of the fiber in ∼10 s (39). The decay of fluorescence intensity is adequately fit to a two-exponential function, with the rapid decay consisting of 49% of the initial intensity occurring with a time constant of 17 s and a slower SRX decay of magnitude 43% with a time constant of 152 s, similar to that published previously for slow-twitch fibers (see Table 1) (22). In addition, there is a small component (∼5%) that does not decay during the experiment in the absence of blebbistatin but can be quite large in its presence (see below). Controls showed that photobleaching is insignificant in these experiments (Methods).
Figure 1.

(A) Fiber fluorescence is shown during the chase phase for a slow-twitch fiber in the absence (lower trace) and presence (upper trace) of blebbistatin. The fiber was first incubated in 250 μM mantATP and subsequently chased with a solution containing 4 mM ATP. The decay of fluorescence was fit by a two-exponential decay function, as described previously (22). For the experiment with blebbistatin, blebbistatin was included in both the incubation and chase solutions. (B) The decay of fiber fluorescence during the chase phase in the presence of blebbistatin is contrasted for a fast- (lower trace) and slow-twitch (upper trace) muscle fiber. As can be seen, the effect of blebbistatin on the rate of release of nucleotides is greater in the slow-twitch muscle fiber than in the fast-twitch muscle fiber.
Table 1.
Nucleotide turnover times and populations of nucleotides with the slow release rate in the absence and presence of blebbistatin
| Sample | P2 | T2 (s) | P2 + BLEBB | T2 + BLEBB (s) |
|---|---|---|---|---|
| Fast fibers | 0.3 ± 0.03 | 250 ± 25 (20) | 0.35 ± 0.05 | 3600 ± 500 (5) |
| Slow fibers | 0.3 ± 0.04 | 150 ± 20 (15) | 0.4 ± 0.05 | >3600 (6) |
| Fast myosin | — | 16 ± 4a | — | 800 ± 100 (6) |
| Slow myosin | — | 26b | — | 820 ± 200 (5) |
Values are expressed as the mean ± SE of 5–20 observations, as indicated by the numbers in parentheses after T2 values. T2, SRX nucleotide turnover time; P2, slow-release-rate nucleotide population.
ATPase rate in the absence of blebbistatin taken from Myburgh et al. (40).
Steady-state ATPase rate of relaxed myofibrils taken from the data of Candau et al. (41).
To observe the effect of blebbistatin on the SRX, the slow-twitch fiber was first incubated in a rigor solution containing 40 μM blebbistatin for 10–20 min to allow blebbistatin to bind to myosin in the fiber. The fiber was next incubated in mantATP and chased with ATP, all in the presence of blebbistatin. As shown in Fig. 1 A, blebbistatin has a dramatic effect on the release of nucleotides in slow-twitch fibers. There was a fast decay, 54% of the initial intensity, similar to that observed in the absence of blebbistatin, that was assumed to come from nonspecific probes and any myosin heads in the DRX (not complexed with blebbistatin). A small component, 5% of the initial fluorescence, decayed with a time constant of 252 s, similar to the decay in the SRX in the absence of blebbistatin. The most dramatic difference from the control fiber is the appearance of a large component of the fluorescence intensity, ∼40% of the initial signal, that decayed with a time constant too slow to be accurately measured with this protocol, ≥60 min (see Fig. 1 A and Table 1). Because blebbistatin is highly specific for the myosin molecule, we can conclude that the nucleotides with a very slow time constant are bound to myosin-blebbistatin complex. Blebbistatin shows a low fluorescence signal, more than an order of magnitude less than that from the mant-nucleotides. This signal remains constant during the experiment and thus does not influence the results.
The effect of blebbistatin on nucleotide turnover in the fibers depends on the fiber type. As described above for slow-twitch muscle fibers, blebbistatin produces a large inhibition in the rate of nucleotide release by myosin in the SRX but does not significantly increase the fraction of probes released slowly. In fast-twitch muscle fibers in the presence of blebbistatin, the rate of nucleotide release was slightly faster but still inhibited, with little change in the population of probes released slowly (Fig. 1 B). This agrees well with small-angle x-ray diffraction findings by Xu et al. (17) of a modest effect of blebbistatin on the affinity of myosin heads for the core of the thick filament at 25°C. The effect of blebbistatin on the thick-filament structure in slow-twitch muscle fibers has not been investigated using x-ray diffraction or cryo-electron microscopy.
In the experiments described above, the chase solution contains ATP and the fiber remains relaxed during the chase phase. In the presence of ADP, myosin heads that are not in the SRX bind tightly to actin and the fiber is no longer relaxed. If the chase solution contains ADP, all nucleotides are released quickly and the slow release of the nucleotides in the SRX is not observed (21). We hypothesized that the tight bond between some myosin heads and actin cooperatively destabilizes nearby myosin heads that are in the SRX, disordering them and allowing them to also bind rapidly to actin and release nucleotides. When fast- and slow-twitch muscle fibers in the presence of blebbistatin were chased with solutions containing ADP, release of nucleotides was only very slightly increased, as shown in Fig. 2.
Figure 2.

Decay of fiber fluorescence late in the chase phase is shown as a function of time. A muscle fiber was first incubated in mantATP and subsequently chased by ATP. The arrow marks the time where the solution was exchanged for one containing 4 mM ADP. All solutions were in the presence of blebbistatin. The two upper traces represent experiments performed with a slow-twitch fiber in 40 μM blebbistatin (solid circles) or in 20 μM blebbistatin (solid diamonds). The lower trace represents the experiment performed with a fast-twitch fiber in 40 μM blebbistatin (open squares). As can be seen, there are only modest changes in the rate of release of fluorescent nucleotides in ADP relative to ATP, showing the stability of the SRX in blebbistatin.
The strong stabilization of the SRX by blebbistatin in both slow and fast fibers under conditions where it is normally destabilized could arise due to the inhibition of the strong bonds between actin and myosin by blebbistatin. We previously hypothesized that such strong bonds are required to destabilize myosin heads in the SRX (21). Another possibility is that blebbistatin could provide such a great degree of stability to the SRX that the formation of strong actomyosin bonds can no longer have a significant effect on it. Reducing blebbistatin concentration from 40 to 20 μM reduces the fraction of myosin heads bound to blebbistatin, thus reducing force inhibition, from 90% to half that amount (42). The larger fraction of myosin heads binding strongly to actin is demonstrated by the observation that fibers shorten under these conditions, requiring immobilization with steel pins to carry out the experiment. As shown in Fig. 2, the myosin found in the long-lived SRX at lower blebbistatin concentrations also remains relatively unchanged during a chase with ADP. These results show that blebbistatin provides such a high degree of stability to the SRX that strong actomyosin bonds cannot destabilize it.
Measuring the orientation of the myosin heads in the SRX using spin-labeled nucleotides
The acquisition of EPR spectra of muscle fibers with a good signal/noise ratio typically involves scanning for 10 min or more. This constraint precludes obtaining spectra of spin-labeled nucleotides in the SRX in fast and slow skeletal fibers, where the lifetime of bound nucleotides is of the order of 2–4 min. However, for tarantula muscle fibers (25) and skeletal fibers in the presence of blebbistatin, the lifetime is far greater, allowing spectral acquisition.
A large number of small bundles of fewer than six fibers were dissected and aligned on the flat cell. The diameter of the bundles needed to be small to provide adequate perfusion for the SLATP, which is in low concentration and cannot be replenished using standard recirculating systems. Fibers were incubated first in rigor and then in relaxing solution containing spin-labeled nucleotides in the presence of blebbistatin (Methods). The fibers were then mounted in a flat cell in which they could be oriented either parallel or perpendicular to the magnetic field. Spectra were obtained and the fibers were then removed from the cell and washed in a relaxing solution containing 4 mM ATP plus blebbistatin to remove any spin-labeled nucleotides not bound at the active site with an extended lifetime. Fig. 3 A shows the strong effect of orientation on the EPR spectrum of spin-labeled nucleotides bound to slow-twitch muscle fibers in the presence of blebbistatin during this chase phase. Each nitroxide spin probe contains one unpaired electron, which gives rise to a three-line EPR spectrum in the presence of a strong magnetic field. If the probes are oriented with respect to the magnetic field (i.e., the long axis of the fiber), the splitting of these three lines is a function of the angle between the principal axis of the spin probe and the magnetic field. If this angle is large, the splitting is small. The three sharp peaks (P2–P4) in the center of the blue spectrum in Fig. 3 A arise from residual free nucleotides. Their sharpness is due to motional narrowing of the lines. These fibers had been washed with a relaxing solution for >5 min, which removes most but not all of the free nucleotides. In addition, some nucleotides are released from myosin during the acquisition of the spectra, adding to the free population. The broad peaks seen at both high (P5) and low magnetic field (P1) arise from nucleotides that are immobilized by binding to proteins, primarily myosin. Both bound and free probes contribute to the central peak, P3, which can be seen as a two-peak superposition in the red spectrum in Fig. 3 A. The red spectrum shows the same fibers aligned with the fiber axis perpendicular to the magnetic field. Due to the stagger of the myosin heads around the thick filament as they project out azimuthally from the thick filament, this spectrum approximates randomly oriented EPR probes. As can be seen, the blue (parallel) and red (perpendicular) spectra are quite different in the high-field (P5) and low-field (P1) regions, indicating that the spin probe bound to myosin is oriented on the myosin molecule. As also can be seen (Fig. 3 A, blue), when fibers are oriented parallel to the magnetic field the splitting between high-field and low-field peaks is large, showing that the angle between probes and the magnetic field is small with probes aligned primarily along the long axis of the fiber (see Discussion).
Figure 3.

(A) EPR spectra of the spin-labeled analog of ATP, 2′3′SLATP, bound to slow-twitch muscle fibers in the presence of blebbistatin. The first derivative of the absorption is shown as a function of the magnetic field. The fibers were oriented with their long axis parallel to the magnetic field (blue) or perpendicular to the magnetic field (red), showing a strong dependence on the orientation. The three sharp peaks (P2–P4) in the central region of the spectrum arise from free nucleotides; the broader peaks at high (P5) and low field (P1) arise from nucleotides bound to specific sites in the fiber, almost entirely to myosin. (B) EPR spectra are shown for slow-twitch muscle fibers aligned parallel to the magnetic field in the presence of blebbistatin (blue) and for tarantula fibers in the absence of blebbistatin (red). The spectra show that the orientation of the spin-labeled nucleotides is very similar for these two fibers and conditions. The spectra from the two muscle types were likewise similar when the fibers were aligned perpendicular to the magnetic field. The center field is 349.1 mT for parallel spectra and 350.0 mT for perpendicular spectra. All spectra are 10 mT wide. Spectra were aligned on the central (P3) peak of unbound nucleotide. To see this figure in color, go online.
Questions concerning the SRX include whether its structure is similar in different fiber types and whether the state stabilized by blebbistatin is similar to the SRX in its absence. Fig. 3 B compares the spectra obtained from slow-twitch vertebrate muscle fibers in the presence of blebbistatin with that of tarantula exoskeletal fibers without blebbistatin, with both preparations aligned parallel to the magnetic field. These two spectra are very similar. As expected, they are also similar when the fibers are aligned perpendicular to the magnetic field (not shown). The almost identical nature of the spectra from different muscles suggests that the orientation of bound nucleotides in the two very diverse fiber types is similar and that the state stabilized by blebbistatin is similar to that in its absence.
The kinetics of nucleotide release measured using spin-labeled nucleotides
The release of spin-labeled nucleotides from the fiber can be monitored in a pulse-chase experiment similar to that performed with the fluorescent nucleotides. Slow-twitch muscle fibers were incubated with spin-labeled nucleotides in the presence of blebbistatin. An aliquot of concentrated ATP was added to the fibers to bring the concentration to 4 mM ATP. The release of bound nucleotides could be monitored either from the decrease in the height of the low-field peak arising from the bound nucleotides or from the increase in the height of the sharp peak arising from free nucleotides.
The change in amplitude of the peak arising from free nucleotides provides a good measure of the release of bound nucleotides, because in this experimental protocol, no nucleotides leave the volume observed after their release. Due to the nature of the EPR spectrum, a given height of the different peaks represents different populations of probes contributing to that peak. For example, a given height for one of the bound peaks (P1 or P5) represents many more spins than does equal height of the peaks arising from the free nucleotides (P2–P4). The exact proportion allotted to each peak can be determined by plotting the intensity of the peak arising from bound probes versus that arising from free nucleotides in each experiment. The slope of this plot, which is a straight line, shows that the bound-peak height represents 23 ± 3 times the number of spins for the same height of a free peak.
Fig. 4 shows the release of bound spin-labeled nucleotides in the presence of blebbistatin from slow-twitch purified myosin (diamonds) and fibers (circles) as a function of time. The myosin samples were in the same buffer used in the fiber experiments, which has an ionic strength such that the myosin has formed synthetic thick filaments. The nucleotide lifetime in slow synthetic thick filaments was 480 s, faster than the three phases found in slow fibers; 45% of the initial signal was released in a more rapid phase with a time constant of ∼900 s, 20% with a time constant of 2400 s, and 35% with a much slower time constant that was too long to be measured. The slower phases are similar to those observed for the fluorescent nucleotides, as shown in Fig. 1 and Table 1. In the absence of blebbistatin, all nucleotides were released rapidly within the time required to add competing ATP and obtain the first spectrum, ∼2 min (Fig. 4 A, squares). Fig. 4 B contrasts the release of spin-labeled nucleotides from fast-twitch myosin and fibers in the presence of blebbistatin, corresponding to lifetimes of 900 and 2000 s, respectively. The release of nucleotides from fast fibers is faster than that from slow fibers, a result also seen with fluorescent nucleotides (see Table 1 and Fig. 1). The rate of nucleotide release was the same for synthetic myosin thick filaments and for myosin subfragment 1. The release was also similar in the standard buffer of ionic strength 250 mM to that observed in buffer of higher ionic strength, 500 mM. The high degree of inhibition of purified myosin by blebbistatin could be due to stabilization of the IHM in the myosin molecules, an effect observed in electron microscopy (2). However, the last two observations rule this possibility out, as the IHM cannot be formed by subfragment 1, nor can it be formed in high ionic strength.
Figure 4.
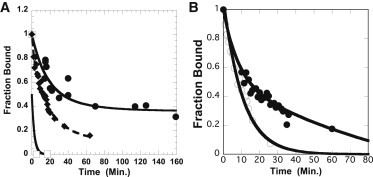
(A) The release of spin-labeled nucleotides from slow-twitch fibers and from myosin is shown as a function of time during a chase with unlabeled ATP. Data obtained from fibers are shown in the presence (solid circles) and absence (open squares) of blebbistatin. Solid diamonds represent data obtained from myosin isolated from slow-twitch fibers in the presence of blebbistatin. In the presence of blebbistatin, the release of nucleotides from fibers is slow, with a time constant similar to that seen with fluorescent nucleotides. A fraction of the probes, 40%, is released fairly quickly, with a time constant of 14 min. Another faction, 31%, is released more slowly, with a time constant of 88 min, and the remaining probes, 29%, are released even more slowly (too slowly to be measured in this assay). In the absence of blebbistatin, all nucleotides are released from the fibers before the first time point at 3 min. The major fraction of nucleotides, 83%, is released from myosin plus blebbistatin with a time constant of 16 min. (B) Release of spin-labeled nucleotides from fast-twitch fibers (solid circles) and from purified myosin (open circles), both in the presence of blebbistatin. Fits to the data show a single-exponential decay with a time constant of 10.6 min for myosin and a two-phase decay for fibers with a slow phase representing 66% of the initial intensity and with a time constant of 31 min.
Monitoring the conformation of the nucleotide-binding pocket in the SRX
One unanswered question concerning the SRX is how the inhibition of ATP turnover is achieved. Is the nucleotide pocket in an unusual conformation, more closed or more open? Previous work in our lab has shown that one can monitor the conformation of the nucleotide pocket using the mobility of spin probes (SLADP) attached to nucleotides that are bound specifically to myosin (30,32). The spin probe undergoes subnanosecond rotational motion that is constrained by the adjoining protein surface. The greater the constraint provided by the protein surface, the less the mobility of the probe, leading to a greater splitting between the low-field peak (P1) and the high-field dip (P5) in the spectra. Baseline probe mobility is obtained from the spectrum of randomly oriented minced samples. We found the low-field to high-field splitting to be 6.6 mT for SLADP bound to randomly oriented slow-twitch skeletal muscle fibers in the presence of blebbistatin; 6.6 mT for randomly oriented tarantula fibers; and 6.5 mT for fast muscle myosin. From the similarity of the spectra, we conclude that there is not a dramatic change in the conformation of the nucleotide pocket of myosin in the SRX.
Discussion
Nucleotide release in the presence and absence of blebbistatin
Nucleotide turnover times for myosin from relaxed fast- and slow-twitch fibers are ∼16 and 25 s, respectively (40,41). In the SRX, nucleotide turnover time increases by a factor of 15 in fast-twitch muscle fibers, from 16 s to 250 s, and by a factor of 6 in slow-twitch muscle fibers, from 25 s to 150 s (21,22) (see Table 1). Blebbistatin is a small molecule that inhibits the ATPase activity of a variety of myosin 2 isoforms, and as such would be expected to inhibit the ATPase activity of myosin in relaxed fibers (13,15,16). Addition of blebbistatin to resting fibers has a strong effect on nucleotide turnover times observed in both fast- and slow-twitch muscle fibers, extending the turnover time from 150–250 s to 30 min or greater. There was a small increase, ∼5%, in the population of myosin in the SRX in slow-twitch fibers. As can be seen in Fig. 1 B, the release of nucleotides in both fiber types is very slow, but it is slower in slow-twitch fibers than in fast-twitch fibers. In fast fibers there is a population of probes that is released with an ∼1 h turnover time, whereas in slow-twitch fibers release is even slower and beyond the ability of the current assay to measure. A faster release of nucleotides in fast-twitch fibers is also seen in the release of spin-labeled nucleotides (Fig. 4). Although the rates of nucleotide release are similar for spin and fluorescent nucleotides, they are not identical due to different protocols, e.g., bundles of fibers in an EPR cell versus single fibers surrounded by solution, and possibly due also to the use of different probes. It has been shown previously that myosin regulatory light chain phosphorylation produces a modest reduction in the stability of the SRX (22). Due to the large degree of stability conferred by blebbistatin, phosphorylation would be expected to have little effect in its presence, but this was not checked here.
Blebbistatin could inhibit nucleotide turnover in muscle fibers by stabilizing the SRX or by simply inhibiting myosin ATP turnover. In the presence of blebbistatin, the time constants for nucleotide release from purified myosin thick filaments are a little slower than those measured previously using steady-state ATPase assays for purified myosins and myosin subfragment 1 (13,15,16). The release rates observed for purified myosin are much faster than those observed in the slow phase for either fast- or slow-twitch fibers in the presence of blebbistatin. These observations suggest that the very slow nucleotide turnover times observed in the muscle fibers are due to stabilization of the SRX by blebbistatin. This stability allowed us to measure orientations and conformations of myosin heads in the SRX, since signals from free nucleotides or nonspecifically bound nucleotides were easily washed out, with little loss of spin-labeled nucleotides bound to myosin during the chase phase.
Correlation between the SRX and structural studies
Our previous work on the SRX has shown that there is a correlation between the lifetime of the SRX and the stability of the IHM (22). As blebbistatin has been shown by electron microscopy and x-ray diffraction to stabilize the IHM (11,17,18), it would be expected to extend nucleotide turnover times in fibers. Our basic observation shows that this is true and again supports the previously proposed correlation between structure and nucleotide turnover in the SRX. The small increase in the population of the SRX observed here correlates well with the lack of change in the stability of myosin heads in the thick filament array observed by x-ray diffraction at 25°C (17). The effect of blebbistatin on thick filament structure in skeletal muscle fibers has not been measured by cryo-electron microscopy. Thus, we conclude that there is a reasonable correlation between the inhibition of myosin ATP turnover observed in functional studies of the SRX and the stability of the array of myosin heads bound to the core of the thick filament observed by cryo-electron microscopy or x-ray diffraction.
Previous work in our laboratory showed that in fast skeletal muscle, a chase with either ADP or an activating solution resulted in rapid release of all nucleotides bound to the fiber (20,21). A similar result was also obtained for slow skeletal fibers. We hypothesized that the binding of myosin to actin synergistically and cooperatively destabilized myosin heads in the SRX, and that this cooperative destabilization was necessary to achieve a very rapid, full activation of muscle fibers. However, in the presence of blebbistatin, a chase with ADP had little effect on the release of nucleotides from myosin heads in the SRX (see Fig. 2). As blebbistatin prevents myosin heads from attaching strongly to actin, this observation could be due to blebbistatin’s prevention of the formation of such bonds, or to blebbistatin stabilizing the SRX to such an extent that strong bond formation no longer affects it. By conducting the experiment at lower levels of blebbistatin, where a chase with ADP will result in strong bond formation, we show that the latter explanation accounts for the data. The great stability of the SRX observed here for fibers in the presence of ADP correlates well with the observation that blebbistatin stabilizes the IHM in ADP, as observed by cryo-electron microscopy (18). Interestingly, skeletal fibers in the presence of blebbistatin resemble cardiac fibers in the absence of blebbistatin in that in both cases, strong bonds between actin and myosin do not affect the SRX (21). Observations of probe orientation, discussed below, also provide support for the conclusion that blebbistatin stabilizes the IHM in skeletal fibers.
Probe orientation in the SRX
Studies of relaxed muscle fibers using spin-labeled nucleotides have been hampered by the fact that these nucleotide analogs do not function with standard ATP-regeneration systems such as creatine kinase, and that the signal from high concentrations of free nucleotides swamps out the signal from the bound nucleotides. To avoid these problems, previous studies of the relaxed state using spin-labeled nucleotides have used the phosphate analogs AlFx, BeFx, or Vi to stabilize a relaxed state. Although all of these analogs produced a mechanically relaxed fiber, the spectra from these states showed completely disordered spin probes (30–32). Although these ligands have been shown by x-ray diffraction to stabilize the binding of myosin to the thick filament in rabbit psoas, they were much less effective than blebbistatin (3,17). They also stabilized thick filament structure of negatively stained tarantula filaments (19). Here, we used blebbistatin to stabilize nucleotides in the SRX in skeletal muscle fibers. The spectra show a clear indication of oriented probes. This is to our knowledge the first observation of oriented EPR probes on the myosin motor domain in relaxed skeletal muscle fibers. Similar spectra were also obtained from tarantula fibers, suggesting that the order seen is intrinsic to the IHM. Thus, we conclude that the IHM is stabilized more effectively by blebbistatin than by phosphate analogs in vertebrate skeletal fibers, in agreement with previous x-ray diffraction studies.
The orientation of the probes shown in Fig. 3 can be determined by comparison with simulations produced by solving the spin Hamiltonian for different probe configurations. Barnett and co-workers simulated spectra for distributions of probes that have a different mean orientation to the magnetic field, Θo, and Gaussian distributions with different full widths at half-maximum, Δθ, about this mean (43). The spectra shown in Fig. 3 are similar to the simulation of a distribution of probes oriented along the fiber axis with Θo = 0°, having a Gaussian distribution of full width Δθ = 90° about this mean. The breadth of the distribution of the probes may arise from the disorder of the probes relative to the myosin heads and from the two different orientations of the nucleotide sites in the IHM.
The conformation of the nucleotide pocket in the SRX is similar to that in Myosin⋅ADP
The EPR spectrum of randomly oriented samples provides information on the rotational mobility of the probes. This in turn provides information on the conformation of the protein surface in the vicinity of the bound probe, in this case, the protein surface in the vicinity of the nucleotide pocket of myosin. There is little change between the spectrum of SLADP bound to myosin and the spectrum of SLADP bound to slow fibers in the presence of blebbistatin during the chase phase. Previous work has shown that the spectrum of SLADP bound to myosin is virtually unchanged between fast myosin and slow myosin and is unchanged upon addition of phosphate analogs AlFx, BeFx, and Vi (31). Together these data suggest that there has been no significant rearrangement of the conformation of the myosin nucleotide pocket in the SRX as measured by spin probes bound to the ribose of the nucleotide. The magnitude of the low-field to high-field splitting can be modeled as the motion of the probe in a cone of revolution, with larger splitting indicating a smaller vertex cone angle (44). The protein surface is most certainly not a cone of revolution, but the analysis serves as an approximation to correlate changes in the magnetic field with a change in the restrictive nature of the protein surface. The splitting observed here, which is equivalent to that previously observed for fast myosin, corresponds to a cone angle of ∼60°, which implies a closed nucleotide site (31). Previous work using cryo-electron microscopy has also suggested that the switch regions of myosin at the nucleotide site are in a closed conformation in the SRX (19). These observations suggest that the inhibition of myosin's ATPase activity seen in the SRX is not due to some special conformation of the nucleotide pocket. Inhibition could be the result of immobilization of the myosin head, which has been observed in previous studies (45–47).
Significance
The SRX may play an important role in whole-body thermogenesis and thus in human health. Human movement is a contributor to caloric expenditure and metabolic rate. Thus, the increasingly sedentary lifestyle in modern human culture may be a major contributor to the public rise in obesity and related diseases. Studies have indicated that low-intensity movement, such as walking periodically throughout the day, can account for as much as 300 Cal, and that for some people, this might be enough to prevent obesity (48). In addition, if regular movement throughout the day prevents muscle from spending as much time in the SRX, muscle, and therefore whole-body metabolic rate, would be higher when not moving, i.e., when relaxed but not super-relaxed. Another unanswered question concerns the kinetics for transitions into and out of the SRX, which, although they have not been measured, are presumed to be fast.
It is not known how frequently movement must occur to avoid the SRX, and the SRX in human muscle fibers has not yet been verified. However, it is found in a broad range within the evolutionary spectrum, and its caloric effects are significant, e.g., helping species that spend significant time lying in wait for prey (such as the tarantula) from dying of starvation while they sit motionless for long periods of time. Thus, avoiding the SRX might contribute as much to caloric expenditure and metabolic rate as low-intensity regular movement itself.
Pharmaceuticals that would cause myosin heads to transition out of the SRX and into the DRX could raise metabolic rate and prove effective for treating obesity and type 2 diabetes (20). The development of such pharmaceuticals requires a better understanding of the properties of the SRX and how to manipulate it. To reach this goal, one must first develop an assay amenable to the high-throughput robotic methods currently used to identify therapeutic molecules. Conditions that stabilize the SRX would be useful in developing high-throughput assays, and blebbistatin is shown here to stabilize the SRX. However, we further show that blebbistatin provides such a high degree of stability to the SRX that mechanisms known to destabilize the SRX are not effective in its presence. Thus, blebbistatin provides a useful tool to aid in the development of an assay. However, screening in the presence of blebbistatin could miss potentially useful compounds.
Conclusion
Blebbistatin stabilizes the SRX, producing a very long-lived myosin-nucleotide complex that facilitates structural and other studies. Using blebbistatin, we show that spin probes bound to the myosin nucleotide site are oriented, and that the conformation of the nucleotide site has not changed. Blebbistatin has been shown in other structural studies to stabilize the IHM (11,18). Thus, the results presented here strengthen the conclusion that the myosin heads in the SRX are also in the IHM. Our observation of the very long life of the SRX in slow-twitch fibers in the presence of blebbistatin suggests that this would make a good model system for further structural studies of this motif in a skeletal muscle.
Acknowledgments
We thank Roger Craig and Raul Padron for comments on the manuscript. Roger Cooke would like to acknowledge that he is on the scientific advisory board of Myokardia, in South San Francisco.
This work was supported by U.S. Public Health Service grant AR062279.
References
- 1.Huxley H.E., Faruqi A.R. Time-resolved x-ray diffraction studies on vertebrate striated muscle. Annu. Rev. Biophys. Bioeng. 1983;12:381–417. doi: 10.1146/annurev.bb.12.060183.002121. [DOI] [PubMed] [Google Scholar]
- 2.Jung H.S., Komatsu S., Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol. Biol. Cell. 2008;19:3234–3242. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S., Offer G., Yu L.C. Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry. 2003;42:390–401. doi: 10.1021/bi026085t. [DOI] [PubMed] [Google Scholar]
- 4.Craig R., Woodhead J.L. Structure and function of myosin filaments. Curr. Opin. Struct. Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Alamo L., Wriggers W., Padrón R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J. Mol. Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodhead J.L., Zhao F.Q., Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 7.Wendt T., Taylor D., Taylor K.A. Visualization of head-head interactions in the inhibited state of smooth muscle myosin. J. Cell Biol. 1999;147:1385–1390. doi: 10.1083/jcb.147.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhead J.L., Zhao F.Q., Craig R. Structural basis of the relaxed state of a Ca2+-regulated myosin filament and its evolutionary implications. Proc. Natl. Acad. Sci. USA. 2013;110:8561–8566. doi: 10.1073/pnas.1218462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig R., Padrón R., Alamo L. Direct determination of myosin filament symmetry in scallop striated adductor muscle by rapid freezing and freeze substitution. J. Mol. Biol. 1991;220:125–132. doi: 10.1016/0022-2836(91)90386-k. [DOI] [PubMed] [Google Scholar]
- 10.Pinto A., Sánchez F., Padrón R. The myosin interacting-heads motif is present in the relaxed thick filament of the striated muscle of scorpion. J. Struct. Biol. 2012;180:469–478. doi: 10.1016/j.jsb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Zoghbi M.E., Woodhead J.L., Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao F.Q., Craig R., Woodhead J.L. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J. Mol. Biol. 2009;385:423–431. doi: 10.1016/j.jmb.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straight A.F., Cheung A., Mitchison T.J. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 14.Allingham J.S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 15.Kovács M., Tóth J., Sellers J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 16.Limouze J., Straight A.F., Sellers J.R. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 17.Xu S., White H.D., Yu L.C. Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophys. J. 2009;96:3673–3681. doi: 10.1016/j.bpj.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao F.Q., Padrón R., Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys. J. 2008;95:3322–3329. doi: 10.1529/biophysj.108.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoghbi M.E., Woodhead J.L., Padrón R. Helical order in tarantula thick filaments requires the “closed” conformation of the myosin head. J. Mol. Biol. 2004;342:1223–1236. doi: 10.1016/j.jmb.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys. Rev. 2011;3:33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooijman P., Stewart M.A., Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys. J. 2011;100:1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart M.A., Franks-Skiba K., Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross R.A., Jackson A.P., Bagshaw C.R. Active site trapping of nucleotide by smooth and non-muscle myosins. J. Mol. Biol. 1988;203:173–181. doi: 10.1016/0022-2836(88)90100-3. [DOI] [PubMed] [Google Scholar]
- 24.Vibert P., Craig R. Structural changes that occur in scallop myosin filaments upon activation. J. Cell Biol. 1985;101:830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naber N., Cooke R., Pate E. Slow myosin ATP turnover in the super-relaxed state in tarantula muscle. J. Mol. Biol. 2011;411:943–950. doi: 10.1016/j.jmb.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas D.D., Ishiwata S., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys. J. 1980;32:873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray J. Temperature dependence of the relaxed pattern from rabbit psoas fibers. J. Muscle Res. Cell Motil. 1987;8:62a. Abstract. [Google Scholar]
- 28.Xu S., Gu J., Yu L.C. The M.ADP.Pi state is required for helical order in the thick filaments of skeletal muscle. Biophys. J. 1999;77:2665–2676. doi: 10.1016/s0006-3495(99)77101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brito R., Alamo L., Padrón R. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J. Mol. Biol. 2011;414:44–61. doi: 10.1016/j.jmb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowder M.S., Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys. J. 1987;51:323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naber N., Purcell T.J., Cooke R. Dynamics of the nucleotide pocket of myosin measured by spin-labeled nucleotides. Biophys. J. 2007;92:172–184. doi: 10.1529/biophysj.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reedy M.K., Lucaveche C., Cooke R. Insect crossbridges, relaxed by spin-labeled nucleotide, show well-ordered 90 degrees state by x-ray diffraction and electron microscopy, but spectra of electron paramagnetic resonance probes report disorder. J. Mol. Biol. 1992;227:678–697. doi: 10.1016/0022-2836(92)90217-8. [DOI] [PubMed] [Google Scholar]
- 33.Karatzaferi C., Franks-Skiba K., Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R948–R955. doi: 10.1152/ajpregu.00541.2007. [DOI] [PubMed] [Google Scholar]
- 34.Naber N., Cooke R., Pate E. Conformational changes at the nucleotide pocket of motor proteins monitored by electron paramagnetic resonance spectroscopy. Pure Appl. Chem. 2011;83:1675–1684. [Google Scholar]
- 35.Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966;5:515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- 36.Weeds A.G., Taylor R.S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975;257:54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- 37.Cremo C.R., Neuron J.M., Yount R.G. Interaction of myosin subfragment 1 with fluorescent ribose-modified nucleotides. A comparison of vanadate trapping and SH1-SH2 cross-linking. Biochemistry. 1990;29:3309–3319. doi: 10.1021/bi00465a023. [DOI] [PubMed] [Google Scholar]
- 38.Woodward S.K., Eccleston J.F., Geeves M.A. Kinetics of the interaction of 2′(3′)-O-(N-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragment 1: characterization of two acto-S1-ADP complexes. Biochemistry. 1991;30:422–430. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]
- 39.Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys. J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myburgh K.H., Franks-Skiba K., Cooke R. Nucleotide turnover rate measured in fully relaxed rabbit skeletal muscle myofibrils. J. Gen. Physiol. 1995;106:957–973. doi: 10.1085/jgp.106.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candau R., Iorga B., Lionne C. At physiological temperatures the ATPase rates of shortening soleus and psoas myofibrils are similar. Biophys. J. 2003;85:3132–3141. doi: 10.1016/S0006-3495(03)74731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart M., Franks-Skiba K., Cooke R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J. Muscle Res. Cell Motil. 2009;30:17–27. doi: 10.1007/s10974-008-9162-9. [DOI] [PubMed] [Google Scholar]
- 43.Barnett V.A., Fajer P., Thomas D.D. High-resolution detection of muscle crossbridge orientation by electron paramagnetic resonance. Biophys. J. 1986;49:144–147. doi: 10.1016/S0006-3495(86)83628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith O.H., Jost P.C. Lipid Spin Labels in Biological Membranes. In: Berliner L.J., editor. Spin Labeling Theory and Applications. Academic Press; New York: 1976. pp. 454–523. [Google Scholar]
- 45.Highsmith S., Duignan K., Cooke R. Reversible inactivation of myosin subfragment 1 activity by mechanical immobilization. Biophys. J. 1998;74:1465–1472. doi: 10.1016/S0006-3495(98)77858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson M., Albet-Torres N., Balaz M. Heavy meromyosin molecules extending more than 50 nm above adsorbing electronegative surfaces. Langmuir. 2010;26:9927–9936. doi: 10.1021/la100395a. [DOI] [PubMed] [Google Scholar]
- 47.White H.D., Rayment I. Kinetic characterization of reductively methylated myosin subfragment 1. Biochemistry. 1993;32:9859–9865. doi: 10.1021/bi00088a042. [DOI] [PubMed] [Google Scholar]
- 48.Levine J.A., Eberhardt N.L., Jensen M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]


