Abstract
Most peptides are generally insufficiently permeable to be used as oral drugs. Designing peptides with improved permeability without reliable permeability monitoring is a challenge. We have developed a supercritical fluid chromatography technique for peptides, termed EPSA, which is shown here to enable improved permeability design. Through assessing the exposed polarity of a peptide, this technique can be used as a permeability surrogate.
Keywords: EPSA, cyclic peptides, permeability, polarity, intramolecular hydrogen bonding
Most peptide drugs are delivered by injection (i.e bortezomib, leuprolerin, octreotide, goserelin, ...).1 Only a handful of peptides are orally bioavailable, cyclosporine 1 being one example, with reported bioavailability values of about 30%. Poor bioavailability is attributed to a conjunction of low systemic stability (proteolytic degradation),2 high clearance, and low intestinal permeability,3 in part due to the peptides’ high polarity, to which the numerous polar side chains and backbone hydrogen bond donors contribute.
Current scientific efforts to affect oral peptide chemical space involve improving peptide permeability and oral bioavailability. Two recent reviews by Craik et al.1 and Bock et al.4 summarize the current state of the art in terms of conformational analyses, intramolecular hydrogen bonding (IMHB), and N-methylation strategies. The consensus learning is that a cyclic backbone, N-methylations, and conformations masking H bond donors are key for membrane permeability.5−10 For instance, N-methylation of key amides of a polyalanine cyclic hexapeptide11,12 or of the somatostatin analogs related to the Veber–Hirschmann peptide 2 improved membrane permeability in a human epithelial colorectal adenocarcinoma cell monolayer (Caco-2) model, along with oral bioavailability in rat, while N-methylation in other positions had no effect.13 Similar behavior was observed for a cyclic hexapeptide 5 (cyclo[Leu-NMe-d-Leu-NMe-Leu-Leu-d-Pro-NMe-Tyr]) containing two d amino acid induced β turns, N-methylated three times, reaching an oral bioavailability in rat of 28% .14
One of the issues in peptide design involves monitoring permeability. In the small molecule Ro5 paradigm, monitoring permeability progress is performed through cell monolayer based screening (such as Caco-215,16 or low efflux Madin Darby canine kidney cells; MDCK-LE; a.k.a. Ralph Russ canine kidney cells; RRCK17) or artificial constructs of lipid bilayer assays (parallel artificial membrane permeation assay; PAMPA).18,19 While reasonable for small molecules, these assays fall short for compounds in beyond Lipinski’s Rule of Five (Ro5) space, such as peptides (presence of peptidases, nonspecific binding, ...). Therefore, it would be valuable to have a reasonably high throughput method that can be reliably used to produce data relating to the permeability of peptides.
Guimarães et al.20 showed passive permeability to be essentially driven by polarity, size, and lipophilicity; for peptides, it is thought that the main obstacle for permeability is polarity. We recently described an experimental tool that is able to highlight IMHB through a matched pair analysis of their relative polarity profiles derived by supercritical fluid chromatography (SFC) retention termed EPSA21 in analogy to the ELogD/ELogP terminology introduced by Lombardo et al.22,23 This method provides, under controlled SFC conditions, a polarity readout that is derived from the retention time of a compound on a specific column, Chirex 3014. This stationary phase was selected for its balance of lipophilic and polar attributes and its capacity to separate compounds with wide polarity differences. SFC, which uses inherently normal phase conditions, provides a low dielectric constant environment conducive to IMHB formation. Polar compounds are retained under those conditions, and a low-slope gradient of methanol achieves adequate separation based on polarity. The presence of an IMHB can therefore be identified, as it can result in a significant reduction in polarity. Results are normalized across runs by the use of calibration standards generating a daily linear relationship between retention time and EPSA values. This method has been successfully utilized for small molecule medicinal chemistry projects to assess exposed polarities of lead compounds.24,25
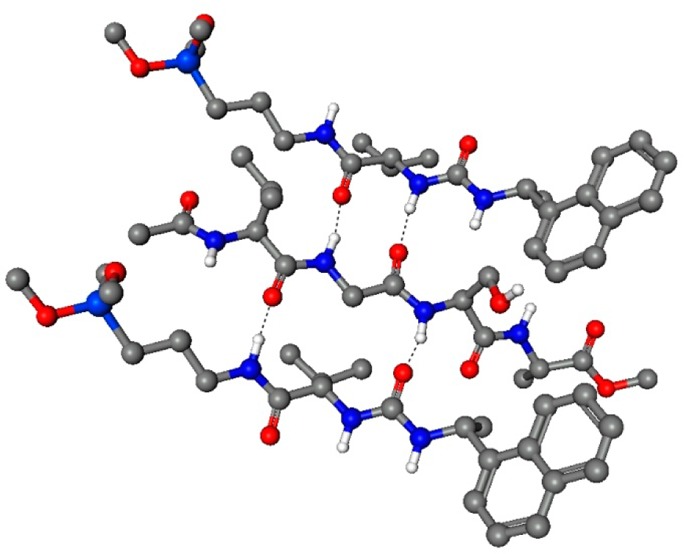
It was envisioned that this unique methodology could be extended to peptides, as illustrated by the tetrapeptide NAc-Val-Gly-Ser-Ala-OMe, in Figure 1. Exemplified in the figure, multiple polar interactions (hydrogen bonds) can in theory occur between the peptide backbone polar groups and the EPSA stationary phase, contributing to retention. Modification of that backbone (i.e., by methylation of amide groups) should therefore impact the retention. In principle, polar side chains are able to interact in a similar fashion with the stationary phase as well. The more exposed the polar groups are, the more interactions are possible and the longer the observed retention.
Figure 1.
Interaction model of an example tetrapeptide NAc-Val-Gly-Ser-Ala-OMe with two EPSA stationary phase molecules (Phenomenex Chirex 3014).
In order to apply this method to peptides, which typically display greater polarity than small molecules, a modification of the reported technique needed to be established. In order to enable the elution of the polar entities within a reasonable time, the existing gradient of MeOH in close to supercritical CO2, ramping from 5% to 50% at 5% MeOH per minute, was extended to reach 60% in 11 min and a significant hold period was added as shown in Figure 2. Additionally, to ensure that EPSA values are consistent across platforms and columns, two highly retained calibration standards were added to the list of standards used for Ro5 compounds, allowing for linearity between retention times and EPSA values across the whole analysis window (Table 1).
Figure 2.
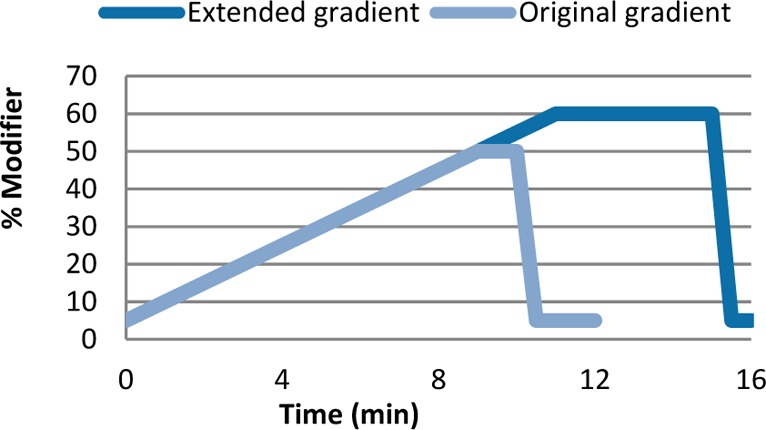
Original EPSA (light blue) and extended EPSA (dark blue) gradients: (Modifier: MeOH with 20 mM ammonium formate) in CO2.
Table 1. Calibration Standards, Retention Times (tR), and Assigned EPSA Values.
| standard name | approx tR (min) | assigned EPSA value |
|---|---|---|
| Antipyrine | 2.2 | 61 |
| Chlorpromazine | 2.6 | 68 |
| Desipramine | 4.0 | 87 |
| Pindolol | 5.1 | 103 |
| Diclofenac | 7.4 | 135 |
| m-Nitrobenzoic acid | 9.0 | 157 |
| Bumetanide | 11.0 | 185 |
| Furosemide | 14.3 | 230 |
We have now investigated over 1500 compounds, and the data is exemplified by the selection shown below. While collecting data for compounds known to be permeable (cyclic peptides developed by Lokey et al.14,26), it appeared that all but one permeable peptide had an EPSA value of 80 or less. Compounds exhibiting additional polarity by EPSA turned out to be nonpermeable by RRCK assessment as well (Figure 3).
Figure 3.
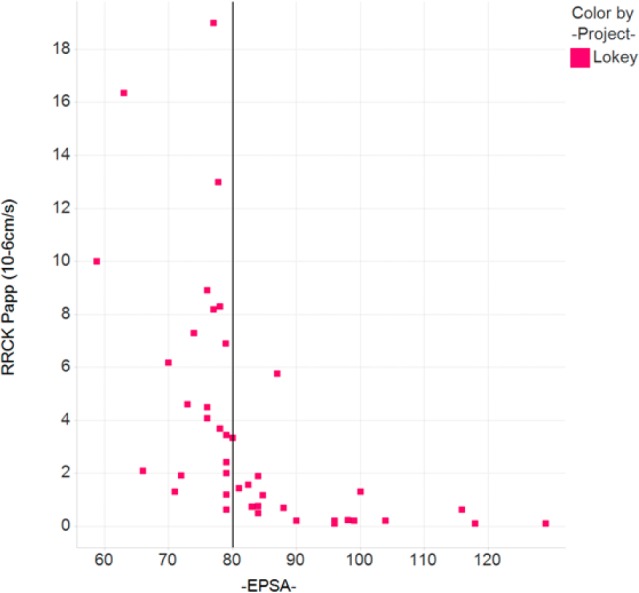
Correlation between RRCK apparent permeability (10–6 cm/s) and EPSA values of cyclic peptides described by Lokey et al.14,26
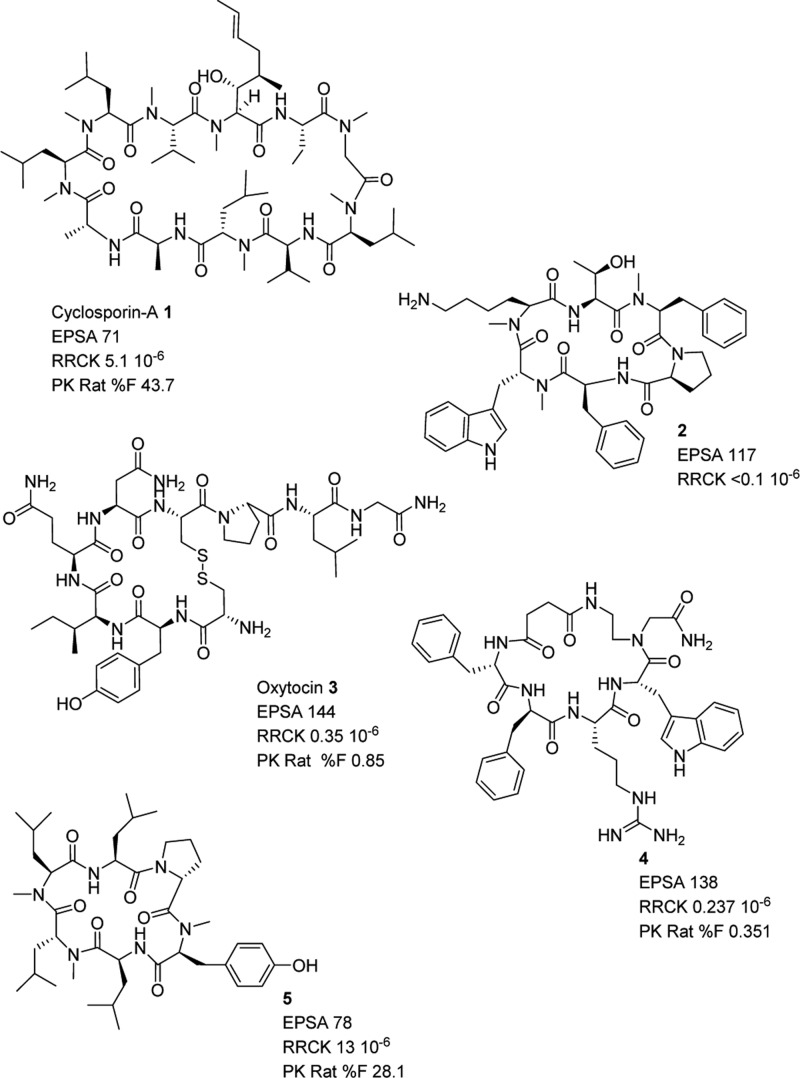
In addition, we looked at other known cyclic peptides (Figure 4), including Cyclosporine A (1), the somatostatin analog related to the Veber–Hirschmann peptide 2,13 Oxytocin (3), the cyclic peptidomimetic melanocortin-4 receptor agonist 4,7 and the Lokey hexapeptide 5,14 and we were able to solidify our hypothesis correlating low EPSA values with permeability. Indeed, 1 and 5 had EPSA values of 71 and 78, respectively, and RRCK Papp values of 5.1 × 10–6 cm/s and 13 × 10–6 cm/s along with bioavailability values of 43.7% and 28%. Compounds 2, 3, and 4 had EPSA values of 117, 144, and 138, correlating with RRCK Papp values of <0.1 × 10–6 cm/s, 0.35 × 10–6 cm/s, and 0.24 × 10–6 cm/s, respectively. Additionally, bioavailability values of 3 and 4 were measured at 0.85% and 0.35%. The poor bioavailability value of 4 (measured in-house) is consistent with low passive permeability and high EPSA measurements, at odds with the originally reported bioavailability value of 8.5%.7
Figure 4.
Selection of representative cyclic peptides.
The EPSA analysis has been extended to all cyclic peptides assessed for permeability by RRCK (814 compounds). Figure 5 illustrates the discriminating power of EPSA in terms of predicting permeability. As seen in the pie charts, a cyclic peptide with an EPSA value below 80 has a much greater chance of being moderately permeable (34%) or permeable (38%) than a compound with a higher EPSA. The box-and-whisker plot shows the median RRCK Papp value in the EPSA < 80 bin to be 3.4 × 10–6 cm/s while it is well below 1 × 10–6 cm/s in all bins where EPSA >80. Furthermore, based on these data, it appears to be clear that a peptide with a measured EPSA above 100 generally will not have significant passive permeability. Therefore, in order to not miss potentially permeable peptides, it is suggested that EPSA could be used as a filter, with a cutoff value of 100.
Figure 5.
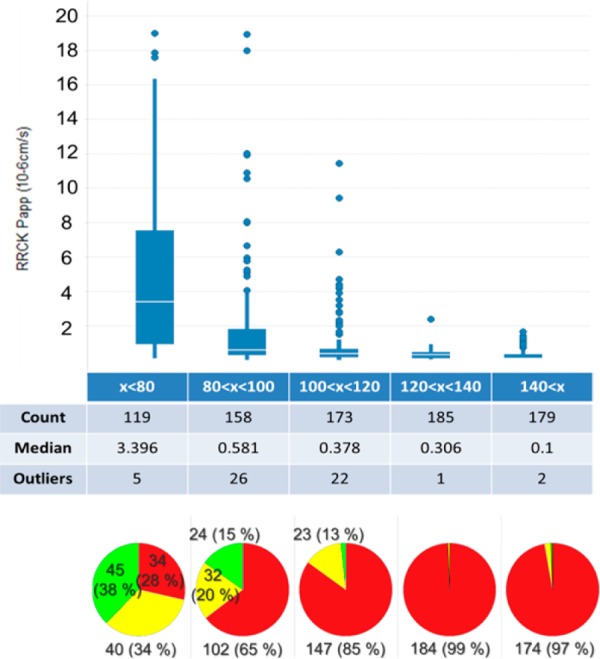
Statistical box-and-whisker plot analysis of RRCK permeability vs binned EPSA, and pie charts of the same data set. (Color coding by permeability assessed by RRCK; cutoffs: Papp < 1 × 10–6 cm/s, not permeable (red); 1 × 10–6 cm/s < Papp < 5 × 10–6 cm/s, moderately permeable (yellow); Papp < 5 × 10–6 cm/s, permeable (green).
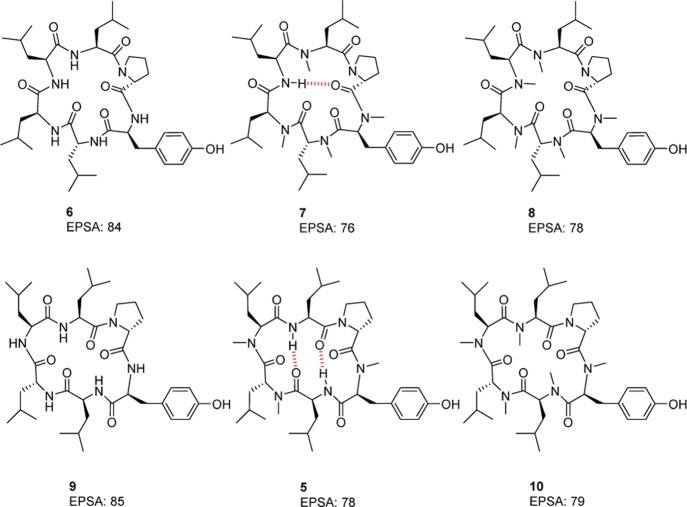
While permeability assessments appear, in our opinion, unnecessary in the high polarity space because of undiscriminating responses of the assay, EPSA on the other hand responds to discrete modifications, such as single amino acid sequence change, amino acid inversion, modification, or N-methylation. As seen in Table 2, showing a few examples described by Lokey et al.,14,26 modification of a single amino acid in cyclic peptides 11 to 17, analogs to 5, impacts the polarity measured by EPSA, which in turn predicts permeability. Similarly, methylations of compounds 6, 7, and 8 impact permeability as predicted by EPSA. Additionally, amino acid inversions (6 and 9) do not impact EPSA and only moderately improve permeability. Finally, methylation of the terminal NH2 of the Lys in 16 does not improve the permeability of 18 as predicted by EPSA.
Table 2. Examples of Discrete Amino Acid Modifications and Impact on EPSA and Permeability.
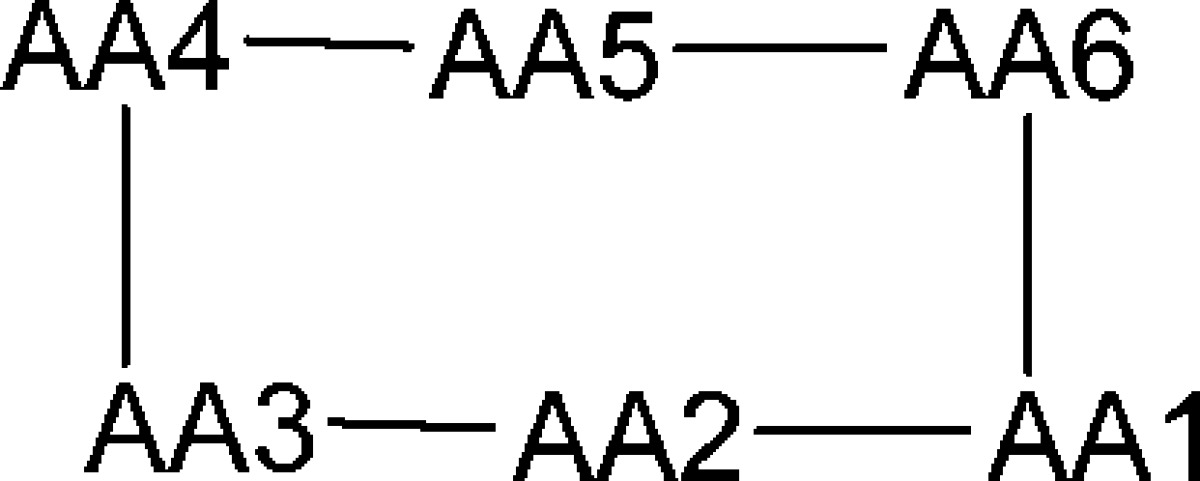
| ID | EPSA | RRCK Papp (10–6 cm/s) | AA1 | AA2 | AA3 | AA4 | AA5 | AA6 |
|---|---|---|---|---|---|---|---|---|
| 11 | 75 | 8.9 | Leu | N-Me-d-Leu | N-Me-Ile | Leu | d-Pro | N-Me-Tyr |
| 12 | 76 | 5.5 | Leu | N-Me-d-Leu | N-Me-Ala | Leu | d-Pro | N-Me-Tyr |
| 5 | 78 | 13.0 | Leu | N-Me-d-Leu | N-Me-Leu | Leu | d-Pro | N-Me-Tyr |
| 13 | 83 | 1.6 | Leu | N-Me-d-Leu | N-Me-Ser | Leu | d-Pro | N-Me-Tyr |
| 14 | 83 | 4.7 | Leu | N-Me-d-Leu | N-Me-Thr | Leu | d-Pro | N-Me-Tyr |
| 15 | 100 | 1.3 | Leu | N-Me-d-Leu | N-Me-Tyr | Leu | d-Pro | N-Me-Tyr |
| 16 | 103 | 0.3 | Leu | N-Me-d-Leu | N-Me-Lys | Leu | d-Pro | N-Me-Tyr |
| 17 | 125 | 0.6 | Leu | N-Me-d-Leu | N-Me-Asp | Leu | d-Pro | N-Me-Tyr |
| 6 | 84 | 0.5 | d-Leu | Leu | Leu | Leu | d-Pro | Tyr |
| 7 | 76 | 4.1 | N-Me-d-Leu | N-Me-Leu | Leu | N-Me-Leu | d-Pro | N-Me-Tyr |
| 8 | 79 | 6.1 | N-Me-d-Leu | N-Me-Leu | N-Me-Leu | N-Me-Leu | d-Pro | N-Me-Tyr |
| 6 | 84 | 0.5 | d-Leu | Leu | Leu | Leu | d-Pro | Tyr |
| 9 | 85 | 1.8 | Leu | d-Leu | Leu | Leu | d-Pro | Tyr |
| 16 | 103 | 0.3 | Leu | N-Me-d-Leu | N-Me-Lys | Leu | d-Pro | N-Me-Tyr |
| 18 | 98 | 0.2 | Leu | N-Me-d-Leu | N-Me-Lys(N-Me) | Leu | d-Pro | N-Me-Tyr |
In a typical design loop (idea–synthesis–potency/permeability assessment–feedback–next idea) the early dual goal is to improve potency and permeability. Permeability is sought after through the strategy of reducing polarity. When starting from a polar lead compound, improving EPSA values will be an indicator of the design advancing in the right direction, even if the permeability readouts have not shown improvement. Project teams internally have successfully applied the strategy of utilizing EPSA as a surrogate for permeability (Figure 6).
Figure 6.
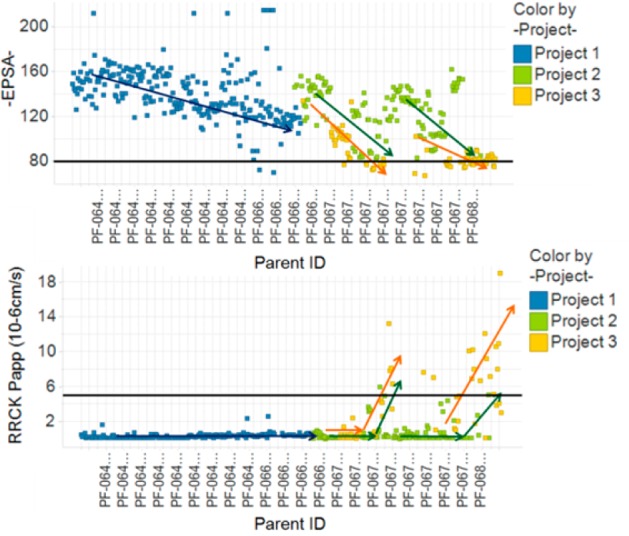
Comparison of EPSA and RRCK values as a function of time for three specific cyclic peptides medicinal chemistry projects (time is measured through sequentially increasing compound ID numbers).
In Figure 6 compounds colored in blue illustrate the design strategy of project team 1 working toward reducing polarity of their lead compounds, whose EPSA value was in the 160s. With each design cycle it was observed that a gradual decrease in polarity (lower EPSA values; purple arrow) was obtained but without any significant improvement in permeability as measured by RRCK. A few compounds were specifically designed for low polarity, and two of them achieved EPSA < 80 (below black threshold line). Those are the two compounds seen emerging from the rest in the RRCK side of the plot. Project team 2 (green) started in a similar polarity space with their first peptide series. Although a gradual decrease in polarity is observed with each design cycle, permeability does not improve, until a threshold in EPSA is reached upon which permeability becomes measurable and increases (green arrows). A backup/second series of peptides followed the same pattern, wherein EPSA readout drove each new design. Plans that increased EPSA were abandoned in favor of those that decreased EPSA. Project team 3 (yellow) started in a slightly less polar space (EPSA value in the 130s), and applying learning from previous projects, their first and second design cycles improved EPSA immediately, first reaching 100 and then 80, which resulted in good permeability (Papp > 5 × 10–6 cm/s; above the black threshold line) by their second cycle. Similar trends in EPSA and improved permeability were observed for their second series as well.
In addition to facilitating polarity reduction in design and improving permeability prediction, EPSA has another major advantage, directly linked to its chromatographic basis; it enables the inferring of IMHB patterns. In the case of the cyclic hexapeptides designed by Lokey et al., the IMHB patterns have been well established.14 Comparing compounds 6 and 7, we observed that methylation of four N–H groups resulted in a significant reduction in EPSA from 84 to 76 (Figure 7). Furthermore, methylation of the only N–H group of compounds 7 (shown by NMR and D2O exchange to be involved in an IMHB)14 to afford compound 8 did not significantly impact the retention and EPSA values (from 76 to 78). Similarly, comparing compounds 9 and 5, we observed that three N–H methylations reduced EPSA from 85 to 78, while additional methylations of 5, which features two distinct IMHBs, did not impact the resulting exposed polarity of 10 (EPSA values 78 vs 79). As can be seen in those examples, EPSA retention is based on polar interactions, independent of molecular weight and lipophilicity.
Figure 7.
Effects of N-methylation on EPSA values of cyclic peptides described by Lokey et al.14
Applying this reasoning, when examining cyclic peptides whose IMHB patterns are unknown, it appears to be possible through targeted systematic N-methylation of each amino acid (methyl walk) and EPSA analysis to assess the relative exposed polarity. A comparative analysis of each methylated analog with the unmethylated peptide could therefore provide an indication of the IMHBs present in the native cyclic peptide. A reduction of EPSA resulting from methylation would indicate an exposed N–H (e.g., 6 → 7 and 9 → 5). Methylations resulting in unchanged EPSAs would indicate a hidden N–H group, possibly due to the presence of an IMHB (e.g., 7 → 8 and 5 → 10). If methylation results in an increased EPSA, it is likely that the conformation has been significantly modified, resulting in a number of previously satisfied hydrogen bonds now exposing polar functionality.
In conclusion we have successfully established EPSA, a relatively high throughput chromatographic method utilizing SFC technology, as a viable polarity monitoring tool for peptide medicinal chemistry programs. These data can now be used to inform peptide design as a permeability predictor and indicator of IMHB patterns in cyclic peptides when combined with synthetic methylation techniques.
Acknowledgments
The authors thank Prof. Scott Lokey, Alan Mathiowetz, and Chris Limberakis for access to peptides, David Griffith, Bill Farrell, and Simone Sciabola for discussions around the EPSA methodology, Mark Flanagan and Peter Jones for critical editing of the manuscript, and Justin Montgomery for assistance with Spotfire DXP.
Glossary
Abbreviations
- IMHB
intramolecular hydrogen bonding
- Ro5
Lipinski’s Rule of 5
- Caco-2
human epithelial colorectal adenocarcinoma cells
- MDCK-LE
low efflux Madin Darby canine kidney cells
- RRCK
Ralph Russ canine kidney cells
- PAMPA
parallel artificial membrane permeation assay
- Papp
apparent permeability
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Craik D. J.; Fairlie D. P.; Liras S.; Price D. The Future of Peptide-Based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [DOI] [PubMed] [Google Scholar]
- Woodley J. F. Enzymatic Barriers for GI Peptide and Protein Delivery. Crit. Rev. Ther. Drug Carrier Syst. 1994, 11, 61–95. [PubMed] [Google Scholar]
- Salamat-Miller N.; Johnston T. P. Current Strategies Used to Enhance the Paracellular Transport of Therapeutic Polypeptides across the Intestinal Epithelium. Int. J. Pharm. 2005, 294, 201–216. [DOI] [PubMed] [Google Scholar]
- Bock J. E.; Gavenonis J.; Kritzer J. A. Getting in Shape: Controlling Peptide Bioactivity and Bioavailability Using Conformational Constraints. ACS Chem. Biol. 2012, 8, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee J.; Gilon C.; Hoffman A.; Kessler H. N-Methylation of Peptides: A New Perspective in Medicinal Chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Hess S.; Ovadia O.; Shalev D. E.; Senderovich H.; Qadri B.; Yehezkel T.; Salitra Y.; Sheynis T.; Jelinek R.; Gilon C.; Hoffman A. Effect of Structural and Conformation Modifications, Including Backbone Cyclization, of Hydrophilic Hexapeptides on Their Intestinal Permeability and Enzymatic Stability. J. Med. Chem. 2007, 50, 6201–6211. [DOI] [PubMed] [Google Scholar]
- Hess S.; Linde Y.; Ovadia O.; Safrai E.; Shalev D. E.; Swed A.; Halbfinger E.; Lapidot T.; Winkler I.; Gabinet Y.; Faier A.; Yarden D.; Xiang Z.; Portillo F. P.; Haskell-Luevano C.; Gilon C.; Hoffman A. Backbone Cyclic Peptidomimetic Melanocortin-4 Receptor Agonist as a Novel Orally Administrated Drug Lead for Treating Obesity. J. Med. Chem. 2008, 51, 1026–1034. [DOI] [PubMed] [Google Scholar]
- Rezai T.; Bock J. E.; Zhou M. V.; Kalyanaraman C.; Lokey R. S.; Jacobson M. P. Conformational Flexibility, Internal Hydrogen Bonding, and Passive Membrane Permeability: Successful in Silico Prediction of the Relative Permeabilities of Cyclic Peptides. J. Am. Chem. Soc. 2006, 128, 14073–14080. [DOI] [PubMed] [Google Scholar]
- Rezai T.; Yu B.; Millhauser G.; Jacobson M. P.; Lokey R. S. Testing the Conformational Hypothesis of Passive Membrane Permeability Using Synthetic Cyclic Peptide Diastereomers. J. Am. Chem. Soc. 2006, 128, 2510–2511. [DOI] [PubMed] [Google Scholar]
- Alex A.; Millan D. S.; Perez M.; Wakenhut F.; Whitlock G. A. Intramolecular Hydrogen Bonding to Improve Membrane Permeability and Absorption in Beyond Rule of Five Chemical Space. Med. Chem. Commun. 2011, 2, 669–674. [Google Scholar]
- Ovadia O.; Greenberg S.; Chatterjee J.; Laufer B.; Opperer F.; Kessler H.; Gilon C.; Hoffman A. The Effect of Multiple N-Methylation on Intestinal Permeability of Cyclic Hexapeptides. Mol. Pharm. 2011, 8, 479–487. [DOI] [PubMed] [Google Scholar]
- Beck J. G.; Chatterjee J.; Laufer B.; Kiran M. U.; Frank A. O.; Neubauer S.; Ovadia O.; Greenberg S.; Gilon C.; Hoffman A.; Kessler H. Intestinal Permeability of Cyclic Peptides: Common Key Backbone Motifs Identified. J. Am. Chem. Soc. 2012, 134, 12125–12133. [DOI] [PubMed] [Google Scholar]
- Biron E.; Chatterjee J.; Ovadia O.; Langenegger D.; Brueggen J.; Hoyer D.; Schmid H. A.; Jelinek R.; Gilon C.; Hoffman A.; Kessler H. Improving Oral Bioavailability of Peptides by Multiple N-Methylation: Somatostatin Analogues. Angew. Chem. 2008, 47, 2595–2599. [DOI] [PubMed] [Google Scholar]
- White T. R.; Renzelman C. M.; Rand A. C.; Rezai T.; McEwen C. M.; Gelev V. M.; Turner R. A.; Linington R. G.; Leung S. S.; Kalgutkar A. S.; Bauman J. N.; Zhang Y.; Liras S.; Price D. A.; Mathiowetz A. M.; Jacobson M. P.; Lokey R. S. On-Resin N-Methylation of Cyclic Peptides for Discovery of Orally Bioavailable Scaffolds. Nat. Chem. Biol. 2011, 7, 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artursson P.Epithelial Transport of Drugs in Cell Culture. I: A Model for Studying the Passive Diffusion of Drugs over Intestinal Absorbtive (Caco-2) Cells. J. Pharm. Sci. 1990, 79. [DOI] [PubMed] [Google Scholar]
- Artursson P.; Magnusson C. Epithelial Transport of Drugs in Cell Culture. II: Effect of Extracellular Calcium Concentration on the Paracellular Transport of Drugs of Different Lipophilicities across Monolayers of Intestinal Epithelial (Caco-2) Cells. J. Pharm. Sci. 1990, 79, 595–600. [DOI] [PubMed] [Google Scholar]
- Di L.; Whitney-Pickett C.; Umland J. P.; Zhang H.; Zhang X.; Gebhard D. F.; Lai Y.; F J. J. III; Davidson R. E.; Smith R.; Reyner E. L.; Lee C.; Feng B.; Rotter C.; Varma M. V.; Kempshall S.; Fenner K.; El-kattan A. F.; Liston T. E.; Troutman M. D. Development of a New Permeability Assay Using Low-Efflux MDCKII Cells. J. Pharm. Sci. 2011, 100, 4974–4985. [DOI] [PubMed] [Google Scholar]
- Avdeef A. The Rise of PAMPA. Expert Opin. Drug Metab. Toxicol. 2005, 1, 325–342. [DOI] [PubMed] [Google Scholar]
- Avdeef A.; Artursson P.; Neuhoff S.; Lazorova L.; Gråsjö J.; Tavelin S. Caco-2 Permeability of Weakly Basic Drugs Predicted with the Double-Sink PAMPA Pka(Flux) Method. Eur. J. Pharm. Sci. 2005, 24, 333–349. [DOI] [PubMed] [Google Scholar]
- Guimarães C. R. W.; Mathiowetz A. M.; Shalaeva M.; Goetz G.; Liras S. Use of 3d Properties to Characterize Beyond Rule-of-5 Property Space for Passive Permeation. J. Chem. Inf. Model. 2012, 52, 882–890. [DOI] [PubMed] [Google Scholar]
- Goetz G. H.; Farrell W.; Shalaeva M.; Sciabola S.; Anderson D.; Yan J.; Philippe L.; Shapiro M. J. High Throughput Method for the Indirect Detection of Intramolecular Hydrogen Bonding. J. Med. Chem. 2014, 57, 2920–2929. [DOI] [PubMed] [Google Scholar]
- Lombardo F.; Shalaeva M. Y.; Tupper K. A.; Gao F.; Abraham M. H. Elogpoct: A Tool for Lipophilicity Determination in Drug Discovery. J. Med. Chem. 2000, 43, 2922–2928. [DOI] [PubMed] [Google Scholar]
- Lombardo F.; Shalaeva M. Y.; Tupper K. A.; Gao F. Elogdoct: A Tool for Lipophilicity Determination in Drug Discovery. 2. Basic and Neutral Compounds. J. Med. Chem. 2001, 44, 2490–2497. [DOI] [PubMed] [Google Scholar]
- Cheng H.; Hoffman J. E.; Le P. T.; Pairish M.; Kania R.; Farrell W.; Bagrodia S.; Yuan J.; Sun S.; Zhang E.; Xiang C.; Dalvie D.; Rahavendran S. V. Structure-Based Design, SAR Analysis and Antitumor Activity of PI3K/mTOR Dual Inhibitors from 4-Methyl-pyridopyrimidinone Series. Biorg. Med. Chem. Lett. 2013, 23, 2787–2792. [DOI] [PubMed] [Google Scholar]
- Wakenhut F.; Tran T. D.; Pickford C.; Shaw S.; Westby M.; Smith-Burchnell C.; Watson L.; Paradowski M.; Milbank Jared; Stonehouse D.; Cheung K.; Wybrow R.; Daverio F.; Crook S.; Statham K.; Leese D.; Stead D.; Adam F.; Hay D.; Roberts L. R.; Chiva J.-Y.; Nichols C.; Blakemore D. C.; Goetz G. H.; Che Y.; Gardner I.; Dayal S.; Pike A.; Webster R.; Pryde D. C. The Discovery of Potent NS5A Inhibitors with a Unique Resistance Profile—Part 2. ChemMedChem 2014, 9, 1387–1396. [DOI] [PubMed] [Google Scholar]
- Rand A. C.; Leung S. S. F.; Eng H.; Rotter C. J.; Sharma R.; Kalgutkar A. S.; Zhang Y.; Varma M. V.; Farley K. A.; Khunte B.; Limberakis C.; Price D. A.; Liras S.; Mathiowetz A. M.; Jacobson M. P.; Lokey R. S. Optimizing PK Properties of Cyclic Peptides: The Effect of Side Chain Substitutions on Permeability and Clearance. Med. Chem. Commun. 2012, 3, 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]