Abstract
The cytochrome bc complexes b6f and bc1 catalyze proton-coupled quinol/quinone redox reactions to generate a transmembrane proton electrochemical gradient. Quinol oxidation on the electrochemically positive (p) interface of the complex occurs at the end of a narrow quinol/quinone entry/exit Qp portal, 11 Å long in bc complexes. Superoxide, which has multiple signaling functions, is a by-product of the p-side quinol oxidation. Although the transmembrane core and the chemistry of quinone redox reactions are conserved in bc complexes, the rate of superoxide generation is an order of magnitude greater in the b6f complex, implying that functionally significant differences in structure exist between the b6f and bc1 complexes on the p-side. A unique structure feature of the b6f p-side quinol oxidation site is the presence of a single chlorophyll-a molecule whose function is unrelated to light harvesting. This study describes a cocrystal structure of the cytochrome b6f complex with the quinol analog stigmatellin, which partitions in the Qp portal of the bc1 complex, but not effectively in b6f. It is inferred that the Qp portal is partially occluded in the b6f complex relative to bc1. Based on a discrete molecular-dynamics analysis, occlusion of the Qp portal is attributed to the presence of the chlorophyll phytyl tail, which increases the quinone residence time within the Qp portal and is inferred to be a cause of enhanced superoxide production. This study attributes a novel (to our knowledge), structure-linked function to the otherwise enigmatic chlorophyll-a in the b6f complex, which may also be relevant to intracellular redox signaling.
Introduction
The integral membrane cytochrome bc lipoprotein complexes (cyt bc complexes) b6f (Fig. 1, A and B) and bc1 function at an intermediate redox position in the photosynthetic and respiratory electron transport chains, where they couple transmembrane proton translocation to electron transfer through redox reactions of lipophilic quinone/-ol, plastoquinone/-ol, and ubiquinone/-ol, respectively (1–6). Quinones (ubiquinone in respiratory membranes and plastoquinone (PQ) in photosynthetic membranes (Fig. 1 C)) contain a redox-active ring that is attached to a 45–50 carbon prenyl tail. On the electrochemically positive (p) side of bc complexes, quinol deprotonation-oxidation reactions within the p-side active (Qp) site involve quinol entry into a portal 11 Å in depth (Qp portal; Figs. 1 B and 2, A–D) that connects the Qp site to the quinone pool in the lipid-filled intermonomer cavity of bc complexes, as described recently in detail for the b6f complex (7). Due to the large size of the substrate quinol/quinone (Fig. 1, C and D), traffic through the Qp portal suggests the possibility of a steric constraint in intraprotein quinone dynamics.
Figure 1.
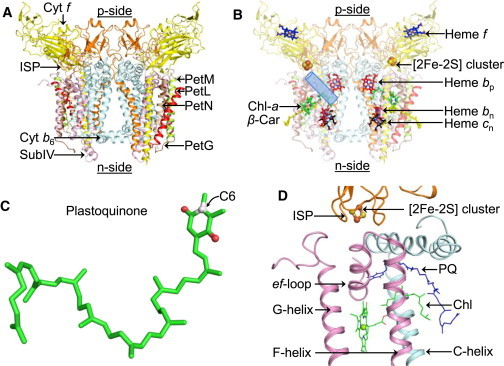
Dimeric cytochrome b6f complex of oxygenic photosynthesis from M. laminosus (PDB ID 2E74). (A) Polypeptide composition of the b6f complex. Color code showing the eight known subunits of the complex: cytochrome b6, cyan; subunit IV, pink; cytochrome f, yellow; ISP, orange; PetL, red; PetM, green; PetG, brown; PetN, wheat. (B) Prosthetic groups in the cytochrome b6f complex are shown as sticks. The Qp portal is highlighted as a blue cylinder. (C) The PQ molecule, which serves as the substrate of cytochrome b6f complex-catalyzed redox reactions. Carbon atoms are shown in green, with the exception of atom C6 (white). The oxygen atoms are shown in red. (D) Overview of the p-side quinone portal, showing both Chl (green sticks) and PQ (blue sticks).
Figure 2.
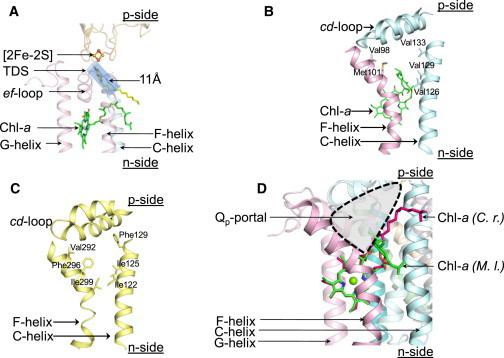
Qp portal in cytochrome bc complexes. (A) The 11-Å-wide Qp portal in the cytochrome b6f complex (PDB ID 4H13). The portal is shown as a blue tunnel around the TDS molecule (yellow/red sticks). For reference, the ef loop of the cyt b6 subunit, and the C, F, and G transmembrane helices are shown as ribbons. (B) Qp portal in the cytochrome b6f complex. C (cytochrome b6) and F (subunit IV) transmembrane helices are shown as ribbons, and residues lining the portal are shown as sticks. The phytyl chain of the Chl molecule (green sticks) is inserted between the C and F transmembrane helices, thereby constricting the space available for substrate diffusion. (C) Qp portal in the cytochrome bc1 complex (PDB ID 3CX5). The C and F transmembrane helices of the cytochrome b subunit are shown as ribbons, and residues lining the portal are shown as sticks. (D) Alternate conformations of the Chl phytyl chain in the cytochrome b6f complex. The Chl phytyl chain (green) is wrapped around the F helix in the cytochrome b6f crystal structure (PDB ID 2E74) obtained from the cyanobacterium M. laminosus (labeled M. l.), whereas it occupies a niche distal to the F helix in the b6f structure (PDB ID 1Q90) obtained from the green alga C. reinhardtii (C. r.). The Qp portal is highlighted.
It has been shown that quinol/quinone redox reactions within the Qp site of bc complexes serve as a significant source of superoxide production (Fig. 3) (8–16). Although the overall architecture and quinol redox chemistry in the p-side active site of cytochrome bc complexes are conserved (17), the rate of superoxide production is at least 10-fold higher in the b6f complex (16). It has been proposed that p-side superoxide generation in bc complexes is a consequence of electron transfer to molecular oxygen from the anionic semiquinone formed through 1), quinol oxidation by the [2Fe-2S] cluster of the iron-sulfur subunit; and/or 2), electron transfer from the reduced heme bp to the oxidized quinone (18). Despite the role of reactive oxygen species (ROS), including superoxide, in stress response, cellular signaling, and maintenance of homeostasis in photosynthetic organisms (19–22), the differences in crystal structure between the b6f and bc1 complexes relevant to superoxide generation have not been defined.
Figure 3.

Electron-proton transfer pathways in the cytochrome b6f complex. The dashed box represents the cytochrome b6f transmembrane core. On the p side, anionic semiquinone (PQ•-) (highlighted in red) is produced by deprotonation-oxidation of the reduced plastoquinol (PQH2), or back-transfer of an electron to the oxidized PQ from the reduced heme bp. The anionic semiquinone has been implicated in the reduction of molecular oxygen to superoxide (O2•-). FNR, ferredoxin-NADP+-oxidoreductase; Fd, ferredoxin. To see this figure in color, go online.
A feature that is unique to the structure of the cytochrome b6f complex Qp portal is the single chlorophyll-a (Chl) molecule in each monomer of the complex, which was first detected spectrophotometrically (23,24) and subsequently in crystal structures of the isolated dimeric b6f complex (Fig. 1, A and B) obtained from the filamentous cyanobacteria Mastigocladus laminosus (PDB IDs 1VF5, 2E74-76, 4H13, and 4H0L) and Nostoc sp. PCC 7120 (PDB IDs 2ZT9, 4H44, and 4OGQ) (6,25–28), and from the green alga Chlamydomonas reinhardtii (PDB ID 1Q90) (29). The presence of a light-harvesting pigment molecule is not expected in the structure of the cytochrome b6f electron-proton transport complex, whose reactions are not directly light dependent. Previous studies have discussed several properties of the unique Chl molecule related to H2O axial ligation (28), the aromatic amino acid environment (30), Chl excited-state lifetimes (31), excited-state triplet energy transfer (32), and a possible function in the regulation of photosynthetic-state transitions (33), as suggested by consideration of the evolution of the complex (34). A possible contact of the Chl phytyl chain with PQ, evident in the original crystal structures of the b6f complex, has been noted (27,29,33). In this study, we consider the consequences of protrusion of the phytyl chain into, and partial occlusion of, the Qp portal (Fig. 2, A–D), which serves as the entrance channel for the plastoquinol electron/proton donor to the complex and the exit for oxidized deprotonated PQ. The Chl phytyl chain appears to provide a quinone/-ol traffic barrier that is absent in the cyt bc1 complex (Fig. 2, B and C). In this regard, we note that a redox-linked conformational change in the quinol/quinone binding position within the bc1 complex Qp site has been implicated in the removal of the semiquinone intermediate (35).
Insights into the architecture of the Qp portal for p-side quinol/quinone entry/exit, and the interaction of the Chl phytyl chain with this portal, have been obtained from previous crystallographic determinations of the position of the Chl molecule in the b6f complex of cyanobacteria (6,25,27,28) and the green alga C. reinhardtii (29). Here, we analyzed further the spatial relation of the Chl phytyl chain and the p-side quinone portal. We report a crystal structure of the cyanobacterial cytochrome b6f complex with the quinone analog p-side inhibitor stigmatellin, which is an effective inhibitor of the bc1, but not the b6f complex, and thus serves as a probe for the relative size of the Qp portal. Results from a comparison with the Qp portal described in a crystal structure of the yeast bc1 complex (38), as well as a novel (to our knowledge) molecular-dynamics analysis of quinone exit from the portal, indicate that the Chl phytyl chain has a role in constraining the exit of the quinol/quinone from its charge-transfer binding site on the p side of the complex, with an implied consequence of increased quinone residence time in its p-side portal and binding site.
Materials and Methods
Protein purification and crystallization
The purification and crystallization of the cyt b6f complex from M. laminosus were described previously (28). Cocrystallization was performed with a stigmatellin/cyt b6f monomer molar ratio of ∼5:1.
X-ray diffraction data collection and data reduction
X-ray diffraction data collection was performed at 100 K using the Advanced Photon Source (Argonne IL) beamline 19-ID-B. Data reduction, including indexing, integration, and scaling, was performed in the HKL2000 suite (39). The high-resolution limit of the data set was determined according to the correlation coefficient (CC∗) method described by Karplus and Diederichs (40) (Table 1). The <I>/<σI> value, which is a measure of the average signal strength within a shell, was found to be 0.6 for the outermost shell of reflections, extending from 3.05 to 3.00 Å, with 38% completeness of the shell (Table 1; Table S1, A–C, in the Supporting Material). An analysis of the reflection intensities showed that the outermost shell was populated mainly by relatively weak reflections (I/<σI> < 2) for 870 reflections (86% of outer-shell reflections; Table 1; Fig. S1; Table S1, A–C). As a result, the average strength of the outer-shell signal was low, even though a significant number of strong reflections were present (146 reflections (14% of outer-shell reflections) with <I>/<σI> ≥ 2). Based on the CC∗ cutoff criterion, all reflections extending up to a resolution of 3.00 Å were utilized for calculation of the electron density map.
Table 1.
Summary of crystallographic data
| Crystal | Cyt b6f with stigmatellin (PDB ID 4PV1) |
|---|---|
| Data collection | |
| Beamline | APS-19-ID |
| Wavelength (Å) | 0.979 |
| Space group | P6122 |
| Cell dimensions | |
| a, b, c (Å) | 159.1, 159.1, 361.3 |
| α, β, γ (°) | 90, 90, 120 |
| Resolution (Å) | 50.00–3.00 (3.05–3.00) |
| CC∗ | 0.96 (0.81) |
| Rpim (%) | 3.5 (64.7) |
| Completeness (%) | 91.4 (38.0) |
| Redundancy | 9.8 (5.0) |
| Refinement | |
| Resolution (Å) | 49.34–3.00 |
| Reflections | 50203 |
| Rwork (%)/Rfree (%)a | 21.6/24.7 |
| Ramachandran statistics | |
| Favored (%) | 91.8 |
| Allowed (%) | 7.8 |
| Unfavored (%) | 0.4 |
| Rotamer outliers (%) | 0.4 |
| Number of atoms | |
| Protein | 7330 |
| Ligand/ion | 703 |
| Water | 16 |
| Wilson B-factor (Å2) | 96.6 |
| B-factor (Å2, average) | |
| Protein | 115.4 |
| Ligand/ion | 109.0 |
| Water | 88.0 |
| Root mean-square deviation | |
| Bond lengths (Å) | 0.004 |
| Bond angles (°) | 0.646 |
| Coordinate error (maximum likelihood, Å) | 0.4 |
Values in parentheses correspond to the outer shell; a5.1% of reflections included in the Rfree test set.
Electron density map calculation and model building
Crystallographic data reduction showed that the unit cell dimensions had similar dimensions (space group P6122, a = b = 159.1 Å, c = 361.3 Å, α = β = 90°, γ = 120°) compared with the cocrystal structure of the cyt b6f complex isolated from M. laminosus with bound quinone analog inhibitor tridecyl-stigmatellin (TDS; PDB ID 2E76, space group P6122, a = b = 157.2 Å, c = 363.3 Å, α = β = 90°, γ = 120°). The polypeptide model of the cyt b6f complex (PDB ID 2E76) was used for rigid-body refinement in REFMAC (41). The prosthetic groups heme bp, bn, cn, and f; [2Fe-2S] cluster; Chl; and β-Car were then built into the model in Coot (42). Restrained refinement was performed in PHENIX (43). After restrained refinement of polypeptide model with the prosthetic groups listed above, electron density was observed in the Fo-Fc and 2Fo-Fc maps at a position axial to heme cn. A simulated annealing OMIT map was calculated around the electron density near heme cn. A stigmatellin molecule was modeled in the electron density, with the chromone ring located proximal to heme cn and the hydrocarbon tail pointing into the intermonomer cavity. Several cycles of manual model building and restrained refinement were performed to improve the fitting of the stigmatellin ligand in the electron density map. It is significant that no electron density was observed for stigmatellin within the Qp site of the b6f complex. An OMIT map (44) was calculated in PHENIX around the Qp and Qn sites using simulated annealing. Translation-libration-screw (TLS) refinement of atomic displacement parameters (ADPs) was performed using 26 TLS groups generated in PHENIX. The figures were generated in PyMol (http://www.pymol.org) and Coot. The model coordinates and structure factors were deposited in the PDB with ID 4PV1.
Simulation of the p-side Qp portal of the cytochrome b6f complex using discrete molecular dynamics
Truncated models of the structure of the b6f complex (28) were prepared by manually removing regions distal from the Qp portal. The truncated model of cytochrome b6f (PDB ID 2E74) contained cytochrome b6 (chain A, Ile-32–Leu-215), subunit IV (chain B, Gly-63–Ala-147) and the Rieske iron-sulfur protein (ISP; chain D, Pro-44–Val-92, Ala-98–Val-179). PQ-9 was manually inserted into the Qp portal of cytochrome b6f using the known binding configuration of TDS (PDB ID 2E76) as a guide. Simulations were performed using parallelized discrete molecular dynamics (πDMD; Molecules in Action) (45) with the Medusa force field (46), which utilizes implicit solvent. Details of the DMD algorithm have been described elsewhere (45,47,48). Briefly, DMD uses discretized energetic step functions in its force field instead of continuous potentials. A discrete representation of the force field provides advantages for the modeling of specific interactions, such as hydrogen bonding (48). The time step used to advance our simulations was 50 fs, compared with 2 fs in traditional MD. In the limit of a shortened time step, the discretized potentials of DMD would become identical to the continuous potentials of MD. The production simulation temperature was 0.4 kcal/mol-kB (where kB is Boltzmann’s constant), with a heat exchange coefficient, which describes the exchange of energy between the system and the implicit solvent, of one. The units of time, temperature, and heat exchange in DMD have been fully described elsewhere (49). Temperature was controlled using the Berendsen thermostat (50). Chains of the protein that were not in direct contact with the quinone were held static with infinite square well constraints. Chains that had residues in direct contact with the quinone were harmonically constrained (spring-like constraints) with a spring constant of 10 kcal/mol × Å2 on each atom of the protein backbone, whereas the side chains were allowed to move freely. Where present, carbon atoms 5–10 of the Chl were placed under a similar harmonic constraint to keep the Chl bound to the protein, whereas the remaining 10 carbon atoms of the phytyl tail, as well as the quinone molecule of each system, were allowed to move freely. Each system was equilibrated at high temperature (0.7 kcal/mol-kB) and high heat exchange (10 exchanges per unit time) for 1,000 DMD time steps (∼50 ps). Simulations were performed for 500,000 DMD time steps (∼25 ns) to observe the behavior of the quinone and Chl phytyl tail in the portal. Production simulations were repeated in 25 replicates for each system of the structure of the b6f complex (PDB ID 2E74) with and without the Chl molecule. The effect of the alternate Chl tail conformation on PQ retention was also investigated by utilizing the Chl conformation from the related eukaryotic b6f structure (PDB ID 1Q90) and analyzing it in the context of the cyanobacterial b6f structure (PDB ID 2E74).
Results
Properties of the p-side quinol-binding niche
The Qp portal provides a connection between the intermonomer cavity (Fig. 1 B) of cytochrome bc complexes, which is believed to be the site of quinone transfer to or from the quinone pool in the membrane bilayer (2,27), as well as the Qp site of quinol deprotonation-oxidation (2), and may contribute to the heterogeneity of the dielectric constant within the complex (51). For the portals in both complexes, the substrate quinols ((plastoquinol in oxygenic photosynthesis, and ubiquinol in anoxygenic photosynthesis and mitochondrial respiration) have a long poly-prenyl chain with nine and ten prenyl units, respectively, which presumably creates a considerable enthalpic and entropic barrier for the diffusion and insertion of the substrate quinol into the narrow Qp portal. Access to the Qp site of cyt bc complexes is defined by the p-side Qp portal that is approximately normal to the plane of the membrane (Fig. 2, B and C). The overall architecture of the Qp portal is conserved between cytochrome bc complexes. The width of the portal (defined by its bordering amino acid residues) is slightly smaller in the cytochrome bc1 complex (10–12 Å; Fig. 2 C), where the portal is lined by aliphatic and aromatic amino acids, than in the b6f complex (13–14 Å; Fig. 2 B), where it is bordered by aliphatic residues (17). The amino acid residues that form the quinone-transfer portal belong to the C and F transmembrane helices of the cytochrome b subunit of bc1, and of the cytochrome b6 subunit and subunit IV in the b6f complex. A sequence and structure comparison shows that the portal is lined in bc1 by the residues Ile-122, Ile-125, and Phe-129 of the C helix, and Val-292, Phe-296, and Ile-299 of the cytochrome b F helix. In b6f, the portal is defined mainly by small hydrophobic amino acids in the C-helix (Val-126, Val-129, and Val-133) and the F-helix (Val-98 and Met-101). Hence, the protein structure implies that the width of the Qp portal should be smaller in the bc1 complex than in the b6f complex. However, the effective diameter of the portal in the b6f complex is significantly reduced from the 13–14 Å defined by the amino acid residues lining the channel due to the presence of the phytyl chain of Chl, as discussed below.
Occlusion of the quinol-transfer portal
The presence of the Chl phytyl chain that occludes the Qp portal in the b6f complex is a significant difference between the p-side quinol-transfer Qp portal in the cytochrome b6f and bc1 complexes. The portal including the phytyl chain (28) is shown in the context of the surrounding amino acid environment in Fig. 2 B, and for the structures obtained from M. laminosus (28) and C. reinhardtii (29) in Fig. 2 D. The different positions of the Chl phytyl chain in the two structures of the b6f complex imply either flexibility of the phytyl chain and/or more than one stable position (Fig. 2 D). In both structures, the presence of the phytyl chain significantly occludes the quinol-transfer portal on the p side of the complex.
Stigmatellin: a probe of the p-side portal space
To probe the accessible space of the Qp portal within the cytochrome b6f complex, we performed a cocrystallization study of the cytochrome b6f complex (PDB ID 4PV1; for a summary of the crystallographic data, see Table 1) with the p-side quinone analog/inhibitor stigmatellin and compared the results with the previously reported structure of the b6f complex with the related inhibitor TDS (PDB ID 2E76). Stigmatellin and TDS have identical cyclic chromone rings (Fig. 4, A and B) attached to a hydrocarbon tail, which is branched in stigmatellin, leading to the expectation that stigmatellin would occupy a larger volume than TDS. Stigmatellin is well known to be a substantially less effective inhibitor of electron transport in oxygenic photosynthesis compared with TDS (53), which contrasts with its well-known efficacy as a p-side inhibitor of electron transport in the bc1 complex (54–56). It was observed that, unlike TDS (6,27–29), stigmatellin was unable to bind tightly within the Qp portal of the b6f complex (PDB ID 4PV1, this study).
Figure 4.
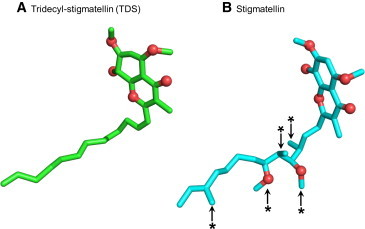
Structure of the quinone analog/inhibitors (A) TDS and (B) stigmatellin. Both inhibitors have identical chromone rings that interact with the Qp site residues. Structural differences lie in the hydrocarbon tails of stigmatellin and TDS. The tail of TDS is unbranched, whereas that of stigmatellin is branched (branching highlighted by arrows and asterisks). The oxygen atoms are shown as red spheres. To see this figure in color, go online.
As can be seen by a comparison of the OMIT maps for stigmatellin at the Qn and Qp sites, respectively (Fig. 5, A and B), although no significant electron density was observed within the Qp site (Fig. 5 B), electron density was clearly defined for stigmatellin at the Qn site (Fig. 5, A and C). At the Qn site, the chromone ring of stigmatellin was modeled and refined proximal to heme cn, whereas the hydrocarbon tail was mostly disordered (Fig. 5 C).
Figure 5.
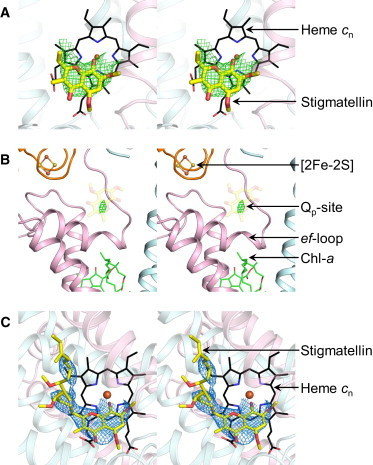
Crystal structure of the cytochrome b6f complex with stigmatellin. (A and B) OMIT map (stereo-pair images) around stigmatellin on the (A) n side and (B) p side. Electron density is observed (mesh, green) on the n side in a position axial to heme cn. However, no significant electron density feature is recorded on the p side. For clarity, only the chromone ring of stigmatellin is shown (semitransparent). The electron density map is contoured at 3.5 σ. (C) Stigmatellin bound axially to heme cn. The 2Fo-Fc map (mesh, blue; contoured at 1.2 σ) shows electron density around the stigmatellin chromone ring. The distal portion of the hydrocarbon tail, located away from the chromone ring, is largely disordered. To see this figure in color, go online.
DMD simulation of quinone exit from the Qp portal of the cytochrome b6f complex
The effect of the presence of the Chl phytyl chain on the dynamics of the lipophilic quinone within the Qp portal of the cytochrome b6f complex from the cyanobacterium M. laminosus (PDB ID 2E74) was analyzed using DMD (45,47,48). Interactions of the PQ molecule with the amino acid residues of the Qp portal were probed in the presence and absence of Chl. To determine whether the presence of the Chl phytyl chain affects the retention of PQ within the Qp portal, a DMD analysis was utilized with an initial simulation on a 25 ns timescale (Table 2).
Table 2.
Kinetics of plastoquinone exit from the Qp portal
| Run | Exit time (with M. laminosus Chl phytyl tail, ns) | Exit time (with C. reinhardtii Chl phytyl tail, ns) | Exit time (without Chl phytyl tail, ns) |
|---|---|---|---|
| 1 | no exit | no exit | no exit |
| 2 | no exit | no exit | no exit |
| 3 | no exit | no exit | no exit |
| 4 | no exit | no exit | 21.9 |
| 5 | no exit | no exit | 16.9 |
| 6 | no exit | no exit | 15.1 |
| 7 | no exit | no exit | 14.3 |
| 8 | no exit | no exit | 10.8 |
| 9 | no exit | 19.5 | 10.1 |
| 10 | no exit | 16.6 | 9.6 |
| 11 | no exit | 16.6 | 8.9 |
| 12 | no exit | 14.9 | 8.0 |
| 13 | 24.4 | 13.1 | 6.2 |
| 14 | 23.4 | 12.3 | 4.8 |
| 15 | 15.4 | 12.0 | 3.7 |
| 16 | 14.7 | 11.8 | 3.3 |
| 17 | 11.2 | 11.1 | 0.2 |
| 18 | 10.7 | 10.2 | 2.1 |
| 19 | 9.9 | 7.7 | 2.0 |
| 20 | 8.4 | 6.9 | 0.8 |
| 21 | 7.3 | 5.9 | 0.7 |
| 22 | 3.8 | 4.9 | 0.5 |
| 23 | 1.9 | 3.7 | 0.4 |
| 24 | 1.9 | 3.4 | 0.3 |
| 25 | 1.5 | 2.8 | 0.2 |
| Avg. | 10.4 ± 2.1 (13) | 10.2 ± 1.2 (17) | 6.4 ± 1.4 (22) |
The M. laminosus cytochrome b6f complex structure (PDB ID 2E74) was utilized for DMD simulations to determine the effect of Chl phytyl tail on plastoquinone (PQ) retention. PQ exit is defined as displacement of the PQ ring (atom C6; Fig. 1C) by 11 Å from its initial position defined proximal to the His-129 ligand of the Rieske ISP. No exit: PQ was displaced by <11 Å within the 25 ns time period of the simulation. Avg., average exit time ± standard error of mean (value in parentheses denotes trials included in calculation).
PQ was initially positioned on the p-side end of the portal proximal (2 Å) to the His-129 ligand to the [2Fe-2S] cluster of the Rieske ISP subunit. PQ translation through the Qp portal was measured as the displacement of the C6 atom of the quinone ring (Fig. 1 C) from its initial position. Exit of the PQ molecule was defined as an 11 Å displacement of the C6 atom of the quinone ring (Fig. 1 C), equal to the length of the Qp portal, within a time interval of 25 ns. In the presence of the Chl-phytyl chain positioned as in the cyanobacterial b6f complex, in 25 independent repetitions of the DMD simulation, PQ exit was observed in only 13 of the 25 simulations (Table 2). In the other 12 simulations, the displacement of the PQ was <11 Å (Table 2) and was considered to be retained within the portal. The time period of PQ displacement by 11 Å was found to be 10.4 ns in the presence of the Chl phytyl tail, averaged over 13 MD trials. The simulation trials that did not result in PQ displacement by at least 11 Å from the initial position within 25 ns were not included in the calculation of the average exit time. When the alternate, extended phytyl-tail conformation was introduced from the eukaryotic C. reinhardtii b6f structure (PDB ID 1Q90) into the cyanobacterial b6f structure (PDB ID 2E74), the quinone exit time was 10.2 ns for the same 11 Å displacement (Table 2), indicating that the Chl phytyl-tail conformations observed in the cyanobacterial and algal b6f structures have a very similar effect on PQ retention. For the 13 fastest exit times from the C. reinhardtii portal that correspond to the number measured for the cyanobacterial b6f complex, the average exit time was 8.2 ns. In the absence of the Chl phytyl tail, the PQ molecule underwent a displacement by >11 Å in 22 of the 25 independent MD trials (Table 2) with an average exit time of 6.4 ns, and an average exit time of 1.9 ns for the 13 trials with shortest exit time. Hence, the PQ molecule was found to undergo exit from the Qp portal not only faster in the absence of the Chl phytyl tail but also substantially more frequently (i.e., in 22 of 25 trials compared with 13 of 25 in the presence of the phytyl chain). It is significant to note that the Chl phytyl tail underwent extensive motion during the course of the DMD simulation (Fig. S2).
Discussion
Longer residence time of quinone within the Qp portal: steric function of the unique Chl
The proximity of the Chl phytyl chain to the Qp portal results in a smaller cross section for quinol/quinone entry and exit via the portal in the b6f complex compared with that in the bc1 complex. The smaller Qp portal in the b6f complex (Fig. 2 B) compared with that of the yeast cytochrome bc1 complex (Fig. 2 C) is consistent with the ability of the b6f complex to bind TDS (28,53), which occupies a smaller volume than stigmatellin (Fig. 4, A and B) (28), within the Qp site. The crystallographic result obtained in this study is consistent with the observation that the binding of the stigmatellin analog TDS to the b6f complex is ∼20- to 30-fold stronger than that of stigmatellin (53). The OMIT map calculated for stigmatellin shows the presence of electron density in a position axial to heme cn (Fig. 5 A). A similar electron density pattern was previously observed proximal to heme cn in the crystal structure of the cyt b6f complex from C. reinhardtii (PDB ID 1Q90), obtained with TDS (29), and in an OMIT map calculated around heme cn, in the M. laminosus cyt b6f-TDS cocrystal structure (PDB ID 2E76) (28). As discussed previously (57), due to the limited availability of a selective amino acid environment, the Qn site in the cyt b6f complex is expected to have a relatively lower selectivity and affinity for substrate binding than the Qp site. These inferences about the smaller aperture of the Qp portal caused by protrusion of the phytyl chain are illustrated by the fact that stigmatellin does not bind effectively at the b6f Qp site where its binding is restricted by the narrow Qp portal, while no steric barrier prevents binding of stigmatellin on the n-side (Fig. 5, A and C).
MD analysis of portal blockage by the Chl phytyl chain
Further insight into the modification of the interactions of the substrate quinone with the cytochrome b6f complex by the Chl molecule was derived from MD simulations that described the effect of Chl on the residence time of the substrate PQ (Table 2). A PQ exit path defined by an 11 Å displacement from the quinol charge-transfer site at the base of the Qp portal, as shown in Fig. 2 A, was defined. In the presence of the phytyl chain, exit through an 11 Å displacement from the initial position at the p-side interface (Figs. 1 D and 2 A), defined statistically through 25 trials, occurred in only one-half to two-thirds of the 25 trials when the Chl phytyl tail conformation was in the position observed in cyanobacterial (28) or C. reinhardtii (29) b6f structures. The average exit time of ∼10 ns was recorded for PQ exit from the Qp portal when the Chl phytyl tail was in either of the orientations observed in the cyanobacterial or C. reinhardtii b6f structures (Table 2). However, in the absence of the Chl, PQ exit through the 11 Å displacement was favored in 22 of 25 trials, with an average exit time of ∼6 ns for the whole set and only 1.9 ns for the set of 13 fastest trials. It is inferred that the presence of the Chl phytyl tail within the Qp portal contributes to the retention of PQ within the Qp site on a timescale that is short compared with the approximately millisecond step(s) associated with proton transfer from the quinol, which is the rate-limiting step in p-side electron-proton transfer (58).
The quinone that exits from the p-side portal would next encounter the intermonomer cavity domain of the dimeric complex (Fig. 1, A and B). This cavity in known to contain lipid-binding sites (7). This lipid presence in the cavity could further impede the exit of the quinone from the portal. Such an effect presumably would be common to both b6f and bc1 complexes.
Consequences of portal blockage by the Chl phytyl chain on the p-side reactions of the b6f complex: enhanced superoxide production
Steric restrictions caused by Chl provide an obstruction to plastoquinol entry into the Qp portal and thus may affect the overall rate of electron transfer through the b6f complex. However, the isolated b6f complex supports an electron transfer rate of ∼250 electrons monomer−1 s−1 at room temperature (25,59,60), which is comparable to the activity of the isolated bc1 complex (61). Therefore, quinol passage through the Chl-obstructed Qp portal is not the rate-limiting step in the photosynthetic linear electron transport chain. Alternatively, it has been proposed that p-side deprotonation of the quinol molecule defines the rate-limiting millisecond step in the redox reactions of the cytochrome bc1-catalyzed reactions (62), which would apply as well to the b6f complex.
Superoxide generation: the alternative p-side reaction
The specific rate of superoxide generation (normalized to the electron transfer rate) is more than an order of magnitude larger in the b6f complex than in the bc1 complex (16). It is inferred from the data described here that this is a consequence of the increased residence time of the PQ and hence, through equilibration, of the plasto-semiquinone within the Qp portal. This inference depends on whether electron transfer to, and reduction of, molecular oxygen in the region of the Qp site occurs on a timescale <10 ns, which corresponds with the time boundary estimated for quinone exit from the quinone portal (Table 2). A nanosecond timescale (approximately that which is associated with electron tunneling through a electron donor-acceptor distance of 8–10 Å (63,64) and thus electron transfer from the plasto-semiquinone to heme bp, separated by a distance of 5–10 Å (6,27–29,35)) characterizes the pathway that is responsible for energy storage in the complex. Hence, electron transfer to dissolved molecular oxygen can occur on a subnanosecond timescale (64), which is faster than the egress time of the quinone from the Qp site. Enhanced quinone retention due to the steric effects of the Chl phytyl tail could then increase superoxide production by providing an increased lifetime of the anionic semiquinone.
Function of the enigmatic Chl in signaling
Intricate pathways have been described for ROS-mediated signaling in photosynthetic organisms in response to environmental stress (19–22). ROS generated in the chloroplast has been implicated in the regulation of nuclear genes through the activation of nuclear response pathways that are essential for homeostasis (65). Our study implies a structure-linked function of the Chl phytyl chain relevant to ROS production. As described here, the Chl phytyl chain impedes quinol/quinone dynamics within the Qp portal of the cytochrome b6f complex, which leads to enhanced retention of the intermediate semiquinone. Superoxide production as a structural consequence of Chl insertion into the cytochrome b6f p-side catalytic site may thus represent an evolutionary modification crucial for stress response and a signaling system necessary for cellular homeostasis.
Acknowledgments
We thank P. Afonine, P. Plevka, V.M. Prasad, M.G. Rossmann, H. Zhang, and members of the CCP4 Workshop 2010 for helpful discussions. Diffraction measurements were facilitated by S. Ginell, J. Lanarz, and F. Rotella at Beamline-19-ID, Structural Biology Center, Advanced Photon Source, Argonne National Laboratory, operated by the University of Chicago, Argonne, LLC, for the Office of Biological and Environmental Research, Department of Energy (DE-AC02-06CH11357).
This study was supported by grants from the NIH (R01-GM038323 to W.A.C. and R01-GM080742 to N.V.D.), a Purdue University Fellowship (S.S.H.), infrastructure support from the Purdue University Center for Cancer Research (NIH P30 CA023168), and an NIH Predoctoral Fellowship from the National Institute on Aging (F31AG039266 to E.A.P.).
Supporting Material
References
- 1.Berry E.A., Guergova-Kuras M., Crofts A.R. Structure and function of cytochrome bc complexes. Annu. Rev. Biochem. 2000;69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 2.Cramer W.A., Zhang H., Smith J.L. Transmembrane traffic in the cytochrome b6f complex. Annu. Rev. Biochem. 2006;75:769–790. doi: 10.1146/annurev.biochem.75.103004.142756. [DOI] [PubMed] [Google Scholar]
- 3.Crofts A.R. The cytochrome bc1 complex: function in the context of structure. Annu. Rev. Physiol. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- 4.Hasan S.S., Yamashita E., Cramer W.A. Conservation of lipid functions in cytochrome bc complexes. J. Mol. Biol. 2011;414:145–162. doi: 10.1016/j.jmb.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trumpower B.L., Gennis R.B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 6.Hasan S.S., Yamashita E., Cramer W.A. Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex. Proc. Natl. Acad. Sci. USA. 2013;110:4297–4302. doi: 10.1073/pnas.1222248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan S.S., Cramer W.A. Internal lipid architecture of the hetero-oligomeric cytochrome b6f complex. Structure. 2014;22:1008–1015. doi: 10.1016/j.str.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cape J.L., Bowman M.K., Kramer D.M. Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci. 2006;11:46–55. doi: 10.1016/j.tplants.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Cape J.L., Bowman M.K., Kramer D.M. A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc. Natl. Acad. Sci. USA. 2007;104:7887–7892. doi: 10.1073/pnas.0702621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dröse S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 11.Dröse S., Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 12.Forquer I., Covian R., Kramer D.M. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2006;281:38459–38465. doi: 10.1074/jbc.M605119200. [DOI] [PubMed] [Google Scholar]
- 13.Lanciano P., Khalfaoui-Hassani B., Daldal F. Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta. 2013;1827:1332–1339. doi: 10.1016/j.bbabio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller F., Crofts A.R., Kramer D.M. Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry. 2002;41:7866–7874. doi: 10.1021/bi025581e. [DOI] [PubMed] [Google Scholar]
- 15.Ghelli A., Tropeano C.V., Rugolo M. The cytochrome b p.278Y>C mutation causative of a multisystem disorder enhances superoxide production and alters supramolecular interactions of respiratory chain complexes. Hum. Mol. Genet. 2013;22:2141–2151. doi: 10.1093/hmg/ddt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baniulis D., Hasan S.S., Cramer W.A. Mechanism of enhanced superoxide production in the cytochrome b(6)f complex of oxygenic photosynthesis. Biochemistry. 2013;52:8975–8983. doi: 10.1021/bi4013534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer W.A., Hasan S.S., Yamashita E. The Q cycle of cytochrome bc complexes: a structure perspective. Biochim. Biophys. Acta. 2011;1807:788–802. doi: 10.1016/j.bbabio.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarewicz M., Borek A., Osyczka A. Discrimination between two possible reaction sequences that create potential risk of generation of deleterious radicals by cytochrome bc1. Implications for the mechanism of superoxide production. Biochim. Biophys. Acta. 2010;1797:1820–1827. doi: 10.1016/j.bbabio.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foyer C.H., Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittler R., Vanderauwera S., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Mittler R., Vanderauwera S., Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Fernández A.P., Strand A. Retrograde signaling and plant stress: plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 2008;11:509–513. doi: 10.1016/j.pbi.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Huang D., Everly R.M., Cramer W.A. Characterization of the chloroplast cytochrome b6f complex as a structural and functional dimer. Biochemistry. 1994;33:4401–4409. doi: 10.1021/bi00180a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierre Y., Breyton C., Popot J.-L. On the presence and role of a molecule of chlorophyll a in the cytochrome b6f complex. J. Biol. Chem. 1997;272:21901–21908. doi: 10.1074/jbc.272.35.21901. [DOI] [PubMed] [Google Scholar]
- 25.Baniulis D., Yamashita E., Cramer W.A. Structure-function, stability, and chemical modification of the cyanobacterial cytochrome b6f complex from Nostoc sp. PCC 7120. J. Biol. Chem. 2009;284:9861–9869. doi: 10.1074/jbc.M809196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J., Kurisu G., Cramer W.A. Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. Proc. Natl. Acad. Sci. USA. 2006;103:69–74. doi: 10.1073/pnas.0504909102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurisu G., Zhang H., Cramer W.A. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science. 2003;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita E., Zhang H., Cramer W.A. Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn. J. Mol. Biol. 2007;370:39–52. doi: 10.1016/j.jmb.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroebel D., Choquet Y., Picot D. An atypical haem in the cytochrome b(6)f complex. Nature. 2003;426:413–418. doi: 10.1038/nature02155. [DOI] [PubMed] [Google Scholar]
- 30.Yan J., Dashdorj N., Cramer W.A. On the structural role of the aromatic residue environment of the chlorophyll a in the cytochrome b6f complex. Biochemistry. 2008;47:3654–3661. doi: 10.1021/bi702299b. [DOI] [PubMed] [Google Scholar]
- 31.Dashdorj N., Zhang H., Savikhin S. The single chlorophyll a molecule in the cytochrome b6f complex: unusual optical properties protect the complex against singlet oxygen. Biophys. J. 2005;88:4178–4187. doi: 10.1529/biophysj.104.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Dashdorj N., Savikhin S. An anomalous distance dependence of intra-protein chlorophyll-carotenoid triplet energy transfer. Biophys. J. 2005;89:28–30. doi: 10.1529/biophysj.105.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lacroix de Lavalette A., Finazzi G., Zito F. b6f-Associated chlorophyll: structural and dynamic contribution to the different cytochrome functions. Biochemistry. 2008;47:5259–5265. doi: 10.1021/bi800179b. [DOI] [PubMed] [Google Scholar]
- 34.Hasan S.S., Cramer W.A. Lipid functions in cytochrome bc complexes: an odd evolutionary transition in a membrane protein. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:3406–3411. doi: 10.1098/rstb.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crofts A.R., Barquera B., Berry E.A. Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry. 1999;38:15807–15826. doi: 10.1021/bi990962m. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted in proof.
- 37.Reference deleted in proof.
- 38.Solmaz S.R., Hunte C. Structure of complex III with bound cytochrome c in reduced state and definition of a minimal core interface for electron transfer. J. Biol. Chem. 2008;283:17542–17549. doi: 10.1074/jbc.M710126200. [DOI] [PubMed] [Google Scholar]
- 39.Otwinowski Z., Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 40.Karplus P.A., Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 42.Emsley P., Lohkamp B., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams P.D., Afonine P.V., Zwart P.H. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terwilliger T.C., Grosse-Kunstleve R.W., Hung L.W. Iterative-build OMIT maps: map improvement by iterative model building and refinement without model bias. Acta Crystallogr. D Biol. Crystallogr. 2008;64:515–524. doi: 10.1107/S0907444908004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirvanyants D., Ding F., Dokholyan N.V. Discrete molecular dynamics: an efficient and versatile simulation method for fine protein characterization. J. Phys. Chem. B. 2012;116:8375–8382. doi: 10.1021/jp2114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding F., Dokholyan N.V. Emergence of protein fold families through rational design. PLOS Comput. Biol. 2006;2:e85. doi: 10.1371/journal.pcbi.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dokholyan N.V., Buldyrev S.V., Shakhnovich E.I. Discrete molecular dynamics studies of the folding of a protein-like model. Fold. Des. 1998;3:577–587. doi: 10.1016/S1359-0278(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 48.Ding F., Tsao D., Dokholyan N.V. Ab initio folding of proteins with all-atom discrete molecular dynamics. Structure. 2008;16:1010–1018. doi: 10.1016/j.str.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding F., Buldyrev S.V., Dokholyan N.V. Folding Trp-cage to NMR resolution native structure using a coarse-grained protein model. Biophys. J. 2005;88:147–155. doi: 10.1529/biophysj.104.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berendsen H.J.C., Postma J.P.M., Haak J.R. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 51.Hasan S.S., Zakharov S.D., Cramer W.A. A map of dielectric heterogeneity in a membrane protein: the hetero-oligomeric cytochrome b6f complex. J. Phys. Chem. B. 2014;118:6614–6625. doi: 10.1021/jp501165k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reference deleted in proof.
- 53.Hope A.B., Valente P. Inhibitor binding to isolated chloroplast cytochrome bf complex. Photosynth. Res. 1996;49:37–48. doi: 10.1007/BF00029426. [DOI] [PubMed] [Google Scholar]
- 54.Iwata S., Lee J.W., Jap B.K. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 55.Kunze B., Kemmer T., Reichenbach H. Stigmatellin, a new antibiotic from Stigmatella aurantiaca (Myxobacterales). I. Production, physico-chemical and biological properties. J. Antibiot. 1984;37:454–461. doi: 10.7164/antibiotics.37.454. [DOI] [PubMed] [Google Scholar]
- 56.Thierbach G., Kunze B., Reichenbach H., Höfle G. The mode of action of stigmatellin, a new inhibitor of the cytochrome b-c1 segment of the respiratory chain. Biochim. Biophys. Acta. 1984;765:227–235. [Google Scholar]
- 57.Saif Hasan S., Yamashita E., Cramer W.A. Transmembrane signaling and assembly of the cytochrome b6f-lipidic charge transfer complex. Biochim. Biophys. Acta. 2013;1827:1295–1308. doi: 10.1016/j.bbabio.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crofts A.R., Wang Z. How rapid are the internal reactions of the ubiquinol:cytochrome c 2 oxidoreductase? Photosynth. Res. 1989;22:69–87. doi: 10.1007/BF00114768. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H., Kurisu G., Cramer W.A. A defined protein-detergent-lipid complex for crystallization of integral membrane proteins: the cytochrome b6f complex of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA. 2003;100:5160–5163. doi: 10.1073/pnas.0931431100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H., Whitelegge J.P., Cramer W.A. Ferredoxin:NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6f complex. J. Biol. Chem. 2001;276:38159–38165. doi: 10.1074/jbc.M105454200. [DOI] [PubMed] [Google Scholar]
- 61.Yu L., Yang S., Yu C.A. Chapter 25 Analysis of electron transfer and superoxide generation in the cytochrome bc1 complex. Methods Enzymol. 2009;456:459–473. doi: 10.1016/S0076-6879(08)04425-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crofts A.R., Guergova-Kuras M., Hong S.J. Proton-coupled electron transfer at the Q(o) site: what type of mechanism can account for the high activation barrier? Biochim. Biophys. Acta. 2000;1459:456–466. doi: 10.1016/s0005-2728(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 63.Moser C.C., Anderson J.L., Dutton P.L. Guidelines for tunneling in enzymes. Biochim. Biophys. Acta. 2010;1797:1573–1586. doi: 10.1016/j.bbabio.2010.04.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moser C.C., Keske J.M., Dutton P.L. Nature of biological electron transfer. Nature. 1992;355:796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- 65.Pfannschmidt T., Schütze K., Oelmüller R. A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem. 2001;276:36125–36130. doi: 10.1074/jbc.M105701200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


