Abstract
The rate constant of the reaction between methanol and the hydroxyl radical has been studied in the temperature range 56-202 K by pulsed laser photolysis-laser induced fluorescence in two separate experiments using either a low temperature flow tube coupled to a time of flight mass spectrometer or a pulsed Laval nozzle apparatus. The two independent techniques yield rate constants which are in mutual agreement and consistent with the results reported previously below 82 K [Shannon et al., Nature Chemistry, 2013, 5, 745-749] and above 210 K [Dillon et al., Phys. Chem. Chem. 2005, 7, 349-355], showing a very sharp increase with decreasing temperature with an onset around 180 K. This onset is also signalled by strong chemiluminescence tentatively assigned to formaldehyde, which is consistent with the formation of the methoxy radical at low temperature by quantum tunnelling, and its subsequent reaction with H and OH. Our results add confidence to the previous low temperature rate constant measurements and consolidate the experimental reference dataset for further theoretical work required to describe quantitatively the tunnelling mechanism operating in this reaction. An additional measurement of the rate constant at 56 K yielded a value of (4.9 ± 0.8) × 10−11 cm3 molecule−1 s−1 (2σ), showing that the rate constant is increasing less rapidly at temperatures below 70 K.
Keywords: Methanol, Hydroxyl Radical, Low Temperature Reactions, Quantum Tunnelling
1. Introduction
The reaction between methanol and the hydroxyl radical has been extensively studied in the past at temperatures above 210 K for its interest in atmospheric and combustion chemistry.1-6 The reaction’s non-Arrhenius behaviour in the temperature range between 210 K and 870K results from characteristic features of the potential energy surface,7 including a loose pre-reaction complex (before the activation barrier), OH…O(H)CH3, and transition states with low barrier heights formed by the roaming OH abstracting either the H atom of the hydroxyl group (TS-H) or one of the H atoms of the methyl group (TS-M).8 Reaction complexes which form after these barriers then fall apart to yield:
| (R1a) |
| (R1b) |
where the enthalpies of reaction have been calculated from up-to-date standard compilations of heats of formation.9-11 Rice-Ramsperger-Kassel-Marcus (RRKM) and transition state theory calculations of the total thermal rate constants of R1 appear to be in good agreement with the experimental results above 210 K.8 According to these theoretical predictions, R1b accounts for more than 80% of the overall products over this range of temperatures owing to the lower barrier height of TS-M.8,12
Very recently Shannon et al.12 carried out the first study on this reaction using a pulsed Laval Nozzle apparatus operating at temperatures below 82 K,13 and found an unexpected enhancement of nearly 2 orders of magnitude of the rate constant relative to the value determined by Dillon et al. at 210 K.6 Observation of the methoxy radical, CH3O, by LIF led them to conclude that at low temperatures the pre-reaction complex would be long lived enough against re-dissociation to increase significantly the probability of tunnelling through the aforementioned barrier. Despite the TS-M barrier being lower than the barrier associated with TS-H, R1a is predicted to be the dominant channel at low temperatures (~90% branching at 150 K) as a result of the larger imaginary frequency of TS-H which has a narrower barrier.12 In the low temperature limit the reaction is expected to reach its limiting gas kinetic rate constant.
These findings indicate the presence of a previously unaccounted mechanism involving long-lived pre-reaction complexes which enable fast hydrogen transfer reactions under the low temperature conditions of interstellar molecular clouds. In the particular case of R1, this mechanism could help to explain the presence of CH3O in interstellar clouds,14 where both the OH radical15-16 and methanol17 have also been observed. It also suggests that this type of reaction could constitute a previously unaccounted sink for the OH radical.
In order to confirm the Shannon et al. results, which were obtained using the pulsed Laval nozzle technique, and to fill the remaining gap in data between 82 K and 210 K, we have performed new experiments on R1 using two independent techniques, namely a low temperature flow tube reactor and the pulsed Laval nozzle apparatus. The experiments with each technique span a mutually overlapping temperature range, and also overlap with the high and low temperature ranges in previous studies. In addition, a new rate constant determination below the temperatures considered by Shannon et al. is reported.
2. Experimental
PLP-LIF-Low temperature reactor
Experiments were conducted in a 38 mm ID Conflat-flanged temperature-controlled reactor (Fig. 1), combining features of the classical flow tube (FT) and pulsed laser photolysis-laser induced fluorescence (PLP-LIF) techniques. This combination was necessary due to the unconventionally low temperatures considered in this study, which caused the excess reactant in R1 (methanol) to freeze out to the reactor walls (the methanol equilibrium vapour pressure is 1.35 mTorr at the melting point temperature, 176 K18) and precluded downstream monitoring of its concentration at the reaction zone. In situ detection would be made challenging by condensation on windows and was not attempted. Accurate quantification of the methanol concentration, which is key to obtaining accurate rate constants from pseudo-first order kinetics ([CH3OH] >> [OH]), relied on the ability to inject the gas mixture at different points in the FT attached to the PLP-LIF 6-way cross (Fig. 1), in such a way that the substantial wall loss of methanol below 200 K could be characterised by a mass spectrometer which sampled downstream. Regarding generation and detection of OH, the experiments were performed in a PLP-LIF configuration by delayed temporal overlapping of the photolysis and probe laser beams, and collecting in situ fluorescence at right angles with a photomultiplier tube.
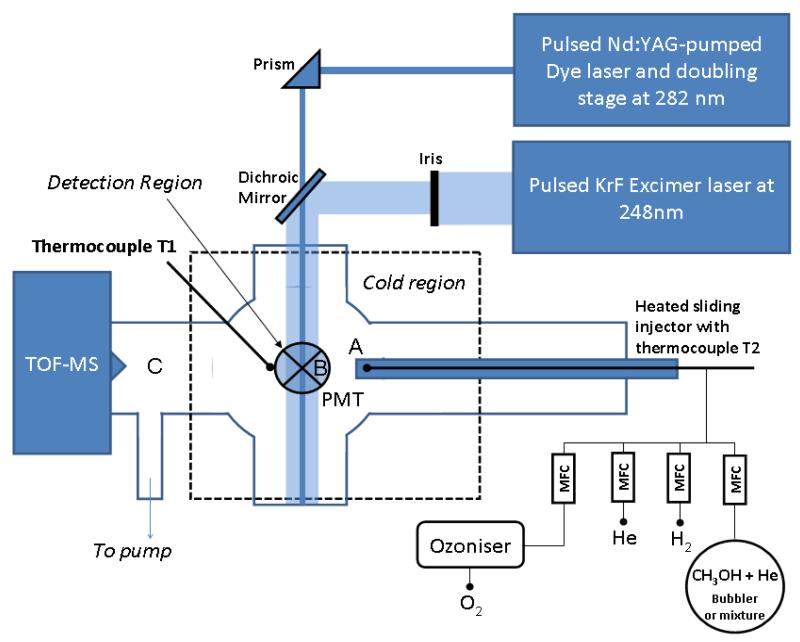
Figure 1.
PLP-LIF-Low temperature reactor. A, B and C indicate respectively the reactant injection point, the reaction zone and the point where methanol concentration is sampled.
The reaction chamber (6-way cross) and a section of the upstream flow tube attached to it were surrounded by copper tubing (OD = 6.25 mm), through which liquid nitrogen could be circulated. The contact between the copper tube and the reactor walls was enhanced by wrapping the walls with Cu foil and then packing Cu shavings in between the cooling coils. The whole block was wrapped in insulating material (neoprene). For temperatures below 190 K, liquid nitrogen stored in a 200 dm3 pressurized dewar was admitted to the cooling circuit via a temperature-controlled solenoid valve. A thermocouple placed inside reaction zone (T1, see Fig. 1) provided the feedback to the temperature controller to activate the valve depending on deviation from the set point temperature. For temperatures above 190 K, a chilled liquid circulator (Huber Unistat 390 W) operating with a thermal bath fluid (DW-Therm) was used. In order to avoid freezing of methanol on the walls of the injection port, the gas mixture was injected using a heated sliding injector (OD = 19 mm), consisting of a jacketed tube, with the outer jacket evacuated and containing a 20 W heating wire and a thermocouple (T2, see Fig. 1). The injection point A, 3 cm upstream of the OH detection volume B, was fixed in such a way that the warm gas mixture injected into the cooled reactor had enough time to cool down to the target temperature, as indicated by thermocouple T1. Experiments where performed at 295 K and in the temperature range between 123 K and 202 K, to bridge the gap between the two temperature ranges for which rate constants of R1 have been reported.6, 12
Gas flows were mixed at room temperature before injection into the temperature-controlled reaction region by delivering gases into a Teflon mixing manifold via calibrated MKS mass flow controllers (MFC). Methanol vapour was introduced at its room temperature equilibrium vapour pressure (96 Torr at 293 K18) by bubbling up to 150 sccm of He through a glass bubbler containing liquid methanol. Alternatively, stored mixtures (typically 4% CH3OH in He) prepared beforehand using calibrated capacitance manometers (MKS Baratron) were flowed through the same MFC. A balance flow of up to 200 sccm of pure He was added via a second MFC to enable controlled variation of methanol concentration. The third MFC delivered 10 sccm of a 3-4% mixture of O3 in O2, which was produced in a 760 Torr corona discharge of O2. Finally, a fourth MFC was used to flow up to 200 sccm of pure H2 in some experiments. The total flow was typically 250 sccm and the pressure in the reactor was in the 0.5 – 3 Torr range.
OH was generated by O3 photolysis at 248 nm using an excimer laser operating on KrF (Lambda Physik, COMPEX 102), followed by reaction of the O(1D) photo-fragment (0.9 quantum yield19) with CH3OH (R3) or H2 (R4):
| (R2) |
| (R3a) |
| (R3b) |
| (R4) |
where the enthalpies of reaction have been calculated from evaluated heats of formation,9-11 except the heat of formation of HOCH2O, which was estimated by Matsumi et al.20
The presence of O2 may cause some loss of O(1D) by quenching:
| (R5) |
However, the impact of R5 can be substantially reduced, provided that the concentration of H2/CH3OH is high enough. This amounts in practice to keeping [O2] equal to or smaller than [H2] and [CH3OH], since the rate constants k3(298 K) = 5 × 10−10 cm3 molecule−1 s−1 and k4(298 K) = 1.1 × 10−10 cm3 molecule−1 s−1 are about one order of magnitude larger than k5(298 K) = 5 × 10−11 cm3 molecule−1 s−1. Despite of formation of OH (v ≥ 0) on a time scale of only a few microseconds, the phenomenological growth of OH(v = 0) takes place on a longer time scale as a result of collisional quenching:
| (R6) |
with M = CH3OH, O2. However, this occurs on a time scale of tens of microseconds and can be discriminated from the slower pseudo-first order removal of OH(v = 0) by R1 and other loss processes such as diffusion (the temporal evolution of OH is shown in Fig. 2). In some experiments, the addition of a sufficiently high concentration of H2 was employed as the OH source (via R4) in order to suppress R3 so that the methoxy radical formed in R1a could be distinguished.
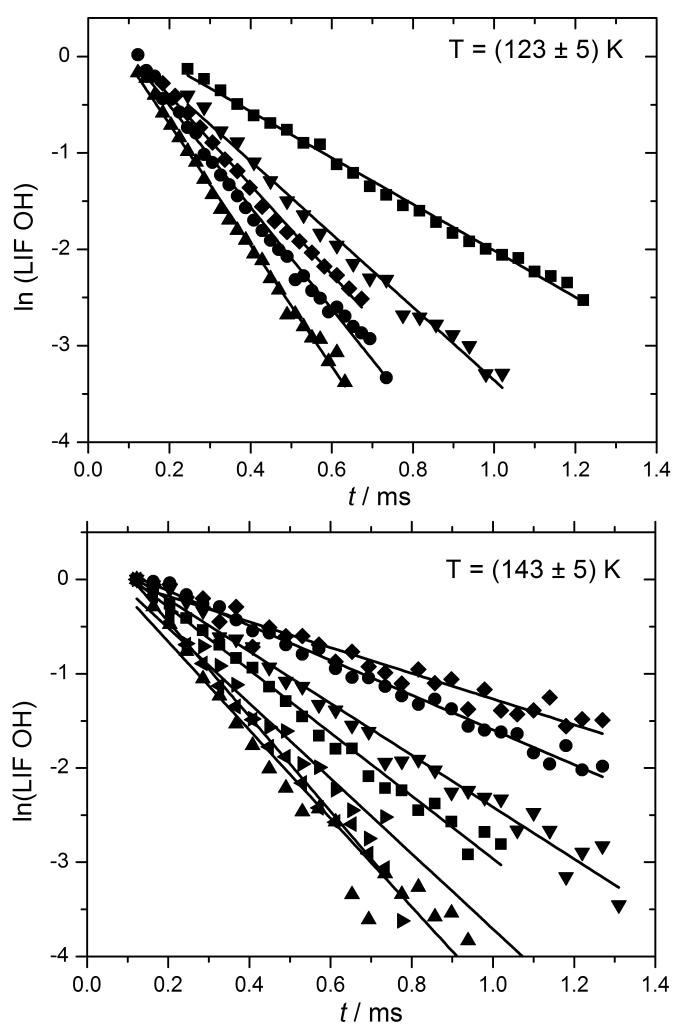
Figure 2.
Decays of OH LIF signal at two different temperatures in the presence of different CH3OH concentrations observed in the PLP-LIF-Low temperature reactor experiments.
OH(X2∏3/2) was observed by time-resolved LIF spectroscopy using a frequency-doubled Nd:YAG-pumped dye laser (Continuum Surelite II and Lambda Physik Cobra Stretch) operated with Rhodamine 6G dye. The output of this laser was frequency doubled with a KDP crystal to probe OH by exciting the (v″ = 0) Q13 or Q11 lines near 282.24 nm. Fluorescence was collected by an f = 5 cm lens through an interference filter centred at 307 nm (10 nm FWHM), focused onto a photomultiplier (Thorn EMI, B-216F) and digitised and integrated using an oscilloscope (LeCroy Waverunner, LT 342). The temporal evolutions of the OH LIF signal were obtained by varying the delay between the dye laser and the 248 nm laser pulse using a delay generator (Quantum Composers, QC9528) controlled via GPIB by a customised LabView data acquisition program. The experiments were run at a laser pulse repetition frequency of 10 Hz.
The OH decay traces consisted of 50 points, each averaged 9 times, as shown in logarithmic form in Fig. 2. In separate experiments, formation the CH3O radical was probed at ~297.65 nm on a sharp feature of its band (v3 = 3 ← 0 and overlapping combination bands), using the KDP-doubled output from Rhodamine B dye. Fluorescence was collected through an interference filter centred at 400 nm (80 nm bandwidth).
Methanol was measured 20 cm downstream of the reaction chamber, out of the cooled region, by sampling on axis through a 200 μm diameter pinhole using an electron impact-time of flight mass spectrometer (Kore). The electron source was operated at 25 eV. Methanol was monitored at several fragmentation peaks (m/z 15, 29, 30), while the parent mass was avoided due to its coincidence with O2. Calibration of these signals was performed at room temperature using stock mixtures of CH3OH in He. Since methanol was always measured at room temperature irrespective of the temperature of the reactor, the methanol concentration in the reaction zone needs to be extrapolated (see below).
PLP-LIF-Laval Nozzle
The experimental setup of the pulsed Laval nozzle apparatus utilised in this study has been discussed in detail previously so only a brief description is given here 12-13, 21-22. Methanol was degassed and its vapour pressure was admitted to an evacuated cylinder where it was diluted with N2 (Ar) and allowed to mix overnight before use. The OH precursor (tert-Butyl Hydroperoxide, t-BuOOH) which was seeded into an N2 (Ar) flow via a bubbler, the methanol mixture and N2 (Ar) bath gas were flowed through a set of calibrated mass flow controllers (MKS instruments) into a 1L stainless steel ballast tank where they were allowed to mix. The experiments were performed under pseudo first order conditions so that [CH3OH] >> [OH]. The mixed gases were then supersonically expanded through a convergent-divergent shaped Laval nozzle into a cylindrical stainless steel chamber which was held at low pressure. Two pulsed solenoid valves positioned prior to a 1 cm3 reservoir behind the nozzle controlled the flow of the gas to the nozzle and limited the expansion to pulses of ~ 15 ms duration. The temperature and density profile of the resultant jet were characterised via impact pressure measurements using two differential pressure transducers positioned in the expanded gas flow and in the reservoir prior to expansion. Two Laval nozzles were utilised in this study. Impact pressure measurements of the jet obtained from the first nozzle, using N2 bath gas, yielded a jet temperature and density of (138 ± 9) K and (7.8 ± 1.0) × 1016 molecule cm−3 respectively. Impact pressure measurements of the same nozzle, where Ar bath gas was utilised, yielded a jet temperature and density of (88 ± 8) K and (9.4 ± 1.3) × 1016 molecule cm−3 respectively. For the second nozzle, Ar bath gas was utilised and a jet temperature and density of (56 ± 4) K and (4.4 ± 0.5) × 1016 molecule cm−3 respectively were obtained.
The rate constant for R1 was obtained by monitoring the temporal evolution of the OH radical via PLP-LIF. During the supersonic expansion, the OH precursor was photolysed along the entire jet by a 248 nm excimer laser (KrF Lambda Physik LPX 200) aligned collinearly with the jet producing a uniform OH density:
| (R7) |
where N′ represents OH which is more rotationally excited than the corresponding Boltzmann distribution at the temperature determined by the impact pressure measurements. Subsequent, rapid collisional relaxation to a thermalised distribution takes place via:
| (R8) |
where M is the bath gas. The OH decay results from R1 and other loss processes, mainly diffusion and reaction with the precursor:
| (R9) |
OH was monitored using the same LIF excitation scheme described above. Fluorescence was collected by a PMT positioned perpendicularly to the axes of the jet and the laser beams. The temporal evolution of OH was acquired by increasing the delay time between the photolysis and probe lasers. The OH decay traces consisted of 170 points each averaged up to 12 times.
Materials
He (99.9999 %, BOC Gases), H2 (99.9999 %, BOC Gases), O2 (99.995 %, BOC Gases), N2 (OFN, BOC Gases), Ar (99.998 %, BOC Gases), t-Butyl Hydroperoxide (70 % wt in H2O, Sigma-Aldrich) and methanol (≥ 99.9%, Sigma Aldrich) were used without further purification. Ozone was made by flowing O2 through a commercial ozonizer (Fischer, OZ500) to produce a 3-4 % O3 in O2 mixture.
3. Results and discussion
PLP-LIF-Low temperature reactor
The major difficulty of measuring rate constants below 200 K in a conventional flow reactor under pseudo-first order conditions lies in quantifying accurately the concentration of the reagent (CH3OH in our case) in the volume where the reactive loss of the species of interest (OH) is measured. Working at such low temperatures, below the freezing point of the reagent, implies heavy losses to walls and aerosol formation, which precludes detection immediately downstream of the volume where the reaction is monitored. In situ techniques for detection of methanol are limited to VUV absorption,6 which at such low temperatures is extremely difficult to measure as a result of reduced transmission of windows due to condensation. To overcome these problems, we have used a technique which allows the wall losses to be characterised with sufficient accuracy. This consists of measuring the methanol reaching the mass spectrometer when the gas is injected inside the cooled reactor (A in Fig. 1) and then placing the head of the heated sliding injector first within the detection volume (B), and second at a point outside the cold region close to the sampling pinhole (C) in order to measure the methanol concentration in the absence of wall losses (at room temperature). In this way the methanol concentration [CH3OH]B(i) at the temperature T at position B for each OH decay (i) can be calculated from the following expression:
| (E1) |
where a and b are the calibration parameters of the mass spectrometer, obtained by injecting known concentrations of CH3OH into the flow tube at room temperature (see above); SA(i) is the methanol signal registered by the mass spectrometer during the kinetic experiment i with the head of the sliding injector at point A; SA, SB and SC are the methanol signals measured respectively at positions A, B and C (see Fig.1) at a fixed methanol concentration after the kinetic experiments; and TA is the temperature of the gas at the MS sampling pinhole (room temperature). The ratio (SC/SA) accounts for the condensational loss between A and C and scales the measured concentration up to the concentration before wall loss at A, while the ratio (SA/SB) accounts for the condensational loss between A and B and scales the concentration at A down to the concentration at B. This scaling approach is supported by the observation that at any temperatures within the reaction volume considered in our study, the CH3OH signal registered downstream by the mass spectrometer varied linearly with the CH3OH flow. This implies that at any temperature in the reaction cell, the decrease in methanol concentration from the injection (A) to the detection point (C) as a result of wall losses and thermalisation is a constant fraction of the concentration injected. One caveat of this approach is that it accounts only for the CH3OH that is permanently lost. If some CH3OH clusters in the cold region and then goes back to CH3OH in the warm region, then we would be potentially underestimating the rate constant.
The ratio SC/SB was in the range 12 - 25 for the three lowest temperatures considered (Table 1), i.e. the concentration of CH3OH decreases by approximately one order of magnitude between B and C as a result of condensation to the walls and aerosol formation. The high sensitivity and wide dynamic range of the mass spectrometer enables the scaling of signals to be performed with enough accuracy. For 1 sec integration time and considering 3 standard deviations of the background noise, the signal to noise ratio is ~ 60 for maximum methanol concentration measured when injecting at point A, which was ~ 60% of the upper limit of the linearity range.
Table 1.
Summary of experimental conditions and measured rate constants
| T /K a | Bath gas ,M | [M]/1016 b | [CH3OH]/1015 b | SC/SB | k1 /10−11 c |
|---|---|---|---|---|---|
| 56 ± 4 d | Ar | 4.4 ±0.5 | 0.07-0.22 | - | 4.9 ±0.8 |
| 88 ± 8 d | Ar | 9.4±1.3 | 0.1-0.6 | - | 3.8 ±0.4 |
| 123 ±5 | He | 3.15 ± 0.15 | 0.3-1.5 | 20 ± 4 | 0.43 ±0.21 |
| 130 ±5 | He | 6.6 ±0.3 | 0.2-1.7 | 13 ± 3 | 0.39 ±0.21 |
| 138 ±9 d | N2 | 7.8 ± 1.0 | 0.1-0.9 | - | 0.53 ±0.17 |
| 143 ± 5 | He | 2.76 ±0.14 | 0.1-1.6 | 17±3 | 0.31 ±0.12 |
| 163 ± 5 | He | 2.46 ±0.13 | 0.3-1.6 | 11 ± 2 | 0.17 ±0.07 |
| 180 ± 10 | He | 1.59 ±0.08 | 0.2-2.0 | 10±2 | 0.07 ±0.04 |
| 202 ± 2 | He | 1.30 ±0.06 | 1.0-3.5 | 2.9 ±0.4 | 0.060 ±0.008 |
| 295 ± 1 | He | 8.9 ±0.4 | 0.4-4.0 | 1 | 0.113 ±0.004 |
Average temperature and range of variation (min-max).
In molecule cm−3.
In cm3 molecule −1 s−1, uncertainty at 2σ confidence level.
Laval Nozzle experiments.
The OH PLP-LIF experiments were performed at low pressure. This was necessary to avoid significant laser scatter observed when the flow tube pressure was above 1 Torr. The scatter was more prominent at the lowest temperatures, which is consistent with the formation of nano-aerosols, presumably from CH3OH. The potential effects of cluster formation on the determination of the rate constant of R1 are discussed below. Under the pseudo-first order conditions employed, and with a clear separation of the time scales of OH growth and decay, the OH signal decays exponentially with time:
| (E2) |
where k′ is the total loss rate and t is time (i.e. the delay between photolysis and probe laser pulses). Linear regression of the ln [OH]t vs. time scatter plots (Fig. 2) yields slopes which are equal to the pseudo-first order loss rates resulting from R1 (for each particular concentration of CH3OH) plus the loss rate corresponding to additional removal processes (e.g. diffusion):
| (E3) |
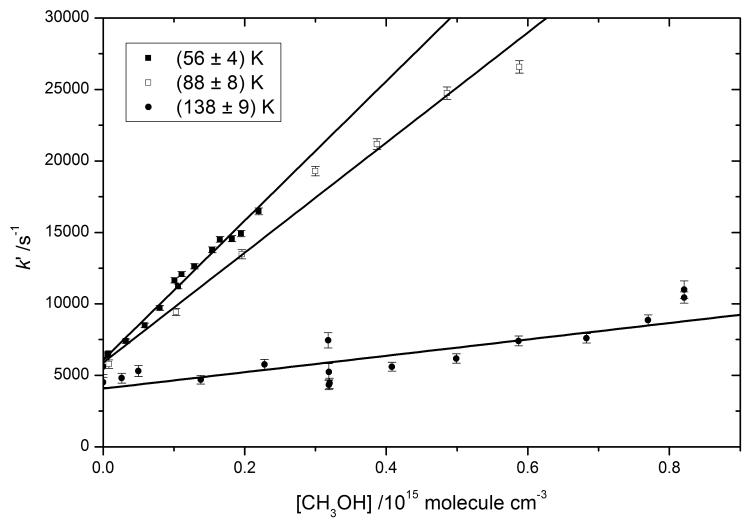
Therefore, a plot of k′ vs. [CH3OH] results in a straight line with intercept equal to the overall loss rate k′loss and slope equal to the bimolecular rate constant, k1. Bimolecular plots for experiments at 123 K, 143 K and 163 K are displayed in Fig. 3.
Figure 3.
Bimolecular plots for 3 different temperatures from the PLP-LIF-Low temperature reactor experiments.
Clustering can reduce the amount of methanol available for reaction with OH. Since the degree of clustering is second-order with respect to [CH3OH], the methanol concentration available for reaction would be particularly overestimated when the methanol concentration is high, causing the bimolecular plots to “fall off”. Such curvature would lead to an underestimation of the rate constant from the slope of a linear fit. Therefore the accessible range of methanol concentration is limited to the range within which the bimolecular plot remains linear, as e.g. for the 123 K dataset, where data points at higher methanol exhibit curvature and therefore were excluded from the analysis. The high precision of the 123 K measurements suggest that deviation from linearity could occur for [CH3OH] > 5 × 1014 molecule cm−3. Comparison to the Laval nozzle determination at 138 K also suggests that the flow tube results at 130 K and 123 K may fall slightly below the trend of the rest of the data points between 180 K and 88 K (see below), which could result from potential methanol overestimation of [CH3OH]. Therefore the flow tube measurements of k1 at these two temperatures may be considered as lower limits.
In Fig. 3 an increase in the slope with decreasing temperature can be unambiguously discerned. The steep increase in reactivity observed between 180 K and 123 K confirms the behaviour of k1 expected in this temperature range from the large rate constant reported at 82 K 12 and the almost 2 orders of magnitude smaller rate constant at 210 K.6 The results obtained for the 7 temperatures considered are summarised in Table 1. Quoted errors are at 95% level of confidence. A breakdown of error contributions is shown in Table 2. As expected, in most cases the major contribution to uncertainty arises from the measurement of the methanol concentration, and in particular from the small signal SB detected by the mass spectrometer during the low temperature kinetic measurements. For the 180 K measurement the major contribution originates from the weighted linear least-squares fit to the bimolecular plot, which is due to the low rate constant and the larger scatter of loss rates, possibly due to the larger range of temperature variability. The 202 K rate constant is also rather small, but the temperature was controlled within only ±2 K.
Table 2.
Breakdown of the uncertainty (in %) reported for the PLP-LIF-Low temperature reactor rate constants.
| T/K | Δ[(a + b ×SA) ×T/TA] a | Δ[SC/SB] b | Δ fit bimolecular plotc | Δ total(1σ) |
|---|---|---|---|---|
| 123 | 5.4 | 18.5 | 14.6 | 24.2 |
| 130 | 5.4 | 25.6 | 8.5 | 27.5 |
| 143 | 5.4 | 18.5 | 5.5 | 20.0 |
| 163 | 5.4 | 18.5 | 6.5 | 20.4 |
| 180 | 5.4 | 7.2 | 27.3 | 28.7 |
| 202 | 5.4 | 13.8 | 6.6 | 16.2 |
A second set of experiments was carried out in an attempt to determine the CH3O yield from R1, by detection of CH3O fluorescence. The experiments were done in the following order: He/O3 ([O3] ~ 2 × 1014 molecule cm−3), He/O3/CH3OH ([CH3OH] ~ 1×1015 molecule cm−3) and He/O3/CH3OH/H2 ([H2] ~ 1×1016 molecule cm−3). Addition of H2 was used to suppress R3 and thus make sure that all CH3O in the system is generated from R1a. The LIF traces obtained in the He/O3 experiment were used as the background and then subtracted from the CH3O traces obtained in the He/O3/CH3OH and He/O3/CH3OH/H2 experiments. The most striking observation in these experiments is the dramatic appearance of a very intense emission between 360 and 440 nm (i.e. a signal independent of the probe laser) for T < 180 K, which coincides with the turnaround in k1. At T > 180 K there was little or no emission, and when the probe laser was present, the CH3O radical was readily observed by LIF in the absence of H2. CH3O was observed via LIF down to about 180 K, and it was always lost when H2 was added to the system, which confirms a very small branching ratio of R1a above the turnaround temperature. Below 180 K the LIF signal was completely overwhelmed by the much larger emission signal. At 163 K the emission from the He/O3/CH3OH system was larger than from He/O3/CH3OH/H2, but at lower temperatures the emission from He/O3/CH3OH/H2 became dominant. In order to qualitatively explain these observations, we introduce the following two reactions:
| (R10) |
| (R11) |
The 1A″ electronic state of formaldehyde (CH2O) occurs at 28188 cm−1 (337.7 kJ mol−1) above the 1A1 ground state, and is therefore accessible from both R10 and R11. The transition gives rise to the well known 350 nm band system of formaldehyde,23-25 which is a likely explanation for the chemiluminescence observed. This mechanism can only account for the emission if the ratio of [H] and [OH] to [CH3OH] is high enough and k10 and k11 are fast. Rate constants for these two reactions (at room temperature) have been reported to be k10 = 3.3 × 10−11 cm3 molecule−1 s−1 26-27 and k11 = 3 × 10−11 cm3 molecule−1 s−1, respectively.27 The estimated O1D initial concentration from O3 photolysis is ~3 × 1013 atom cm−3, therefore k′10 ~ k′11 ~ 1000 s−1, which yields a growth time constant within the time scale of our observations (< 2 ms). Another requirement is that R3 has a small CH3O yield, since if this were not the case then emission following R11 would be observed for O3/CH3OH at all temperatures. Even though we have not quantified the quantum yield for CH3O from R3, a semi-quantitative comparison to the LIF signal obtained from R3 and from 248 nm photolysis of CH3ONO 12 indicates that CH3O generation via R3a is very minor.
In principle this appears to be in contradiction with the branching ratio of 0.82 for R3a reported by Matsumi et al.,20 based on their own measured H/D isotopic ratios and the OH/OD ratios measured by Goldstein and Wiesenfeld.28 However, CH3O formed from R3a has a large energy excess resulting from the exothermicity of this channel (ΔH°298= −180.9 kJ mol−1), and should fall apart quickly:
| (R12) |
Note also that although the reaction:
| (R13) |
where CH2OH is a possible product from R1, is also exothermic enough to make CH2O(1A″) and estimated to be fast 29 (k13 = 4×10−11 cm3 molecule−1 s−1), it cannot compete with:
| (R14) |
with k13 = 9.6 × 10−12 cm3 molecule−1 s−1 30 and [O2]~ 1014 molecule cm−3. The analogous reaction of CH3O with O2 is slower by 3 orders of magnitude at room temperature.27
The decay of the chemiluminescence signal (inset in Fig. 5), which is proportional to the concentration of CH2O(1A″), is mostly determined by R1 and R10-R11 and the short lifetime of the excited state (k′ ~ 107 s−1).24 A numerical model including reactions R1-6 and R10-14 was used to confirm qualitatively the onset of the rapid increase in k1 and the chemiluminescence at temperatures below 180 K. In this model, the temperature dependence of the relevant rate constants is considered, including the rate constants measured in this work. The temperature-dependent branching ratios of the title reaction calculated by Shannon et al.12 from RRKM theory are used and it is assumed that the effective quantum yield for CH3O production from R3 is very small. The model is numerically integrated using a standard ODE integrator starting from known initial concentrations of OH and CH3OH, to generate concentration vs. time curves of the different species. As shown in Fig. 5, there is an onset of the integrated (2 ms) emission intensity with temperature that coincides with the dramatic increase in k1. However, attempting to quantify the kinetics of the chemiluminescence decay by fitting to simulated curves is not possible given the limited range of data available and the large number of free parameters. Also the fact that the early times of the chemiluminescence traces are lost due to overloading of the PMT from the scattered excimer radiation renders an assignment of growth kinetics from these traces impossible.
Figure 5.
Integrated chemiluminescence (over 2 ms) as a function of temperature. Black squares: Signal collected at (400 ± 40) nm in the low temperature reactor CH3O experiments (H2 present); open squares: model simulation, assuming a small yield of CH3O from R3 and the branching ratios for R1 calculated by Shannon et al.12
PLP-LIF-Laval Nozzle
The OH traces were fitted with an expression accounting for the reaction of OH with methanol and other loss processes of OH such as diffusion and reaction with the OH precursor (R9), as well as the early time relaxation of a rotationally excited distribution of OH formed via t-BuOOH photolysis (R7) which yielded an initial increase in the LIF signal in the level probed by the probe laser:
| (E4) |
where [OH]0 and [OH(N′)]0 are respectively the initial concentrations of ground state and rotationally hot OH formed from R7, k′rel = k8 [M] (M = N2, Ar) is the rate of collisional relaxation via R8 and k′ is the experimentally observed pseudo-first order loss rate, which is equal to:
| (E5) |
and encompasses the rates for all losses of OH including diffusion (k′diff), reaction with the OH precursor (R9) and reaction with methanol. A plot of k′ vs. [CH3OH] yields a straight line with a slope equal to the bimolecular rate constant, k1 (shown in Fig. 4 for the three temperatures studied, 56, 88 and 138 K), and an intercept equal to the total background loss rate k′loss. The results obtained for these three temperatures together with the corresponding uncertainties (95% confidence) are compiled in Table 1 together with the low temperature flow-reactor results.
Figure 4.
Bimolecular plots for 3 different temperatures from the PLP-LIF-Laval Nozzle experiments.
The rotational relaxation time (1/k′rel) is about 10 μs, which corresponds to a rate constant of ~ 10−11 cm−3 molecule−1 s−1 typical for rotational energy transfer processes. Since this initial growth of the OH signal is short compared to its subsequent decay (~200 μs), excluding it from the analysis and fitting a simple exponential decay through the kinetic traces yields essentially the same results for k1.
The typical time span of the flash photolysis experiments (~1 ms) is longer than for the Laval nozzle setup (~200 μs). Since clustering of methanol is kinetically controlled, this implies that the extent of clustering for a given temperature should be higher in the former. Only at the lowest temperature studied with the Laval nozzle system, 56 K, the range of CH3OH concentrations was clearly limited owing to the bimolecular plot becoming curved at high CH3OH concentrations. A hint of curvature can also be observed in the 88 K bimolecular plot, although closer inspection shows that the data points for [CH3OH] ~ 4 × 1014 and 5 × 1014 molecule cm−3 are well in line with the points for the lowest three concentrations, while the absolute deviations from the line of the [CH3OH] ~ 3×1014 and 6×1014 molecule cm−3 points are similar. This suggests that it is random scatter that is creating the apparent curvature. Excluding the highest methanol concentration point from the fit yields a rate constant only 8% larger than the value obtained with the full dataset. This falls well within the reported 2σ uncertainty interval.
4. Conclusions
Rate constants for the CH3OH+OH reaction in the previously unexplored temperature range between 82 and 210 K have been determined and found to be consistent with data in the literature below 82 K and above 210K. The combination of two independent techniques yields results which agree with one another, providing confidence in the pulsed Laval nozzle technique, which previously reported large rate constants at temperatures below 90 K.
This reaction shows an unusual behaviour, where the rate constant dramatically increases at low temperatures. This marked increase is observed below 180 K, which is matched by the onset of a chemiluminescent signal tentatively attributed to excited formaldehyde formed from the reaction between the methoxy radical and H and/or OH. This result, while qualitative, adds support to the previous observation of CH3O radical formation from this reaction at low temperatures.12 An additional measurement of the rate constant at 56 K indicates that below 90 K the rate constant does not increase as rapidly with decreasing temperature when compared to the 100 – 180 K temperature range. Further pulsed Laval measurements at molecular cloud temperatures (T ≤ 20 K) are required to avoid relying on very uncertain extrapolations in astrochemical models. However, clustering and wall losses of oxygenated reagent might limit the lowest temperature at which such experiments can be carried out.
The dramatic increase of the rate constant was modelled semi-quantitatively by Shannon et al.12 using statistical rate theory. An increased lifetime of the pre-reaction complex at low temperature followed by quantum mechanical tunnelling was invoked to explain the increased rate constant and switch to the CH3O product formed over the higher barrier. Full quantitative theoretical modelling of this reaction over the whole range of temperatures remains a real challenge for theory, and until then prevents reliable extrapolation to molecular cloud temperatures.
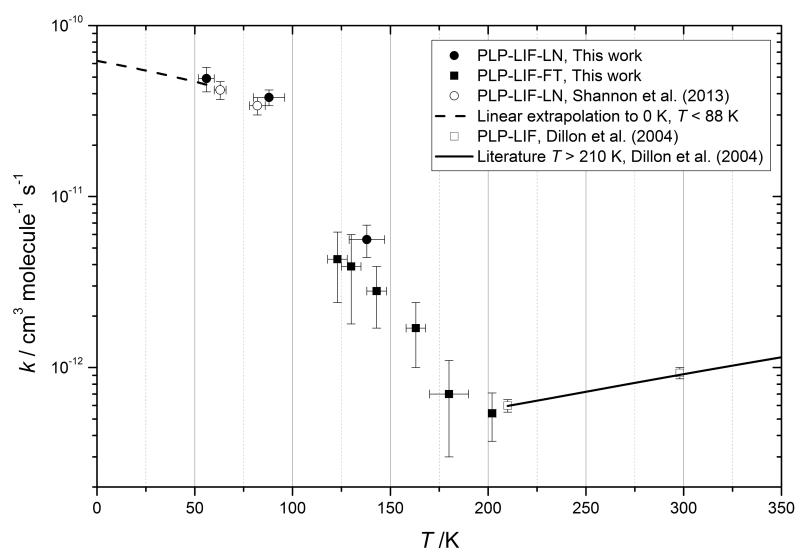
Figure 6.
Summary plot of the temperature dependent rate constants of CH3OH+OH reported in this work and in the literature. Uncertainty ranges are given a 95 % confidence. For reference, the dashed line shows a linear extrapolation towards 0K of the available Laval nozzle data below 88 K.
Acknowledgements
This study was supported by project number 291332 (CODITA – Cosmic Dust in the Terrestrial Atmosphere) from the European Research Council. R.L.C. wishes to acknowledge the Natural Environment Research Council for funding of a PhD studentship.
REFERENCES
- 1.Hess WP, Tully FP. Hydrogen-Atom Abstraction from Methanol by Hydroxyl Radical. J. Phys. Chem. 1989;93:1944–1947. [Google Scholar]
- 2.Jiménez E, Gilles MK, Ravishankara AR. Kinetics of the Reactions of the Hydroxyl Radical with CH3OH and C2H5OH Between 235 and 360 K. J. Photochem. Photobiol., A. 2003;157:237–245. [Google Scholar]
- 3.Wallington TJ, Kurylo MJ. The Gas Phase Reactions of Hydroxyl Radicals with a Series of Aliphatic Alcohols over the Temperature Range 240–440 K. Int. J. Chem. Kinet. 1987;19:1015–1023. [Google Scholar]
- 4.Meier U, Grotheer HH, Just T. Temperature Dependence and Branching Ratio of the CH3OH + OH Reaction. Chem. Phys. Lett. 1984;106:97–101. [Google Scholar]
- 5.Bott JF, Cohen N. A Shock Tube Study of the Reactions of the Hydroxyl Radical with Several Combustion Species. Int. J. Chem. Kinet. 1991;23:1075–1094. [Google Scholar]
- 6.Dillon TJ, Holscher D, Sivakumaran V, Horowitz A, Crowley JN. Kinetics of the Reactions of HO with Methanol (210-351 K) and with Ethanol (216-368 K) Phys. Chem. Chem. Phys. 2005;7:349–355. doi: 10.1039/b413961e. [DOI] [PubMed] [Google Scholar]
- 7.Sims IR. Low-temperature Reactions: Tunnelling in space. Nature Chem. 2013;5:734–736. doi: 10.1038/nchem.1736. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Lin MC. Theoretical Study on the Kinetics for OH Reactions with CH3OH and C2H5OH. Proc. Combust. Inst. 2007;31:159–166. [Google Scholar]
- 9.Gurvich LV, Veyts IV, Alcock CB. Thermodynamic Properties of Individual Substances. Fourth ed Hemisphere Pub. Co.; New York: 1989. [Google Scholar]
- 10.Ruscic B, Boggs JE, Burcat A, Császár AG, Demaison J, Janoschek R, Martin JML, Morton ML, Rossi MJ, Stanton JF, et al. IUPAC Critical Evaluation of Thermochemical Properties of Selected Radicals. Part I. J. Phys. Chem. Ref. Data. 2005;34:573–656. [Google Scholar]
- 11.Ruscic B, Pinzon RE, Morton ML, Srinivasan NK, Su M-C, Sutherland JW, Michael JV. Active Thermochemical Tables: Accurate Enthalpy of Formation of Hydroperoxyl Radical, HO2. J. Phys. Chem. A. 2006;110:6592–6601. doi: 10.1021/jp056311j. [DOI] [PubMed] [Google Scholar]
- 12.Shannon RJ, Blitz MA, Goddard A, Heard DE. Accelerated Chemistry in the Reaction Between the Hydroxyl Radical and Methanol at Interstellar Temperatures Facilitated by Tunnelling. Nature Chem. 2013;5:745–749. doi: 10.1038/nchem.1692. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SE, Goddard A, Blitz MA, Cleary PA, Heard DE. Pulsed Laval Nozzle Study of the Kinetics of OH with Unsaturated Hydrocarbons at Very Low Temperatures. Phys. Chem. Chem. Phys. 2008;10:422–437. doi: 10.1039/b711411g. [DOI] [PubMed] [Google Scholar]
- 14.Cernicharo J, Marcelino N, Roueff E, Gerin M, Jiménez-Escobar A, Caro GMM. Discovery of the Methoxy Radical, CH3O, toward B1: Dust Grain and Gas-phase Chemistry in Cold Dark Clouds. Astrophys. J. Lett. 2012;759:L43. [Google Scholar]
- 15.Weinreb S, Barrett AH, Meeks ML, Henry JC. Radio Observations of OH in the Interstellar Medium. Nature. 1963;200:829–831. [Google Scholar]
- 16.Harju J, Winnberg A, Wouterloot JGA. The distribution of OH in Taurus Molecular Cloud-1. Astron. Astrophys. 2000;353:1065–1073. [Google Scholar]
- 17.Herbst E. Atoms, Ions and Molecules: New Results in Spectral Line Astrophysics. In: Haschick AD, Ho PTP, editors. Astron. Soc. Pacif; 1991. pp. 313–322. ASP Conference Series Vol. 16. [Google Scholar]
- 18.Goodwin RD. Methanol Thermodynamic Properties From 176 to 673 K at Pressures to 700 Bar. J. Phys. Chem. Ref. Data. 1987;16:799–892. [Google Scholar]
- 19.Matsumi Y, Comes FJ, Hancock G, Hofzumahaus A, Hynes AJ, Kawasaki M, Ravishankara AR. Quantum Yields for Production of O(1D) in the Ultraviolet Photolysis of Ozone: Recommendation Based on Evaluation of Laboratory Data. J. Geophys. Res. [Atmos.] 2002;107 ACH 1-1-ACH 1-12. [Google Scholar]
- 20.Matsumi Y, Inagaki Y, Kawasaki M. Isotopic Branching Ratios and Translational Energy Release of H and D Atoms in the Reaction of O(1D) with CH3OD and CD3OH. J. Phys. Chem. 1994;98:3777–3781. [Google Scholar]
- 21.Shannon RJ, Taylor S, Goddard A, Blitz MA, Heard DE. Observation of a Large Negative Temperature Dependence for Rate Coefficients of Reactions of OH with Oxygenated Volatile Organic Compounds Studied at 86-112 K. Phys. Chem. Chem. Phys. 2010;12:13511–13514. doi: 10.1039/c0cp00918k. [DOI] [PubMed] [Google Scholar]
- 22.Shannon RJ, Caravan RL, Blitz MA, Heard DE. A Combined Experimental and Theoretical Study of Reactions Between the Hydroxyl Radical and Oxygenated Hydrocarbons Relevant to Astrochemical Environments. Phys. Chem. Chem. Phys. 2014;16:3466–3478. doi: 10.1039/c3cp54664k. [DOI] [PubMed] [Google Scholar]
- 23.Job VA, Sethuraman V, Innes KK. The 3500 A1A2 - X1A1 Transition of Formaldehyde-h2, d2, and hd: Vibrational and Rotational analyses. J. Mol. Spectrosc. 1969;30:365–426. [Google Scholar]
- 24.Sheinson RS, Williams FW. Chemiluminescence Spectra from Cool and Blue flames: Electronically Excited Formaldehyde. Combust. Flame. 1973;21:221–230. [Google Scholar]
- 25.Henderson JR, Muramoto M. 3546 Å System of Formaldehyde. J. Chem. Phys. 1965;43:1215–1219. [Google Scholar]
- 26.Hoyermann K, Loftfield NS, Sievert R, Wagner HG. Mechanisms and Rates of the Reactions of CH3O and CH2OH Radicals with H Atoms. Symp. (Int.) Combust., [Proc.] 1981;18:831–842. [Google Scholar]
- 27.Tsang W, Hampson RF. Chemical Kinetic Data Base for Combustion Chemistry. Part I. Methane and Related Compounds. J. Phys. Chem. Ref. Data. 1986;15:1087–1279. [Google Scholar]
- 28.Goldstein N, Wiesenfeld JR. Dynamics of O(1D2) Reactions with Bifunctional Substrates: Alcohols and Thiols. J. Chem. Phys. 1983;78:6725–6731. [Google Scholar]
- 29.Tsang W. Chemical Kinetic Data Base for Combustion Chemistry. Part 2. Methanol. J. Phys. Chem. Ref. Data. 1987;16:471–508. [Google Scholar]
- 30.Radford HE. The Fast Reaction of CH2OH with O2. Chem. Phys. Lett. 1980;71:195–197. [Google Scholar]