Abstract
The cause of airway smooth muscle (ASM) hypercontractility in asthma is not fully understood. The relationship of spontaneous intracellular calcium oscillation frequency in ASM to asthma severity was investigated. Oscillations were increased in subjects with impaired lung function abolished by extracellular calcium removal, attenuated by caffeine and unaffected by verapamil or nitrendipine. Whether modulation of increased spontaneous intracellular calcium oscillations in ASM from patients with impaired lung function represents a therapeutic target warrants further investigation.
Keywords: airway hyperresponsiveness, airway smooth muscle, asthma, calcium, oscillations
Asthma affects over 300 million people worldwide. It is characterized by variable airflow obstruction (AFO) and airway hyperresponsiveness as a consequence of increased airway smooth muscle (ASM) contractility.1,2 There is emerging evidence that ASM from asthmatics is hypercontractile as demonstrated by an increased velocity of contraction in response to electrical field stimulation at the single cell level3 and in cell populations using gel contraction assays.4,5
Spontaneous ASM contraction is considered of fundamental importance in foetal lung development.6 Spontaneous intracellular calcium ([Ca2+]i) oscillations are reported in foetal ASM and are implicated in ASM contraction. Agonist-induced [Ca2+]i oscillations are also reported in adult ASM and are implicated in augmenting ASM contraction and Ca2+-dependent transcriptional regulation.7,8 Spontaneous Ca2+ oscillations have not hitherto been reported in ASM from adults. We hypothesized that spontaneous [Ca2+]i oscillations are maintained in ASM derived from subjects with asthma and are related to disease severity and disordered airway physiology.
Primary ASM was derived from bronchial biopsies obtained from well-characterized volunteers. All subjects gave written informed consent. The Leicestershire, Northamptonshire and Rutland Ethics committee approved the study. ASM cells were cultured and characterized as previously described9 and used at passages 2–5. To measure changes in [Ca2+]i, subconfluent ASM cells were loaded with 2 μmol/L Fura-2AM in the presence of 2.5 mmol/L probenecid and 0.04% w/v pluronic F-127, and visualized on an inverted epifluorescence microscope (Nikon Diaphot 200, Nikon Instruments, Kingston, UK). Fura-2 fluorescence (F) emission intensity at 510 nm was measured following excitation at wavelengths of 340 and 380 nm, and reported as a ratio, R = F340/F380, such that R is directly proportional to [Ca2+]i. [Ca2+]i oscillatory behaviour was analysed using two methods. First, the [Ca2+]i oscillation frequency was measured by a single-blinded observer counting the number of oscillations reaching a 340/380 ratio >10% greater than the baseline derived for each donor for 10 min. Second, the [Ca2+]i oscillation dominant frequency (and its amplitude) were determined by fast Fourier transform (FFT) spectral analysis using software previously developed for MATLAB.10 The two methods were significantly correlated (rs = 0.58; P = 0.0002). The FFT-derived Ca2+ oscillation dominant frequency data are reported here. The effect of removal of extracellular Ca2+, IP3 receptor inhibition (caffeine, 10 mmol/L) and voltage-gated L-type Ca2+ channel inhibition (1 μmol/L verapamil and 1 μmol/L nitrendipine) on [Ca2+]i oscillations in highly oscillating cells were investigated.
Statistical analyses were performed using Prism version 6 (GraphPad, San Diego, CA, USA) and IBM SPSS version 20 (SPSS, Inc., Chicago, IL, USA). Data are presented as median (interquartile ranges) or maximum dominant frequency for the cells for an individual donor. Comparisons before and after pharmacological interventions used paired Student's t-tests, and across groups Kruskal–Wallis test and post-hoc pairwise comparisons using Dunn's test. Spearman rank correlation coefficients were used to assess the correlations. Previously, Ressmeyer et al.7 suggested that the oscillation frequency induced by histamine in ASM of lung slices relates to the degree of ASM contraction. Their work suggests that a dominant frequency of 60 mHz is equivalent to 30% contraction. We used this cut-off to identify the proportion of patients with cells that had dominant frequencies above this threshold. Proportions were analysed using chi-square test. A P value less than 0.05 was considered statistically significant.
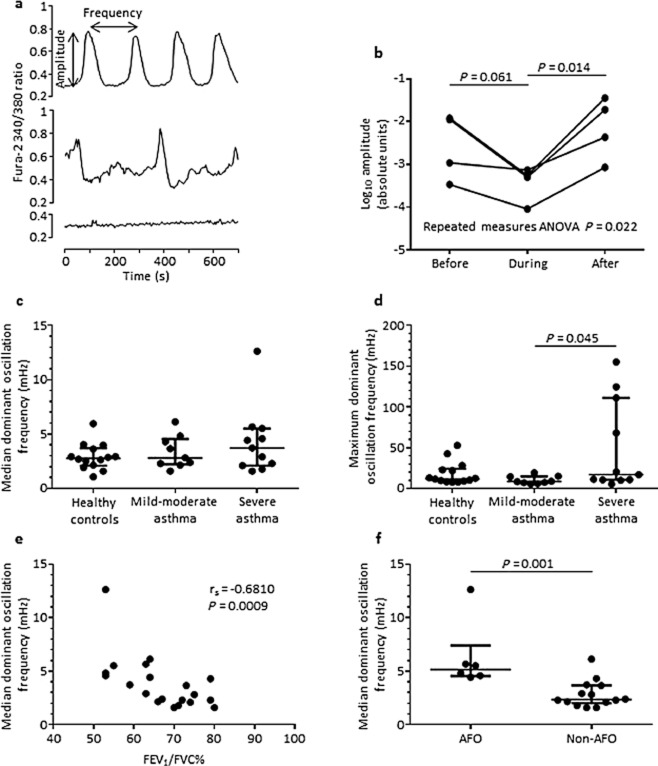
We recruited 34 volunteers (14 healthy subjects (8 male, 6 female), 9 with mild-moderate asthma (4 male, 5 female) and 11 severe asthmatics (6 male, 5 female)). Subjects were age-matched: 60 (5) years healthy, 46 (5) years mild/moderate asthma and 48 (5) years severe asthma (P = 0.71). The spirometry measurements for all subjects (healthy, mild-moderate asthma Global Initiative for Asthma (GINA) I-III and severe asthma GINA IV and V respectively (mean (standard error of the mean)) were: forced expiratory volume in 1 s (FEV1)% predicted: 97 (3), 91 (8) and 77 (6) (P < 0.05); FEV1/forced vital capacity (FVC)%: 79 (2), 70 (3) and 64 (3) (P < 0.05). The dominant [Ca2+]i oscillation frequency was determined for each cell (minimum 5 cells per donor). Spontaneous ASM [Ca2+]i oscillations were observed in both health and disease. Example traces are shown (Fig. 1a). Spontaneous [Ca2+]i oscillations were abolished by removal of extracellular Ca2+ (99.4% ± 0.4; data not shown). The amplitude of spontaneous [Ca2+]i oscillations were attenuated by caffeine, being restored following its washout (n = 4 donors, 91 individual cells assessed; ANOVA P = 0.022; Fig. 1b) but was not significantly affected by verapamil or nitrendipine (data not shown). Spontaneous ASM [Ca2+]i oscillation median dominant frequency was not different between asthma and health, or across disease severity (Fig. 1c). The maximum dominant frequency was increased in severe asthma compared with mild-moderate and healthy controls (P = 0.048 Kruskal–Wallis; Fig. 1d). Four of 11 severe asthmatic patients and none of the healthy subjects or patients with mild/moderate asthma had a maximum [Ca2+]i oscillation dominant frequency greater than 60 mHz (P = 0.009; Fig. 1d). There were no differences in the amplitude of the dominant frequency across groups (data not shown). Interestingly, the median [Ca2+]i oscillation dominant frequency was significantly correlated with FEV1% predicted (rs = −0.54, P = 0.014) and with FEV1/FVC% (rs = −0.68, P = 0.0009; Fig. 1e) in those with asthma. Furthermore, in those asthmatics with persistent AFO (FEV1% predicted <80% and FEV1/FVC <70%) compared with those without persistent AFO the median [Ca2+]i dominant oscillation frequency was significantly increased (5.17 (4.5–7.4) vs 2.4 (2.0–3.7) mHz), P = 0.001; Fig. 1f). Similar observations were made between oscillation frequency determined by >10% greater than baseline and lung function (correlation FEV1/FVC% and oscillation frequency r = −0.47, P = 0.005; other data not shown).
Figure 1.
(a) Examples of intracellular calcium ([Ca2+]i) traces showing a low oscillating cell, a pronounced oscillating cell with high amplitude, regular frequency and little background, and an oscillating cell with higher background interference. (b) Median amplitude of [Ca2+]i oscillations before, during and after caffeine (10 mmol/L) application for four donors derived from 91 cell traces. Repeat measures analysis of variance (ANOVA) and pairwise t-test P-values are as shown. (c) Median dominant oscillation frequencies of subjects with asthma were compared with healthy controls. Each point is an individual subject. Horizontal bars represent medians. (d) Maximum dominant oscillation frequencies of subjects with asthma were compared with healthy controls. Each point is an individual subject. Horizontal solid bars represent medians. Comparisons made using Kruskal–Wallis test (P = 0.048) and post-hoc pairwise comparisons using Dunn's test as shown. (e) Median dominant oscillation frequency was correlated negatively with forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)% Spearman's rank correlation coefficient. (f) Dominant oscillation frequency for asthmatic subjects with and without persistent airflow obstruction (FEV1% predicted <80%, FEV1/FVC <70%). Horizontal bars represent medians. Comparison between groups by Mann–Whitney test.
We report here for the first time that spontaneous [Ca2+]i oscillations are observed in primary ASM cells in asthma and health. The maximum dominant frequency of these spontaneous [Ca2+]i oscillations was increased in severe asthma. The median dominant frequency was related to impaired lung function and was increased in asthmatics with persistent AFO. Agonist-induced [Ca2+]i oscillations have been consistently observed, and previous reports have primarily implicated the intracellular Ca2+ stores.7,8 However, other reports have implicated both intracellular Ca2+ stores and influx pathways.11 Altered Ca2+ homeostasis has previously been reported as a consequence of altered mitochondrial biogenesis12 and SERCA2 downregulation,13 suggesting that intracellular Ca2+-handling in ASM in asthma might be altered via a number of interrelated mechanisms. The consequences of spontaneous [Ca2+]i oscillations in asthma is not fully understood but have been implicated in increased ASM contraction and Ca2+-transcriptional coupling.7,8 Indeed, agonist-induced ASM hypercontractility is reported in primary ASM from asthmatics.4,5 Ressmeyer and colleagues demonstrated a relationship between the agonist-induced [Ca2+]i oscillation frequency and percentage airway contraction in human airway slices.7 The frequency of the spontaneous [Ca2+]i oscillations we describe here, predominantly in patients with severe asthma, might be sufficient to induce contraction and may also enhance the response to agonists. In addition to hypercontractility, primary ASM from asthmatics have increased capacity to release several important pro-inflammatory chemokines and matrix proteins compared with ASM derived from healthy volunteers.5,14 Although the potential role of spontaneous [Ca2+]i oscillations in these mechanisms warrants further study, the associations observed here with asthma severity and disordered airway physiology suggests that these observations might be clinically important. The presence of spontaneous [Ca2+]i oscillations in foetal ASM6 presents the intriguing possibility that this phenomenon might reflect either persistence or regression towards a foetal ASM phenotype in asthma. Whether this altered ASM is a consequence of the inflammatory effects of the asthmatic milieu or represents a mechanism independent of inflammation requires further study.
In conclusion, we have observed increased frequency of spontaneous [Ca2+]i oscillations in ASM cells from subjects with impaired lung function. The mechanisms of this aberrant spontaneous [Ca2+]i oscillatory behaviour in asthma need to be fully elucidated and might identify new therapeutic targets.
Glossary
- AFO
airflow obstruction
- ASM
airway smooth muscle
- [Ca2+]i
intracellular calcium
- F
fluorescence
- FEV1
forced expiratory volume in 1 s
- FFT
fast Fourier transform
- FVC
forced vital capacity
- GINA
Global Initiative for Asthma
References
- Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J. Allergy Clin. Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2011. GINA Report, Global Strategy for Asthma Management and Prevention. [Accessed 3 Mar 2014.] Available from http://www.ginasthma.org/
- Ma X, Wang Y, Stephens NL. Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am. J. Physiol. 1998;274:1206–1214. doi: 10.1152/ajpcell.1998.274.5.C1206. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Moir LM, Oliver BG, Burgess JK, Roth M, Black JL, McParland BE. Comparison of gel contraction mediated by airway smooth muscle cells from patients with and without asthma. Thorax. 2007;62:848–854. doi: 10.1136/thx.2006.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am. J. Respir. Crit. Care Med. 2012;185:267–274. doi: 10.1164/rccm.201107-1281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards IS, Kulkarni A, Brooks SM. Human fetal tracheal smooth muscle produces spontaneous electromechanical oscillations that are Ca2+ dependent and cholinerically potentiated. Dev. Pharmacol. Ther. 1991;16:22–28. [PubMed] [Google Scholar]
- Ressmeyer AR, Bai Y, Delmotte P, Uy KF, Thistlethwaite P, Fraire A, Sato O, Ikebe M, Sanderson MJ. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am. J. Respir. Cell Mol. Biol. 2010;43:179–191. doi: 10.1165/rcmb.2009-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savineau JP, Marthan R. Cytosolic calcium oscillations in smooth muscle cells. News Physiol. Sci. 2000;15:50–55. doi: 10.1152/physiologyonline.2000.15.1.50. [DOI] [PubMed] [Google Scholar]
- Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, Bradding P, Brightling CE. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am. J. Respir. Crit. Care Med. 2006;174:1179–1188. doi: 10.1164/rccm.200603-394OC. [DOI] [PubMed] [Google Scholar]
- Uhlén P. Spectral analysis of calcium oscillations. Sci. STKE. 2004;258:pl15. doi: 10.1126/stke.2582004pl15. [DOI] [PubMed] [Google Scholar]
- Hamada H, Damron DS, Hong SJ, Van Wagoner DR, Murray PA. Phenylephrine-induced Ca2+ oscillations in canine pulmonary artery smooth muscle cells. Circ. Res. 1997;81:812–823. doi: 10.1161/01.res.81.5.812. [DOI] [PubMed] [Google Scholar]
- Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, et al. Bronchial smooth muscle remodelling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrashdan YA, Alkhouri H, Chen E, Lalor DJ, Poniris M, Henness S, Brightling CE, Burgess JK, Armour CL, Ammit AJ, et al. Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L1118–1127. doi: 10.1152/ajplung.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]