Abstract
The genetic factors involved in the regulation of physical activity are not well understood. The dopamine system has been implicated in the control of voluntary locomotion and wheel running (WR) in mice and is thus a likely candidate as a genetic/biological system important to the regulation of physical activity. This study evaluated the effects of four different dopaminergic acting drugs on WR in differentially active inbred strains of mice. High active C57L/J (n=7, 3 controls, 4 experimental) and low active C3H/HeJ (n=8, 3 controls, 5 experimental) were analyzed for baseline wheel-running indices of distance (km/day), duration (mins/day), and speed (m/min) for 21 days. Experimental mice received increasing doses over four days of each of the following drugs: SKF 81297 (D1 agonist), SCH 23390 (D1 antagonist), GBR 12783 (DAT inhibitor), and AMPT (tyrosine hydroxylase inhibitor). Each drug dose response treatment was separated by three days of recovery (no drug injections). WR indices were monitored during drug treatments and during drug wash-out phases. SKF 81297 significantly reduced (p=0.0004) WR in the C57L/J mice, but did not affect WR in the C3H/HeJ mice. GBR 12783 significantly increased (p=0.0005) WR in C3H/HeJ mice, but did not affect WR in C57L/J mice. Only duration (not overall WR) was significantly reduced in C57L/J mice in response to SCH 23390 (p=0.003) and AMPT (p=0.043). SCH 23390 (p=0.44) and AMPT (p=0.98) did not significantly affect WR in C3H/HeJ mice. These results suggest that genetic differences in dopamine signaling may play a role in the WR response to dopaminergic-acting drugs in inbred strains of mice. The high activity in the C57L/J strain appears most responsive to D1-like receptor acting drugs, while in the C3H/HeJ strain, dopamine re-uptake appears to have an influence on activity level.
Keywords: dopamine, dopamine signaling, physical activity, inbred mice, genetics, regulation
It is well known that physical activity improves human health by decreasing the risk of obesity, cardiovascular diseases, Type II Diabetes, depression, certain types of cancer, and overall mortality (1). Although the physiology of exercise has been well studied over the past 40 years, the genetic and biological regulating factors of physical activity have yet to be fully investigated and understood. It has been estimated that physical inactivity is a leading cause of mortality, and contributes to increasingly higher health care costs in developed countries (2). Therefore, in order to prevent disease and improve human health it is vital to understand the regulating factors of physical activity.
It has been shown that physical activity patterns are at least moderately inherited and thus partially regulated by genetic factors (3-7). At least two studies have identified both single-gene and epistatic quantitative trait loci (QTL) involved in the regulation of physical activity in mice; in particular, significant single-gene QTL have been found on chromosomes 9 and 13 (8, 9). However, the exact genes involved in regulation of physical activity are yet to be fully elucidated. A different model from inbred mice, selective breeding studies conducted by Garland and colleagues also illustrate a significant genetic component involved in the regulation of physical activity. After 35 generations of selective breeding for running wheel activity, selected animals ran over 170% farther than control mice (10). Selection acting on genetic variation in the original outbred population of mice highlights a definite genetic component to the regulation of voluntary physical activity in mice.
The central nervous system may play a key role in the genetic/biological regulation of physical activity in rodents (11-14). The dopamine system, part of the central nervous system, located in the mid-brain, has two main tracks. The nigrostriatal track mediates locomotion (15) while the mesolimbic track is involved with emotion and motivation (16). For example, it is known that depletion of dopamine neurons in the mid-brain are a major cause of the motor deficits seen in Parkinson’s disease (17). Also, the hyperactive phenotype common in Attention Deficit Hyperactivity Disorder (ADHD) is also mediated through dysfunctions in dopamine signaling in the brain (18). Pharmacological studies in rodents confirm dopaminergic involvement in locomotor behavioral responses to stimuli such as psychostimulant drugs (19, 20); however, compelling evidence from wheel running studies in mice also implicates the dopamine system in mediating general voluntary physical activity levels. Specifically, Rhodes and Garland (2003) investigated the effects of Ritalin (a DAT inhibitor), apomorphine (a non-selective dopamine agonist), SCH 23390 (a selective D1-like antagonist), and raclopride (a selective D2-like antagonist) on wheel running in both selected and control animals (12). At high doses of apomorphine, and all doses of raclopride, both control and selected animals markedly decreased their wheel running by the same proportion. However, in response to SCH 23390 control line mice decrease wheel running more than selected animals. A differential response to Ritalin was seen where the selected animals decreased wheel running in response to Ritalin, while the control animals increased wheel running. A differential response to Ritalin, a drug that acts by increasing circulating dopamine (by inhibiting the re-uptake via DAT), indicates genetic differences in dopamine signaling between the selectively bred animals and the controls. Additionally, recent results from our lab exhibiting an independent relationship of dopamine D1 receptors and tyrosine hydroxylase genes with differentially active inbred mice in the nucleus accumbens and striatum area of the brain (21) indicate that D1-like receptors as well as the amount of dopamine present in the mid-brain may influence wheel running in mice.
Wheel running in animals has been suggested as a good model for daily physical activity in humans (22, 23). Thus, studying wheel running responses to dopaminergic drugs may prove useful in elucidating the proposed independent mechanism by which the dopamine system mediates physical activity behavior. Therefore, the purpose of this study was to investigate the wheel running responses to several dopaminergic acting drugs in differentially active inbred mice. This study is another step in the understanding of the central genetic and biological regulation of physical activity, and will be important for future studies investigating the mechanisms of this regulation and importance to human health and performance.
MATERIALS AND METHODS
Animals
Differentially active strains of inbred mice were used in this study: C3H/HeJ mice (n=8 males) previously identified as low active (30), and C57L/J mice (n=6 females, n=l male) previously identified as high active (30). The use of primarily female C57L/J mice, while not optimal, was unavoidable due to the extremely limited supply of these highly active mice (see below). However, whereas comparisons are made primarily within mouse and versus control mice of the same sex, appropriate conclusions can be drawn from the use of both male and female mice in this study. The C3H/HeJ mice were purchased from Jackson Laboratories; however, given that C57L/J mice are no longer available from Jackson Laboratories (nor from other suppliers), the C57L/J mice used in this study were taken from a small breeding colony our lab maintains. These mice were the first generation inbred offspring from C57L/J breeder pairs purchased from Jackson Laboratories in Spring 2008.
Running wheel data were collected from the mice beginning at 63 days (9 weeks) of age which corresponds to the most active period in the lifespan for mice (24). All mice were housed in the University Vivarium with 12-hour light/dark cycles (light 6am-6pm, dark 6pm-6am) and were provided with food (Harlan Teklad 8604 Rodent Diet, Madison, WI) and water ad libitum. All procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee. Additionally, all animals were weighed twice weekly.
Measurement of voluntary activity (wheel running)
Daily wheel running was measured using methods described previously (6, 8). Briefly, mice were housed individually in standard rat sized cages, each equipped with a solid surface running wheel (450 mm circumference; Ware Manufacturing, Phoenix, AZ, USA) mounted on the cage top. A magnet was mounted on the outside surface of each wheel and the cage top was equipped with a magnetic sensor (BC500; Sigma Sport, Olney, IL, USA). Each computer was calibrated with wheel dimensions to allow for accurate measurement of distance (km/day) and time (duration-mins/day) each mouse ran on the wheel. Speed of running (m/min) was then calculated from the distance and duration data. Mice were monitored and data was collected every 24 hours at approximately 9am during baseline and drug wash-out phases of the protocol. During drug treatments, data was collected immediately before drug treatment at 6pm (the beginning of the dark/active phase for mice), at 12am (6 h post-drug treatment), and again at 6am (12 h post drug treatment). Negligible running was recorded during the light cycle (6am-6pm) so all data reported is 24 hour data.
Drug treatment
Evidence from our lab (21) and others (12, 14) suggest physical activity in the form of wheel running in mice is at least partially regulated by the D1-like receptors, the dopamine transporter (DAT), as well as possibly the expression and/or function of the tyrosine hydroxylase enzyme. Tyrosine hydroxylase is the enzyme that converts L-dopa into dopamine and thus plays a role in dopamine production. Therefore, in this study, we designed 5 different treatments: SKF 81297 (D1-like agonist; Tocris Bioscience, Ellisville, MO), SCH 23390 (D1-like antagonist; Tocris Bioscience, Ellisville, MO), GBR 12783 (DAT inhibitor; Tocris Bioscience, Ellisville, MO), and DL-2-Methyl-3-(4-hydroxyphenyl) alanine (AMPT) (Tyrosine Hydroxylase inhibitor; Sigma Aldrich, St. Louis, MO), and placebo (saline injections only). All drugs have been shown to be centrally active after intraperitoneal (IP) injection and were administered IP in a volume of 0.3 mL per mouse. Dose responses were investigated using the following consecutive drug doses (mg/kg): SKF 81297 (0.5, 0.75, 1.0, 1.25), SCH 23390 (0.5, 0.75, 1.0, 1.25), GBR 12783 (15, 20, 25, 30), and AMPT (85,90,95, 100). All doses were based on previous literature investigating locomotion responses in mice to these particular drugs.
Treatment procedures
At nine weeks of age, mice were housed with a wheel, and baseline activity pattern was assessed for 21 consecutive days in all mice. Five mice from the C3H/HeJ strain and 4 mice (3 females, and 1 male) from the C57L/J strain were randomly chosen for the experimental drug treatment group, leaving three mice in each strain serving as controls. Control mice received saline injections only. The experimental animals received one injection (according to the dose schedule described above) at 6pm, at increasing doses for 4 consecutive days, followed by three full days of drug wash-out (i.e. no injections). Wheel running was monitored at 12am and 6am during drug treatment, and every 24 hours during drug wash-out. This pattern was repeated for all four drugs in succession.
Injection methods
Each drug injection solution was made fresh each day immediately prior to injections and all drugs were dissolved in 0.9% sterile saline. Once the appropriate dose was dissolved, the solution was placed in a sterile syringe and filtered through a 0.2 micron filter during injection. Because drugs were made fresh daily and were kept in sterile conditions, the C57L/J mice received the drugs in the following order: SKF 81297 (83-87 days old), SCH 23390 (90-94 days old), GBR 12783 (97-101 days old), and finally AMPT (103-106 days old). Due to age differences upon arrival and the need to keep drug injections sterile the C3H/HeJ mice received the drug treatments in the following order: GBR 12783 (83-87 days old), AMPT (90-94 days old), SKF 81297 (97-101 days old), and SCH 23390 (103-106 days old).
Statistics
Given the differential drug injections at differing ages, (e.g. the C57L/J mice received SKF 81297 at 83-87 days old, but the C3H/HeJ mice received this drug at 97-101 days old), each strain was analyzed in a separate ANOVA for the effects of the four drugs on wheel running indices. The alpha value was set a priori at 0.05. Within a strain, each drug was analyzed separately with a two-way ANOVA with group (control vs. experimental) and dose (repeated measure) as main effects. Three dependent variables were analyzed including distance (km/day), duration (mins/day), and speed (m/min). Tukey’s HSD post-hoc tests were used to evaluate main effects and group by dose interactions within the ANOVA model. There were no statistical differences between wheel running indices taken at 6 hours post-injection or 12 hours post-injection (data not shown) and thus, only wheel-running data from 12 hour post-injection will be presented. Differences in weight at baseline measurements between strains, as well as differences in weights between group within strains, were analyzed using independent t-tests, and correlation analysis was used to investigate relationships between weight and distance run. Although wheel running in mice has been shown to be highly repeatable (25), whole model post hoc power analysis revealed a value of .74.
RESULTS
Weights
Mice were weighed twice weekly during this study to encompass one weight measurement during each drug treatment, as well as one weight measurement during drug wash-out. C3H/HeJ (n=8 males) mice as a whole group were significantly heavier than C57L/J (n=6 females, n=l male) mice at baseline, and at all time points throughout the study (p<0.001). Weight of the control versus the experimental animals did not differ across the treatments (C3H/HeJ, p=0.20; C57L/J, p=0.66). As has been shown in previous studies (6, 8) during baseline activity measurements, weight was not correlated with distance run in either strain (C3H/HeJ: p=0.11, r2=0.43; C57L/J: p=0.12, r2=0.36). Speed was also not correlated with weight in either strain (C3H/HeJ: p=0.66, r2=0.03; C57L/J: p=0.93, r2=0.002). Duration was significantly correlated with weight in both strains (C3H/HeJ: p=0.04, r2=0.54; C57L/J: p=0.02, r2=0.69). Weight did not significantly increase over the course of the study in C3H/HeJ mice (p=0.69; beginning: 28.0±1.6g; end: 29.9±2.2g), while weight did significantly increase in C57L/J mice over the course of the study (p=0.02; beginning: 23.6±1.1; end: 25.1±1.0).
Baseline physical activity results
Baseline wheel running indices for both strains of mice are illustrated in Fig. 1. As was expected from previous literature, the C57L/J mice ran 191% farther, 177% longer, and 84% faster than C3H/HeJ mice (p<0.0001). There was no difference between control and experimental mice at baseline in distance (p=0.52), duration (p=0.52), or speed (p=0.74) in the C57L/J mice. Likewise, there was no difference between groups of C3H/HeJ mice at baseline in distance (p=0.22), duration (p=0.33), or speed (p=0. 16).
Fig. 1.
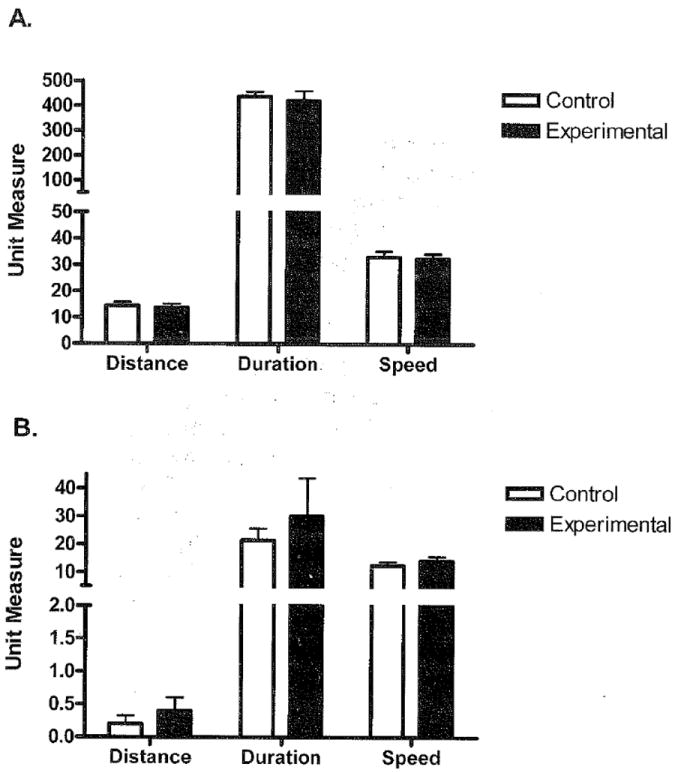
Baseline values of distance, duration, and speed in control and experimental mice. A) Running wheel data at baseline for C57L/J mice (n=7) is shown. No differences in distance (km) (p=0.52), duration (mins) (p=0.52), or speed (m/min) (p=0.74) were found between control and experimental groups; however, C57L/J mice ran significantly farther, longer, and faster than C3H/HeJ mice at baseline (p<0.0001). B) Running wheel data at baseline for C3H/HeJ mice (n=8). No differences in distance (p=0.22), duration (p=0.23), or speed (p=0.44) were found between control and experimental groups.
Drug effects on WR in C57L/J mice
Wheel-running distance, the product of duration of activity and speed of activity, responses in C57L/J mice to all four drugs are shown in Fig. 2. No significant dose response was seen in distance run after treatment with the D1 agonist SKF 81297 (p=0.72); however, SKF 81297 significantly reduced wheel running distance regardless of dose (Fig. 2; p=0.0004). No significant differences in distance were observed between group or by dose for the D1-antagonist SCH 23390 (p=0.12), the DAT inhibitor GBR 12783 (p=0.89), or the TH inhibitor AMPT (p=0.37). Similar responses for duration and speed for all four drugs were observed and are reported in Table I.
Fig. 2.
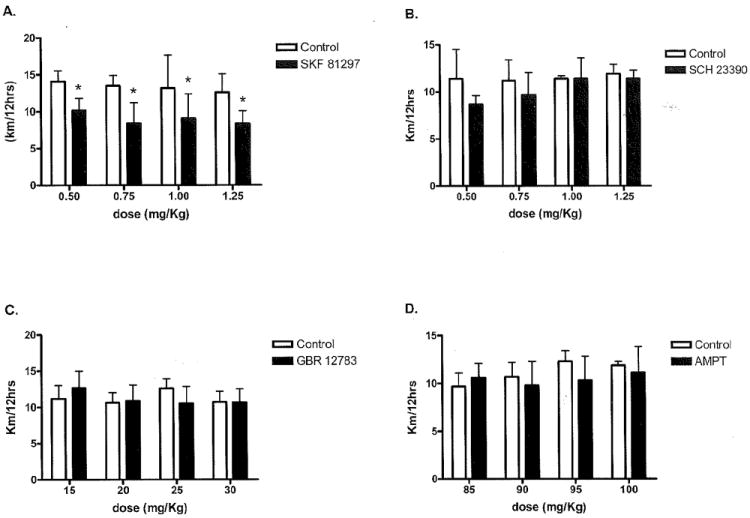
Distance responses to all four dopaminergic drugs in C57L/J mice. Distance responses to all four drugs in the C57L/J mice. A) Dose response after administration of SKF 81297 is shown. No significant dose response was seen; however, all four doses significantly reduced wheel running distance in experimental mice compared to controls (p=0.0004). B) Dose response to SCH 23390 is shown. No significant changes in distance run between groups were seen for any dose (p=0.12). C) Dose response to GBR 12783 is shown. No significant differences in distance run were seen between groups for any dose (p=0.89). D) Dose response to AMPT is shown. No significant differences in distance run between grousp were seen for any of the doses (p=0.37).
Table I.
Duration and speed responses to dop
| Drug | Dose (mg/Kg) | Duration (mins/12hrs) | Speed (m/min) | ||
|---|---|---|---|---|---|
|
| |||||
| Control | Experimental | Control | Experimental | ||
| SKF 81297 | 0.5 | 425±13 | 358±82 | 33.3±4.4 | 29.1±5.7 |
| 0.75 | 416±17 | 327±65 | 32.5±4.5 | 25.3±4.6 | |
| 1 | 389±50 | 346±69 | 33.3±7.7 | 25.7±5.8 | |
| 1.25 | 401±13 | 294±45 | 31.4±5.4 | 28.3±3.6 | |
| p=0.002* | p=0.015* | ||||
| SCH 23390 | 0.5 | 396±63 | 312±47 | 28.5±4.1 | 28.2±5.4 |
| 0.75 | 389±45 | 324±42 | 28.7±3.7 | 30.0±5.7 | |
| 1 | 395±29 | 364±15 | 29.0±2.8 | 31.1±5.3 | |
| 1.25 | 402±41 | 371±24 | 29.7±3.0 | 30.7±3.1 | |
| p=0.003* | p=0.54 | ||||
| GBR 12783 | 15 | 392±12 | 382±35 | 28.4±4.0 | 33.1±3.5 |
| 20 | 375±27 | 339±39 | 28.4±4.5 | 31.9±4.2 | |
| 25 | 431±24 | 369±70 | 29.3±2.4 | 28.4±1.3 | |
| 30 | 378±10 | 373±60 | 28.5±3.4 | 28.5±2.0 | |
| p=0.091 | p=0.18 | ||||
| AMPT | 85 | 341±28 | 343±36 | 28.6±4.6 | 30.9±4.0 |
| 90 | 362±27 | 320±82 | 29.6±3.4 | 30.7±2.8 | |
| 95 | 396±25 | 323±67 | 31.0±2.4 | 32.0±3.1 | |
| 100 | 393±37 | 342±40 | 30.6±2.5 | 32.3±4.2 | |
| p=0.043* | p=0.29 | ||||
Duration and speed data for C57L/J mice. p values reported indicate significant differences between groups within strain.
Drug effects on WR in C3H/HeJ mice
Wheel-running distance responses in C3H/HeJ mice (low active) to all four drugs are shown in Fig. 3. No significant dose response was seen in distance run after treatment with the DAT inhibitor GBR 12783 (p=0.73); however, injection of GBR 12783 did significantly increase wheel running independent of dose (Fig. 3; p=0.0005). No other drugs used in this study significantly affected wheel running the C3H/HeJ mice: the D1-agonist SKF 81297 (p=0.91), the D1-antagonist SCH 23390 (p=0.44), and the TH-inhibitor AMPT (p=0.98). Data for duration and speed for all four drugs for C3H/HeJ mice showed similar responses as distance and are reported in Table II.
Fig. 3.
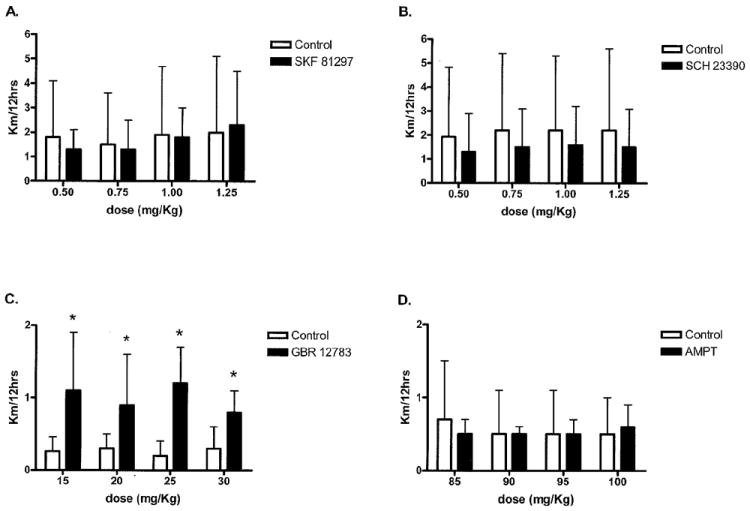
Distance responses to all four dopaminergic drugs in C3H/HeJ mice. Distance responses to all four drugs in the C3H/HeJ mice. A) Dose response after administration of SKF 81297 is shown. No significant differences in distance between groups were seen for any dose (p=0.91). B) Dose response to SCH 23390 is shown. No significant changes in distance run between groups were seen for any dose (p=0.44). C) Dose response to GBR 12783 is shown. No significant dose response was observed, however, distance was significantly increased in the experimental group compared to control following treatment with all four doses (p=0.0005). D) Dose response to AMPT is shown. No significant differences in distance run between group were seen for any of the doses (p=0.98).
Table II.
Duration and speed responses to dopaminergic drugs in C3H/HeJ mice.
| Drug | Dose (mg/Kg) | Duration (mins/12hrs) | Speed (m/min) | ||
|---|---|---|---|---|---|
|
| |||||
| Control | Experimental | Control | Experimental | ||
| SKF 81297 | 0.5 | 97±106 | 73±33 | 15.2±4.8 | 17.2±2.6 |
| 0.75 | 83±111 | 75±56 | 14.4±3.6 | 16.4±2.6 | |
| 1 | 101±145 | 103±56 | 14.5±4.2 | 16.5±2.8 | |
| 1.25 | 104±150 | 124±99 | 15.0±4.4 | 17.1±2.9 | |
| p=0.95 | p=0.11 | ||||
| SCH 23390 | 0.5 | 95±133 | 74±79 | 15.7±5.0 | 16.0±2.8 |
| 0.75 | 110±141 | 84±78 | 15.3±5.5 | 16.3±2.5 | |
| 1 | 111±142 | 91±80 | 15.6±4.6 | 16.3±2.9 | |
| 1.25 | 110±147 | 89±80 | 15.5±5.6 | 16.1±2.6 | |
| p=0.56 | p=0.63 | ||||
| GBR 12783 | 15 | 20±16 | 66±41 | 12.5±1.4 | 16.3±1.7 |
| 20 | 21±14 | 53±36 | 12.5±1.0 | 15.4±2.0 | |
| 25 | 19±12 | 69±25 | 12.1±1.2 | 16.4±1.8 | |
| 30 | 23±19 | 53±15 | 13.1±1.0 | 15.7±1.1 | |
| p=0.0005* | p<0.0001* | ||||
| AMPT | 85 | 39±43 | 35±12 | 14.3±3.4 | 15.3±1.6 |
| 90 | 34±32 | 36±6 | 14.4±2.9 | 15.0±1.3 | |
| 95 | 33±30 | 35±10 | 14.2±2.7 | 15.3±1.4 | |
| 100 | 29±26 | 37±15 | 15.3±3.0 | 15.5±1.6 | |
| p=0.82 | p=0.31 | ||||
Duration and speed data for C3H/HeJ mice. p values reported indicate significant differences between groups within strain.
DISCUSSION
This study investigated four different dopaminergic acting drugs on a high active strain of mice and a low active strain of mice to determine the role of D1-like receptors, DAT, and tyrosine hydroxylase in regulating physical activity level. We observed strain dependent effects of the D1-like receptor agonist (SKF 81297) and the DAT inhibitor (GBR 12783) (Figs. 1 and 2). The D1-like agonist significantly reduced overall distance, duration, and speed in C57L/J mice (high active), while the DAT inhibitor significantly increased overall distance, duration, and speed in the C3H/HeJ (low active) mice.
It is becoming well accepted that a significant genetic component exists in the regulation of physical activity in both rodents (3-7,26-28) and humans (29, 30). Using wheel-running as a model of physical activity in mice, both single-gene and epistatic QTL associated with physical activity have been found (8, 9). However, the genes and gene interactions that regulate physical activity behavior are still unclear. Interestingly, haplotype analysis conducted in the study by Lightfoot and colleagues identified a suggestive QTL on chromosome 13 that contains the Drd1 gene which codes for the D1 receptor (8), and research conducted in our lab (21) indicate C57L/J inbred mice (high active) have significantly lower expression of Drd1 mRNA compared to low active C3H/HeJ inbred mice. The current study was designed to investigate several aspects of dopamine signaling in relation to physical activity in mice.
Wheel running in response to DAT and tyrosine hydroxylase inhibitors
GBR 12783 and AMPT were used in this study to investigate wheel running responses to drugs affecting either dopamine re-uptake or dopamine production, respectively. Treatment of low active C3H/HeJ mice with the DAT inhibitor (dopamine re-uptake inhibitor) significantly increased wheel running distance, duration, and speed independent of dose compared to control mice (Fig. 3, Table II). The DAT inhibitor did not affect wheel running in the C57L/J strain. This finding corresponds to previous research with animal models of ADHD and treatment with Ritalin [also a dopamine re-uptake inhibitor] (14). Responses to Ritalin in humans can vary depending on the status of the neurotransmitter systems (31-33). Specifically, it has been proposed that the response to drugs such as Ritalin depends largely on baseline values of the response in question (33).
The only effect of the TH inhibitor was a slight, but significant decrease in duration in the high active C57L/J mice (Table I). We observed no significant group by dose interactions for this drug in C57L/J mice, with no difference reflected in distance or speed (Fig. 2, Table I). In our previous study, we observed decreased expression of tyrosine hydroxylase mRNA in the mid-brain of C57L/J mice compared to C3H/HeJ mice (21). If decreased expression of tyrosine hydroxylase, and subsequent decreased dopamine production and downstream dopamine signaling mediate the high active phenotype, inhibiting this enzyme further would theoretically lead to further increased physical activity; however, this high active strain may have already been running at a “physiological maximum”. Rhodes and Garland (12) have suggested a possible “ceiling effect” in response to high doses of apomorphine in mice selectively bred for high wheel running.
Wheel-running in response to Dl-like agonist and antagonist
In contrast to determining the response to generalized alteration in dopamine levels through the use of reuptake inhibitors or dopamine synthesis inhibitors, we used SKF 81297 (D1-like agonist) and SCH 23390 (D1-like antagonist) to investigate the effects of manipulation of dopamine signaling specifically through the D1-like receptors. The D1 agonist caused significant reduction in distance, duration, and speed in the high active C57L/J mice (Fig. 2 and Table I). Observation that high active C57L/J mice in the current study reduced wheel sunning in response to a D1 agonist supports the hypothesis that decreased function and/or expression of D1-like receptors may mediate running wheel activity in high active inbred strains (21). In contrast, the low active C3H/HeJ mice did not decrease wheel running in response to the D1 agonist used in this study. The largely lack of response to SCH 23390 (a selective D1-like antagonist) would again suggest a possible floor or ceiling effect with these two unique strains of mice (Figs. 2 and 3, Tables I and II).
In summary, strain differences in the response to a D1 receptor agonist demonstrate that D1-like receptors may play a role in mediating the high active phenotype in C57L/J mice. Likewise, differential strain responses to a dopamine re-uptake inhibitor suggest that the amount of dopamine present in the synapse may be important in mediating the low active phenotype in C3H/HeJ mice. However, full elucidation of the role of dopaminergic functioning in these strains purposely selected for their divergent activity responses is difficult because of the possibility of physiological ceiling and floor effects in physical activity levels. Similarly, baseline genetic differences in dopamine signaling between inbred strains are a potential explanation for the differences in wheel sunning responses to dopaminergic drugs. It is also possible that low activity may be a different phenotype than high activity and regulated by slightly different pathways (JT Lightfoot, personal correspondence). Further investigations should use strains of mice that fall in the middle of the spectrum of voluntary physical activity.
Acknowledgments
The authors would like to thank Dr. Trudy Moore-Harrison and David Ferguson for assistance in wheel-running data collection. We also thank the UNC Charlotte Vivarium staff and Dr. Yvette Huet for assistance in preparation of sterile injectables. This work was funded by NIH NIAMS AR050085.
Footnotes
DISCLOSURE: ALL AUTHORS REPORT NO CONFLICTS OF INTEREST RELEVANT TO THIS ARTICLE.
References
- 1.Heitmann BL, Hills AP, Frederiksen P, Ward LC. Obesity, leanness, and mortality: effect modification by physical activity in men and women. Obesity (Silver Spring) 2009;17(1):136–42. doi: 10.1038/oby.2008.479. [DOI] [PubMed] [Google Scholar]
- 2.Oldridge NB. Economic burden of physical inactivity: healthcare costs associated with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2008;15(2):130–9. doi: 10.1097/HJR.0b013e3282f19d42. [DOI] [PubMed] [Google Scholar]
- 3.Festing MF. Wheel activity in 26 strains of mouse. Lab Anim. 1977;11(4):257–8. doi: 10.1258/002367777780936530. [DOI] [PubMed] [Google Scholar]
- 4.Lauderdale DS, Fabsitz R, Meyer JM, Sholinsky P, Ramakrishnan V, Goldberg J. Familial determinants of moderate and intense physical activity: a twin study. Med Sci Sports Exerc. 1997;29(8):1062–8. doi: 10.1097/00005768-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Lerman I, Harrison BC, Freeman K, et al. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Applied Physiol. 2002;92(6):2245–55. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 6.Lightfoot ST, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19(3):270–6. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- 7.Stubbe JH, Boomsma DI, De Geus EJ. Sports participation during adolescence: a shift from environmental to genetic factors. Med Sci Sports Exerc. 2005;37(4):563–70. doi: 10.1249/01.mss.0000158181.75442.8b. [DOI] [PubMed] [Google Scholar]
- 8.Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR, Leamy LJ. Quantitative trait loci (QTL) for physical activity traits in mice. Physiol Genomics. 2008;32(3):401–8. doi: 10.1152/physiolgenomics.00241.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leamy LJ, Pomp D, Lightfoot JT. An epistatic genetic basis for physical activity traits in mice. J Hered. 2008;99(6):639–46. doi: 10.1093/jhered/esn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zoo1. 2006;79(1):83–99. doi: 10.1086/498187. [DOI] [PubMed] [Google Scholar]
- 11.Bronikowski AM, Rhodes JS, Garland T, Jr, Prolla TA, Awad TA, Gammie SC. The evolution of gene expression in mouse hippocampus in response to selective breeding for increased locomotor activity. Evolution Int J Org Evolution. 2004;58(9):2079–86. doi: 10.1111/j.0014-3820.2004.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2003;167(3):242–50. doi: 10.1007/s00213-003-1399-9. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117(6):1243–56. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- 14.Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T., Jr Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2001;158(2):120–31. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- 15.Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology (Berl) 1992;107(2-3):160–74. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- 16.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137(1-2):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Goodlett DR. Proteomic approach to studying Parkinson’s disease. Mol Neurobiol. 2004;29(3):271–88. doi: 10.1385/MN:29:3:271. [DOI] [PubMed] [Google Scholar]
- 18.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(S):7–12. [PubMed] [Google Scholar]
- 19.Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102(4):544–52. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- 20.Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knab AM, Bowen RS, Hamilton AT, Gulledge AA, Lightfoot JT. Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res. 2009;204(1):147–52. doi: 10.1016/j.bbr.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eikelboom R. Human parallel to voluntary wheel running: exercise. Anim Behav. 1999;57(3):F11–F12. doi: 10.1006/anbe.1998.1045. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56(1):11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 24.Swallow JG, Garland T, Jr, Carter PA, Zhan WZ, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus) J Appl Physiol. 1998;84(1):69–76. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Knab AM, Bowen RS, Moore-Harrison T, Hamilton AT, Turner MJ, Lightfoot JT. Repeatability of exercise behaviors in mice. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leamy LJ, Pomp D, Lightfoot JT. A search for quantitative trait loci controlling within-individual variation of physical activity traits in mice. BMC Genet. 2010;11:83. doi: 10.1186/1471-2156-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lightfoot JT. Current understanding of the genetic basis for physical activity. J Nutr. 2011;141(3):526–30. doi: 10.3945/jn.110.127290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lightfoot JT, Leamy L, Pomp D, et al. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. J Appl Physiol. 2010;109(3):623–34. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rankinen T, Bray MS, Hagberg JM, et al. The human gene map for performance and health-related fitness phenotypes: the 2005 update. Med Sci Sports Exerc. 2006;38(11):1863–88. doi: 10.1249/01.mss.0000233789.01164.4f. [DOI] [PubMed] [Google Scholar]
- 30.Garland T, Jr, Schutz H, Chappell MA, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214(Pt 2):206–29. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwald MK, Schuster CR, Johanson CE, Jewel1 J. Automated measurement of motor activity in human subjects: effects of repeated testing and d-amphetamine. Pharmacol Biochem Behav. 1998;59(1):59–65. doi: 10.1016/s0091-3057(97)00387-0. [DOI] [PubMed] [Google Scholar]
- 32.Robbins TW, Sahakian BJ. “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacology. 1979;18(12):931–50. doi: 10.1016/0028-3908(79)90157-6. [DOI] [PubMed] [Google Scholar]
- 33.Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1976;4(1):73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]


