Abstract
Plants can recognize and resist invading pathogens by signaling the induction of rapid defense responses. Often these responses are mediated by single dominant resistance genes (R genes). The products of R genes have been postulated to recognize the pathogen and trigger rapid host defense responses. Here we describe isolation of the classical resistance gene N of tobacco that mediates resistance to the well-characterized pathogen tobacco mosaic virus (TMV). The N gene was isolated by transposon tagging using the maize Activator (Ac) transposon. We confirmed isolation of the N gene by complementation of the TMV-sensitive phenotype with a genomic DNA fragment. Sequence analysis of the N gene shows that it encodes a protein with an amino-terminal domain similar to that of the cytoplasmic domains of the Drosophila Toll protein and the interleukin 1 receptor in mammals, a putative nucleotide-binding site and 14 imperfect leucine-rich repeats. The presence of these functional domains in the predicted N gene product is consistent with the hypothesis that the N resistance gene functions in a signal transduction pathway. Similarities of N to Toll and the interleukin 1 receptor suggest a similar signaling mechanism leading to rapid gene induction and TMV resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Raghunath M., Bishayee S., Das M. Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: role for interreceptor interaction in kinase regulation. Mol Cell Biol. 1989 Feb;9(2):671–677. doi: 10.1128/mcb.9.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Dawson W. O. Tobamovirus-plant interactions. Virology. 1992 Feb;186(2):359–367. doi: 10.1016/0042-6822(92)90001-6. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Heguy A., Baldari C. T., Macchia G., Telford J. L., Melli M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila toll protein are essential for IL-1R signal transduction. J Biol Chem. 1992 Feb 5;267(4):2605–2609. [PubMed] [Google Scholar]
- Hehl R., Baker B. Properties of the maize transposable element Activator in transgenic tobacco plants: a versatile inter-species genetic tool. Plant Cell. 1990 Aug;2(8):709–721. doi: 10.1105/tpc.2.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
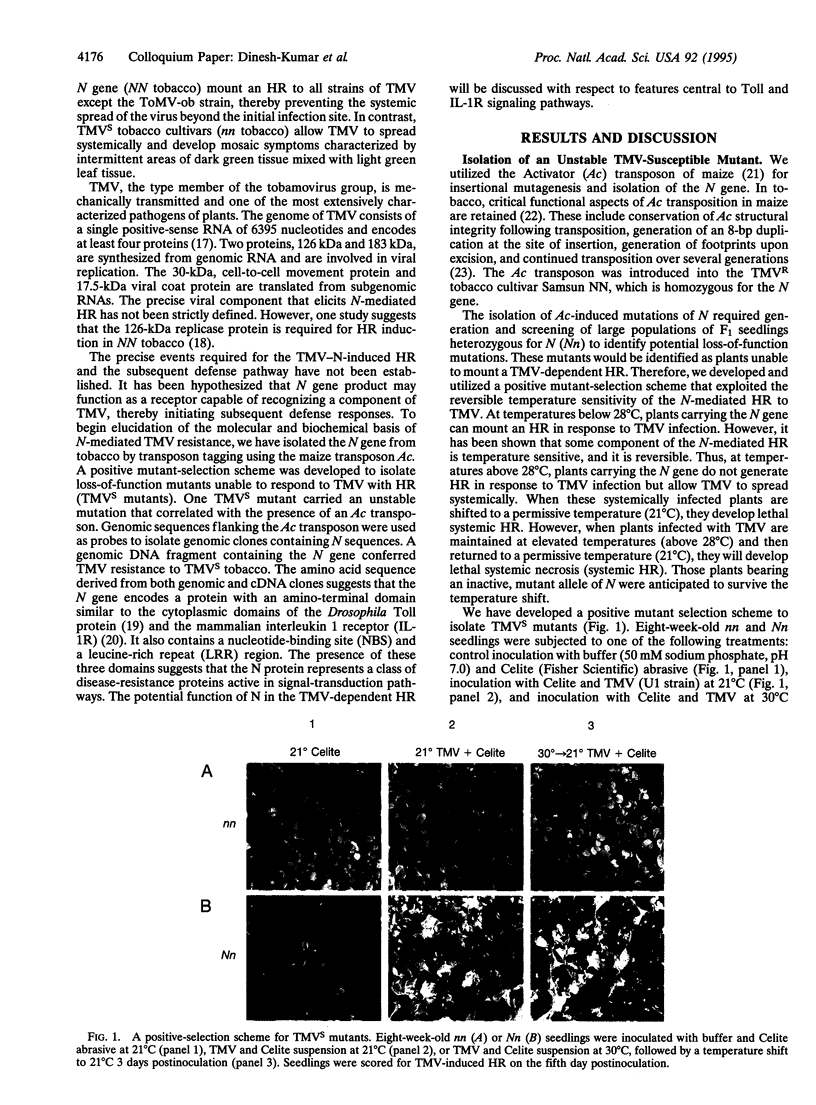
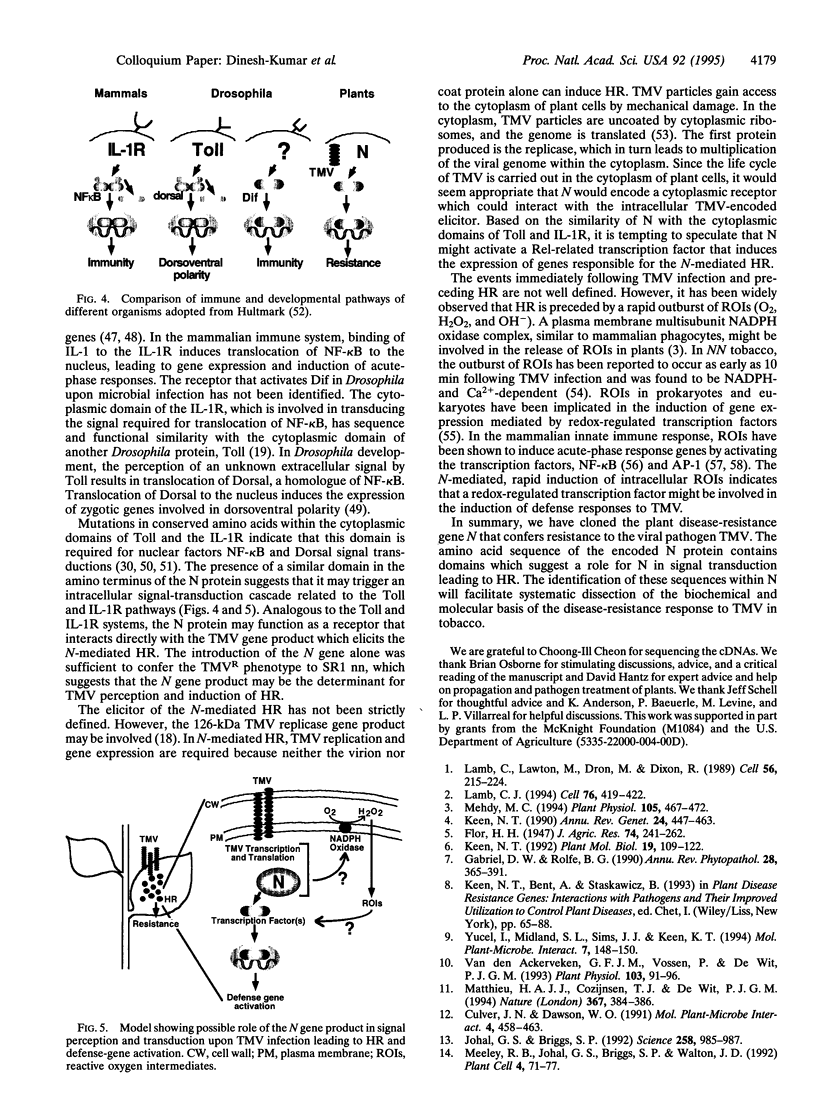
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Insect immunology. Ancient relationships. Nature. 1994 Jan 13;367(6459):116–117. doi: 10.1038/367116a0. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Johal G. S., Briggs S. P. Reductase activity encoded by the HM1 disease resistance gene in maize. Science. 1992 Nov 6;258(5084):985–987. doi: 10.1126/science.1359642. [DOI] [PubMed] [Google Scholar]
- Joosten M. H., Cozijnsen T. J., De Wit P. J. Host resistance to a fungal tomato pathogen lost by a single base-pair change in an avirulence gene. Nature. 1994 Jan 27;367(6461):384–386. doi: 10.1038/367384a0. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Keen N. T. Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet. 1990;24:447–463. doi: 10.1146/annurev.ge.24.120190.002311. [DOI] [PubMed] [Google Scholar]
- Keen N. T. The molecular biology of disease resistance. Plant Mol Biol. 1992 May;19(1):109–122. doi: 10.1007/BF00015609. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lamb C. J. Plant disease resistance genes in signal perception and transduction. Cell. 1994 Feb 11;76(3):419–422. doi: 10.1016/0092-8674(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Lee F. S., Vallee B. L. Modular mutagenesis of human placental ribonuclease inhibitor, a protein with leucine-rich repeats. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1879–1883. doi: 10.1073/pnas.87.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Betts J. C., Xu L., Nabel G. J. The cytoplasmic domain of the interleukin-1 receptor is required for nuclear factor-kappa B signal transduction. J Biol Chem. 1994 Jan 21;269(3):1579–1582. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990 Jul;5(7):1095–1097. [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993 Nov 26;262(5138):1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Meeley R. B., Johal G. S., Briggs S. P., Walton J. D. A Biochemical Phenotype for a Disease Resistance Gene of Maize. Plant Cell. 1992 Jan;4(1):71–77. doi: 10.1105/tpc.4.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy M. C. Active Oxygen Species in Plant Defense against Pathogens. Plant Physiol. 1994 Jun;105(2):467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Komatsu N., Nakauchi H. A truncated erythropoietin receptor that fails to prevent programmed cell death of erythroid cells. Science. 1992 Aug 21;257(5073):1138–1141. doi: 10.1126/science.257.5073.1138. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Nakauchi H. A truncated erythropoietin receptor and cell death: a reanalysis. Science. 1994 Apr 22;264(5158):588–589. doi: 10.1126/science.8160019. [DOI] [PubMed] [Google Scholar]
- Padgett H. S., Beachy R. N. Analysis of a tobacco mosaic virus strain capable of overcoming N gene-mediated resistance. Plant Cell. 1993 May;5(5):577–586. doi: 10.1105/tpc.5.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L., Baeuerle P. A. Oxygen and the control of gene expression. Bioessays. 1994 Jul;16(7):497–502. doi: 10.1002/bies.950160709. [DOI] [PubMed] [Google Scholar]
- Pohlman R. F., Fedoroff N. V., Messing J. The nucleotide sequence of the maize controlling element Activator. Cell. 1984 Jun;37(2):635–643. doi: 10.1016/0092-8674(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Reinke R., Krantz D. E., Yen D., Zipursky S. L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell. 1988 Jan 29;52(2):291–301. doi: 10.1016/0092-8674(88)90518-1. [DOI] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Schneider D. S., Hudson K. L., Lin T. Y., Anderson K. V. Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev. 1991 May;5(5):797–807. doi: 10.1101/gad.5.5.797. [DOI] [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991 Aug;1(2-3):39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Choe H. R., Nishida Y., Yamawaki-Kataoka Y., Ohnishi S., Tamaoki T., Kataoka T. Leucine-rich repeats and carboxyl terminus are required for interaction of yeast adenylate cyclase with RAS proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8711–8715. doi: 10.1073/pnas.87.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur J Biochem. 1994 May 15;222(1):9–19. doi: 10.1111/j.1432-1033.1994.tb18835.x. [DOI] [PubMed] [Google Scholar]
- Valon C., Smalle J., Goodman H. M., Giraudat J. Characterization of an Arabidopsis thaliana gene (TMKL1) encoding a putative transmembrane protein with an unusual kinase-like domain. Plant Mol Biol. 1993 Oct;23(2):415–421. doi: 10.1007/BF00029017. [DOI] [PubMed] [Google Scholar]
- Van Vactor D., Jr, Krantz D. E., Reinke R., Zipursky S. L. Analysis of mutants in chaoptin, a photoreceptor cell-specific glycoprotein in Drosophila, reveals its role in cellular morphogenesis. Cell. 1988 Jan 29;52(2):281–290. doi: 10.1016/0092-8674(88)90517-x. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken G. F., Vossen P., De Wit P. J. The AVR9 race-specific elicitor of Cladosporium fulvum is processed by endogenous and plant proteases. Plant Physiol. 1993 Sep;103(1):91–96. doi: 10.1104/pp.103.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel I., Midland S. L., Sims J. J., Keen N. T. Class I and class II avrD alleles direct the production of different products in gram-negative bacteria. Mol Plant Microbe Interact. 1994 Jan-Feb;7(1):148–150. doi: 10.1094/mpmi-7-0148. [DOI] [PubMed] [Google Scholar]