Abstract
Patients with the autoimmune polyendocrine syndrome type I (APS-I), caused by mutations in the autoimmune regulator (AIRE) gene, and myasthenia gravis (MG) with thymoma, show intriguing but unexplained parallels. They include uncommon manifestations like autoimmune adrenal insufficiency (AI), hypoparathyroidism (HP), and chronic mucocutaneous candidiasis (CMC) plus autoantibodies neutralizing IL-17, IL-22 and type I interferons. Thymopoiesis in the absence of AIRE is implicated in both syndromes. To test whether these parallels extend further, we screened 247 patients with MG and/or thymoma for clinical features and organ-specific autoantibodies characteristic of APS-I patients, and assayed 26 thymoma samples for transcripts for AIRE and 16 peripheral tissue-specific autoantigens (TSAgs) by quantitative PCR. We found APS-I-typical autoantibodies and clinical manifestations, including CMC, AI and asplenia, respectively in 49/121 (40%) and 10/121 (8%) thymoma patients, but clinical features seldom co-occurred with the corresponding autoantibodies. Both were rare in other MG subgroups (N=126). In 38 APS-I patients, by contrast, we observed neither autoantibodies against muscle antigens nor any neuromuscular disorders. Whereas relative transcript levels for AIRE and 7 of 16 TSAgs showed the expected under-expression in thymomas, levels were increased for 4 of the 5 TSAgs most frequently targeted by these patients’ autoAbs. Hence the clinical and serologic parallels to APS-I in patients with thymomas are not explained purely by deficient TSAg transcription in these aberrant AIRE-deficient tumors. We therefore propose additional explanations for the unusual autoimmune biases they provoke. Thymoma patients should be monitored for potentially life-threatening APS-I manifestations such as AI and HP.
Keywords: thymoma; myasthenia gravis; central tolerance; autoimmune polyendocrine syndrome type I; APECED; autoantibodies, type I interferon
Introduction
Much is being learnt about pathogenetic pathways from two human autoimmune syndromes and from the unexpected parallels between them. In autoimmune polyendocrine syndrome type I (APS-I), the autoimmunity against endocrine and ectodermal targets results from recessive mutations in the Autoimmune Regulator (AIRE) gene (1, 2). Expressed mainly in medullary thymic epithelial cells (mTECs), wild-type AIRE is one factor that normally ensures that mTECs ‘promiscuously’ express peripheral tissue-specific autoantigens (TSAgs) that then induce self-tolerance in thymocytes maturing nearby (3-5). According to current hypotheses, potentially autoreactive T-cells escape this negative selection in AIRE-deficient thymi, emigrate and cause autoimmune damage to target tissues (3, 4).
Starting in infants or young children, the typical diagnostic triad of APS-I comprises hypoparathyroidism (HP), autoimmune adrenal insufficiency (AI) and chronic mucocutaneous candidiasis (CMC). Many patients develop other autoimmune manifestations, e.g. premature ovarian insufficiency (POI), vitiligo, alopecia, autoimmune hepatitis, keratitis, enamel dysplasia, and/or intestinal malabsorption (Table I)(4, 5). Phenotypes vary widely, even within families; some patients are first recognized in adulthood (4, 5).
Table I.
APS-I-like manifestations given as number (%) in the different patient groups2.
| Manifestation | APS-I n=38 | Thymoma | LOMG n=63 | EOMG n=55 | |
|---|---|---|---|---|---|
| with MG n=114 | no MG n=7 | ||||
| Any APS-I-like manifestation | 38 (100) | 9 (8) | 1 (14) | 2 (3) | 0 (0) |
| Hypoparathyroidism | 26 (68) | ||||
| Addison’s disease | 25 (66) | 1 (14) | |||
| Gonadal failure | 8 (21) | ||||
| Autoimmune thyroiditis | 5 (13) | 2 (2) | 2 (3) | ||
| Type I diabetes | 3 (8) | 1 (1) | |||
| Alopecia | 12 (32) | 5 (4) | |||
| Vitiligo | 6 (16) | 2 (2) | 1 (2) | ||
| Nail pitting | 5 (13) | 1 (1) | |||
| Keratitis | 3 (8) | ||||
| Enamel dysplasia | 12 (32) | ||||
| Malabsorption | 8 (21) | ||||
| Autoimmune hepatitis | 3 (8) | ||||
| Pernicious anemia | 2 (3) | ||||
| Dry eyes/mouth | 2 (5) | 1 (2) | |||
| Squamous carcinoma | 1 (3) | ||||
| Chronic mucocutaneous candidiasis | 28 (74) | 3 (3) | 1 (2) | ||
| Hypogammaglobulinemia | 1 (14) | ||||
| Pure red cell aplasia | 2 (2) | ||||
| Asplenia | 2 (5) | 1 (1) | |||
See Tables II and Supplemental Table III for more details on the patients.
Numerous autoantibodies (autoAbs) recognize organ-specific autoantigens (autoAgs)(6-10), and often correlate with the clinical manifestations in APS-I patients. For example, autoAbs to NACHT leucine-rich-repeat protein 5 (NALP-5) correlate with HP (6), 21-hydroxylase (21OH) with AI (10) and side-chain cleavage enzyme (SCC) with POI (9). Remarkably, at diagnosis almost 100% of these patients have autoAbs neutralizing type I interferons (IFNs), especially IFN-α2, IFN-α8 and the related IFN-ω (11-13). Moreover, autoAbs against the Th17-mediators IL-17A, IL-17F and/ or IL-22, involved in mucous membrane defenses against Candida albicans, are prevalent (14, 15) and correlate with the CMC (14).
There are intriguing parallels and differences in the autoimmune associations with thymic epithelial neoplasms. Overall, myasthenia gravis (MG) occurs in ≥30% of all patients with thymomas, especially of the thymopoietic types B2 and B3, plus characteristic autoAbs against the acetylcholine receptor (AChR), titin and other muscle antigens (16-19). Other autoimmune features in thymoma patients (with or without MG) are also sharply focused, especially on hemopoietic targets (in ~5%), causing various bone marrow aplasias (20-23) and possibly hypogammaglobulinemia. At diagnosis, ~70% have autoAbs neutralizing type I IFNs (24, 25), similar to those in APS-I, and again with an IgG4 bias (26). Occasional thymoma patients have been reported with autoimmune endocrine (27-29) or ectodermal (30) manifestations, or even CMC plus autoAbs neutralizing IL-17 and IL-22/ deficiencies in Th17 and Th22 cells (14), very similar to those observed in APS-I (4, 5, 14). Whereas autoAbs against AChRs are clearly pathogenic in MG, the organ-specific Abs in APS-I are useful diagnostic markers, and typically directed against intracellular tissue specific autoAgs (TSAgs), as are those against titin and ryanodine receptor in many late-onset MG (LOMG; onset after age 45) and most thymoma patients (16-19).
The most obvious link between these disparate syndromes is that, in nearly all thymomas, the neoplastic TECs fail to express AIRE detectably, implying reduced expression of TSAgs like AChR, insulin and GAD65 (31, 32). The currently prevailing hypothesis is that AIRE-deficient thymi or thymomas fail to express target autoAgs, and consequently generate and export non-tolerant T-cells that eventually cause autoimmune damage in the periphery (3). An alternative explanation suggests biased selection or even active autoimmunization against certain common targets in aberrant thymic tissue (33). To distinguish between these hypotheses, we assessed whether the clinical and serologic similarities between APS-I and thymoma patients extend to APS-I-typical organ-specific autoAbs, and whether these correlate with under-expression of AIRE and TSAg transcripts in their thymomas. These tumors must hold clues to autoimmunizing mechanisms, which are otherwise very hard to study in humans.
Materials and methods
Patients
All samples were taken with informed consent and Ethics Committee approval in each referral center (Bergen, London, Oxford, and Tartu). The demographics of the patients and controls are shown in Supplemental Table I. Over 200 of the 247 patients with MG and/ or thymomas were seen (and many followed for long periods) by a single UK neurology team (34); all clinical information was from hospital and autopsy records. Another 43 were assessed similarly in Bergen. They are grouped into those with:- thymoma (with or without MG), LOMG, early-onset MG (EOMG; onset before age 45) and ocular MG. The 38 APS-I patients were from the Norwegian panel detailed previously (35).
Thymus and thymoma samples
Thymoma samples were snap-frozen as blocks from 26 patients and stored at −80 °C until use (34). Nearly all thymomas were encapsulated and could be clearly separated from any adjacent thymic remnants (n=5), which were often minimal or absent in older or steroid pre-treated cases. At least one remnant showed follicular hyperplasia, and so did ≥38 of the 48 EOMG thymi removed. Most MG thymomas resemble disorganized infant thymic cortex (17, 34), so pediatric thymi seem the most suitable and available controls.
Assays for autoAbs
All samples were tested in the same lab with radioligand binding assays against in vitro transcribed and translated proteins (Promega, Fitchburg, WI) for autoantibodies against 21OH, 17-hydroxylase (17OH), SCC, GAD65, tryptophan hydroxylase 1 (TPH-1), aromatic L-amino acid decarboxylase (AADC), tyrosine hydroxylase (TH), and NALP-5 (7, 13). The threshold for positivity was set as the mean of the indices for all healthy blood donors tested (n=57-150) + 3SD. We assayed autoAbs against thyroid peroxidase (TPO) with Immulite 2000 solid-phase chemiluminescence immunoassays (FDA Clears Siemens, Malvern, PA), against titin by ELISA (DLD diagnostika GmbH, Hamburg, Germany)), and against AChR by RIA (IBL International, Hamburg, Germany)(18). Anti-TH and anti-TPO autoAbs were only analyzed in patients from whom we had thymoma samples.
AIRE mutations
Both AIRE alleles were sequenced using standard protocols and primers as described elsewhere (35).
RNA extraction from thymomas and real-time PCR
Tissue samples were homogenized in Trizol (Thermo Scientific, Waltham, MA ) using AutoMACS with M-tubes (Miltenyi Biotech, Bergisch Gladbach, Germany), followed by RNA extraction according to the manufacturer’s protocol. RNA concentrations were measured with NanoDrop (Thermo Scientific, Waltham, MA ); 5 μg of total RNA was reverse-transcribed using Superscript III (Invitrogen), 10mM dNTP Mix, RiboLock RNase inhibitor and random hexamers (Thermo Scientific, Waltham, MA ). Real time quantitative PCR (qPCR) was performed using Applied Biosystems® ViiA™ 7 Real-Time PCR System with 384-Well Block (Life Technologies) and Maxima SYBR Green /ROX qPCR Master Mix (Thermo Scientific, Waltham, MA). Every sample was run in 3 parallel reactions in two separate series of experiments; their results were broadly consistent and have been combined. We detected reliable signals for all transcripts tested except NALP-5.
Every transcript signal was expressed as 2−ΔΔCt (where Ct represents the threshold cycle), and normalized relative to the value for β-actin in the same sample, and then to its (β-actin-normalized) KRT8 value. For Table V and Figs 2 and 3, the resulting AIRE or TSAg values were next expressed relative to that in one control infant thymus. Primers are listed in Supplemental Table II.
Table V.
Tissue-specific autoantigen transcript values in paired thymoma ÷ thymic remnant 6
| Gene | Patient no. | ||||
|---|---|---|---|---|---|
| P24 non-MG | P17 | P25 | P26 | P15 | |
| AIRE | 0.02 | 0.04 | 0.01 | 0.02 | 0.00 |
| 21OH | 4.87 | 0.11 | 0.38 | 0.22 | 13.90 |
| 17OH | 7.87 | 0.11 | 0.26 | 0.88 | 1.17 |
| SCC | 0.22 | 0.32 | 0.49 | 1.44 | 0.12 |
| AADC | 0.00 | 1.40 | 0.00 | 0.04 | |
| TPH-1 | 0.40 | 0.15 | 0.19 | 0.11 | |
| HDC | 0.02 | 0.01 | 0.04 | 0.37 | 0.65 |
| TG | 0.58 | 0.11 | 0.14 | 0.46 | |
| TPO | 0.01 | 0.32 | 0.02 | 1.55 | 0.03 |
| GAD65 | 0.01 | 0.06 | 0.00 | 38.30 | |
| INS | 0.31 | 0.19 | 0.02 | 2.31 | 0.07 |
| IA-2 | 0.16 | 0.10 | 1.54 | 2.71 | 2.63 |
| TDRD6 | 0.07 | 0.06 | 0.05 | 0.17 | 0.37 |
| H/K ATPase | 0.01 | 0.05 | 0.01 | 0.04 | |
| SOX9 | 1.51 | ||||
| AChR-α | 0.00 | 0.35 | 3.49 | ||
Each number is the TSAg value in the patient’s thymoma block divided by the value in the autologous thymic remnant.
Red: transcript values >4.00 (>4x higher in thymomas than in thymic remnants)
Green: transcript values <0.25 (>4x lower in thymomas than in thymic remnants)
Yellow: intermediate transcript values (from 0.26 to 4.00).
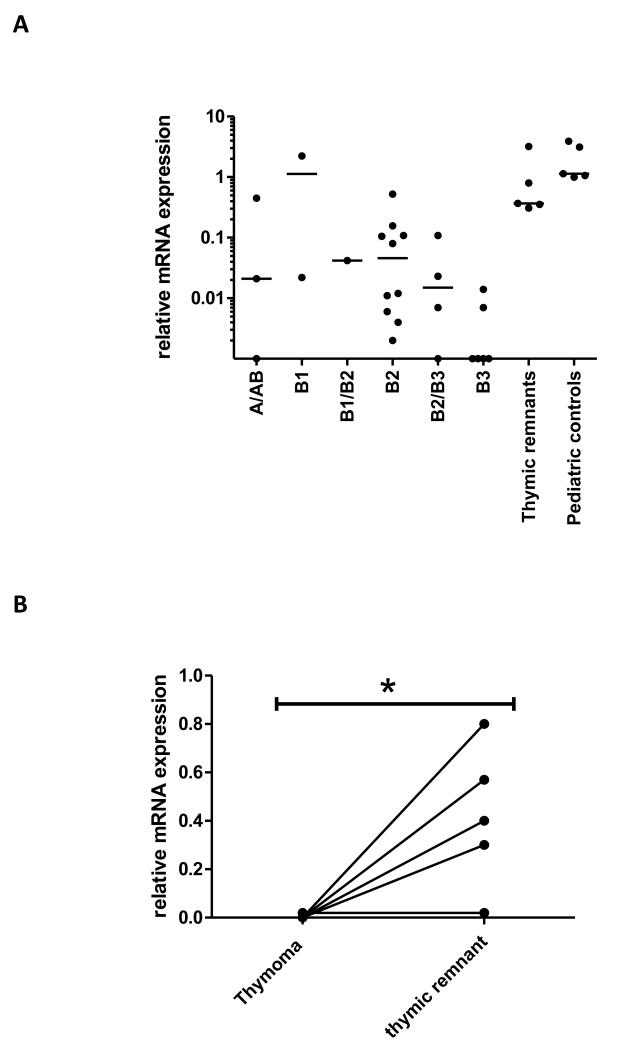
FIGURE 2.
Relative AIRE expression in thymoma types and adjacent thymic remnants. A. AIRE expression in blocks from different WHO thymoma types using Keratin-8 (KRT-8) to adjust for epithelial cell content, and a pediatric control sample as calibrator. B. AIRE expression in paired thymomas/ thymic remnants for 5 informative patients. Expression was calculated as for A. * p < 0.05.
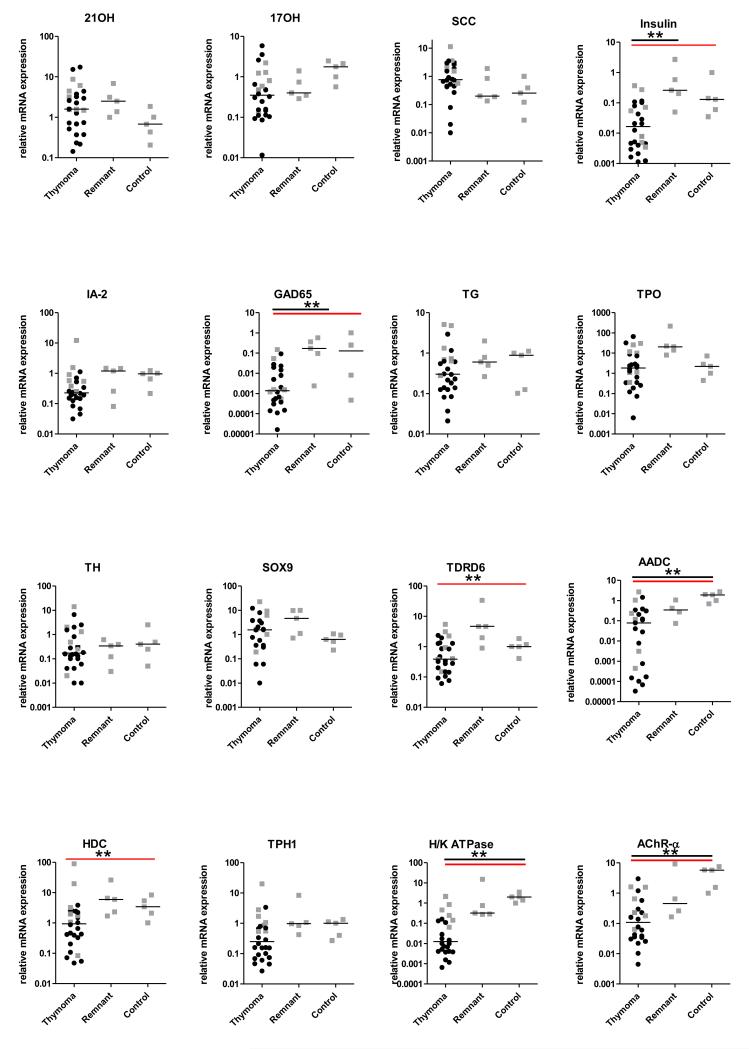
FIGURE 3.
Relative transcript signals for APS-I target autoAgs (normalized to KRT-8) and shown as fold change compared to one pediatric control sample. The bars for each group represent the medians; gray squares and black circles, samples with AIRE expression >0.1 or <0.1, respectively. Black horizontal lines above indicate where the thymomas differed significantly from remnants or from control groups (one-way ANOVA (Kruskal-Wallis) with Dunn’s multiple Comparison correction), and the red horizontal lines where they differed significantly from the combined remnants plus controls (Mann-Whitney U tests. ** p < 0.01
Statistics
We evaluated differences in autoAb prevalences between patients and controls using Pearson χ2 tests/ program SPSS v. 15, and in transcript values between different groups using non-parametric one-way ANOVA (Kruskal-Wallis) with Dunn’s multiple comparison tests and Mann-Whitney U tests (Fig 3) (GraphPad Prism, La Jolla, CA). For TSAg transcript values, the threshold for significance was set at p= 0.01. Differences between thymoma and thymus remnant expression of AIRE were evaluated using paired t-tests. We also calculated z-scores to show the number of SDs by which each thymoma TSAg signal differed from the corresponding mean of the 5 infant control thymi, whether above it (positive z-scores) or below (with – signs).
Results
APS-I-typical clinical manifestations in patients with MG and/or thymomas
Among the 121 patients with thymomas (with or without MG), 15 have APS-I-typical manifestations, including AI in non-MG patient P2 (Tables I and II). Several of these disorders occurred together, even including asplenia or nail dystrophy (in patients P1 or P3). Both are unusual in autoimmune patients like these who had no genomic AIRE mutations (asterisked in Table II and Supplemental Table III). So is the CMC (plus IL-22 autoAbs) that severely afflicted P8-10. Many of the APS-I-typical manifestations presented long after the thymomas, for example, in 6 of 29 UK cases with intervals >5 years (21%) versus only 5/70 (7%) of patients with shorter intervals (p = 0.051).
Table II.
Thymoma and MG patients with both APS-I-like clinical features and autoantibodies 3
| Patient (sex, MG onset-age) | MG status | Thymoma:- | Clinical features (age at onset or †death) | APS-I-type autoAbs | Other autoAbs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO Type1 | duration2 | 21OH | 17OH | NALP5 | SCC | GAD65 | TPH-1 | AADC | IFN-αs | IL-17/22 | ||||
| P 1 (F, 40)* | MG | B2, invasive | ≥8yr | Urt (42), T (48), As (50), V (56), SLE (59), NMT (56) | ++ | ++ | ++ | − | IL-12, ANA | |||||
| P 2 (F) | Not MG | B2 (50) | ≥14yr | Hypo-γ (35), TC (41), AI, urticaria, TLP (50) | ++ | − | nd | |||||||
| P 3 (M, 46)* | MG | “malignant” recurred (56) | ≥4yr ≥23yr |
T1D (43), T, Alo (50), V, Nail dystr3, SPS (65)(†65) | + | + | ++ | ++ | ++ | nd | IL-12 | |||
| P 4 (F, 41)* | MG | B2 | Alo (38) | ++ | ++ | − | IL-12 | |||||||
| P 5 (M, 28) | MG | B1 | ~3yr | Alo (5 and 31), myocardial infarct (†42) | ++ | ++ | ++ | − | IL-12 | |||||
| P 6 (F, 50) | MG | B1 | Alo (51) | + | ++ | nd | IL-12 | |||||||
| P 7 (F, 64) | MG | B3 | Alo (45), RBC aplasia (74) | + | + | − | nd | IL-12± | ||||||
| P 8 (F, 46)* | MG | B3 | ≥12yr | CMC (58) (†73) | + | ++ | ++ | + | ++ | ++ | IL-12 | |||
| P 9 (M, 27)* | MG | B2; metastatic | ≥16yr | CMC (44), NMT (50) (†59) | ++ | ++ | ++ | |||||||
| P 10 (F, 35) | MG | B2/ B3 | ~9.5yr | CMC (44) (†47) | ++ | ++ | IL-12 ± | |||||||
|
| ||||||||||||||
| P 11 (M, 74)* | LOMG | None found4 | V (60), PA (70), CMC, T, dry eyes, pulm fibr (74)4 | ++ | ++ | thyroid ++ ANA | ||||||||
| P 12 (F, 53) | LOMG | None found | PA (48), T (52), strong family history of T | ++ | nd | IL-12 | ||||||||
Common APS-I manifestations are marked in bold.
No genomic AIRE-mutations;
WHO Classification;
delay between first signs of thymoma and of APS-I-type features;
noted at autopsy;
lost to follow-up at age 74;
AutoAb positive but index <200;
AutoAb index >200
Abbreviations:- AI, adrenal insufficiency; Alo, alopecia; ANA, anti-nuclear antibodies, As, asplenia; CMC, chronic mucocutaneous candidiasis; hypo-γ, hypogamma-globulinemia; IFNs, autoantibodies to type 1 interferons; Nail dystr, nail dystrophy; NMT; neuromyotonia; PA, pernicious anemia; pulm.fibr, pulmonary fibrosis; RBC aplasia, red blood cell aplasia; SLE, systemic lupus erythematosus; SPS, stiff-man syndrome; T, thyroid autoimmunity; T1D, type 1 diabetes mellitus; Th17, autoantibodies to IL-17A, IL-17F or IL-22; TLP, tongue lichen planus; Urt, Urticaria; V, vitiligo.
Of the 121 MG patients without thymomas at diagnosis, only one with LOMG (P11) showed clear APS-I-typical features, while another (P12) had pernicious anemia and thyroid disease, and a strong family history of thyroid autoimmunity (Table II).
Although this study is retrospective, all patients were assessed similarly and thoroughly by the same experts in each referral center. Thus the EOMG and LOMG groups are ideally matched ‘disease controls’ for the thymoma patients – for their geographic origins as well as the assessors.
APS-I-typical organ-specific autoAbs in patients with MG and/or thymomas
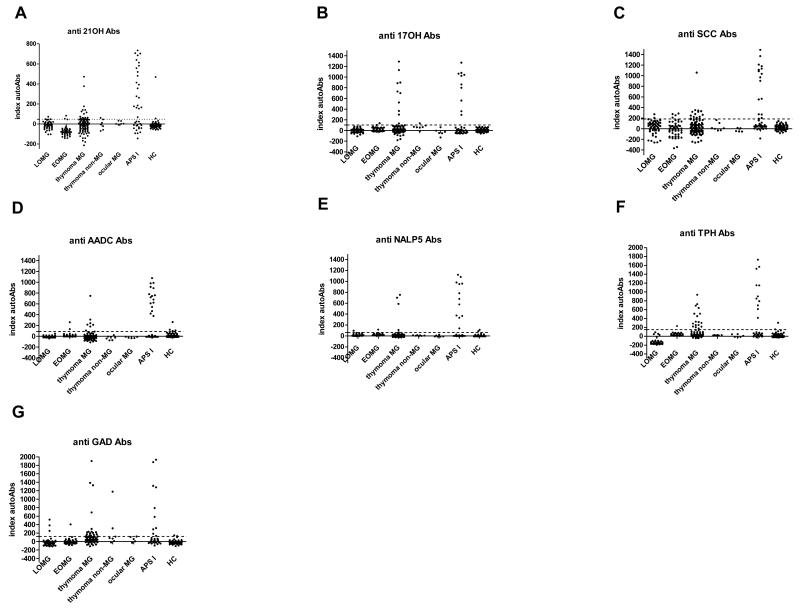
In the thymoma patients – with or without MG – we found significantly higher prevalences of autoAbs against 21OH, 17OH, SCC, TPH-1 and GAD65 than in either the healthy controls or the other MG subgroups (Fig 1, Table III); against TPH-1 and GAD65, prevalences were approximately half of those found in the APS-I patients. Many of the binding signals were in the same range as in APS-I (Fig 1), as also against AADC and NALP-5 in occasional cases. AutoAbs against AADC, TPH and NALP-5 were considered APS-I-specific (7).
FIGURE 1.
APS-I-related autoantibodies in MG subgroups, APS-I patients and controls against the indicated autoAgs. Thresholds for positivity are marked by horizontal lines.
Table III.
Prevalences (%) of APS-I-type organ-specific autoantibodies in the different cohorts 4 a.
| Autoantibody (Ab) | APS-I | Thymoma ± MG | LOMG | EOMG | Healthy controls | AI/APS-IIb |
|---|---|---|---|---|---|---|
| 21OH | 55** | 13* | 1.6 | 3.6 | 3.5 | 86 |
| 17OH | 24** | 8.8* | 0 | 1.8 | 0 | 11 |
| SCC | 40** | 11** | 3.2* | 7.3* | 0 | 6.5 |
| AADC | 41** | 6.2 | 0 | 3.6 | 4.4 | 2.2 |
| NALP-5 | 35** | 3.5 | 1.8 | 1.8 | 1.6 | NA |
| TPH-1 | 28** | 14** | 0 | 1.8 | 0 | NA |
| GAD65 | 29** | 15* | 4.8 | 1.8 | 3.4 | 21 |
↓Numbers of sera assayed for the various autoAbs varied slightly; we tested sera from ≥37 patients with APS-I, ≥113 with thymomas ± MG, ≥56 with LOMG, and ≥55 with EOMG, and ≥57 controls. We tried to test the earliest bleeds from each patient, but many with thymomas were already taking immunosuppressive drugs for their MG.
Data from 426 Norwegian AI/APS-II patients fully characterized by MM Erichsen et al (1). APS II is defined as AI plus thyroid disease and/or type I diabetes.
p < 0.05, patient group versus control;
p < 0.01, patient group versus control. NA, not analyzed.
1. Erichsen, M. M., K. Lovas, B. Skinningsrud, A. B. Wolff, D. E. Undlien, J. Svartberg, K. J. Fougner, T. J. Berg, J. Bollerslev, B. Mella, J. A. Carlson, H. Erlich, and E. S. Husebye. 2009. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab 94:4882-4890.
In total, 49/121 thymoma patients (40%) had ≥1 APS-I-related organ-specific autoAbs, and they tended to co-occur with each other (Table IV), though often not with the corresponding clinical manifestations (Table II). For example, the one patient with AI (P2) tested negative against 17OH or 21OH, but positive against GAD-65. She was one of the 7 thymoma patients without MG, of whom 2 others also had GAD65 autoAbs, and one gave low signals vs 17OH and 21OH. Notably, APS-I-type autoAbs were found in more patients with disease durations >5 years (13/25 (52%) than with more recent onset (20/65 (31%), although not quite significantly (p = 0.061).
Table IV.
Percentages of patients without and from 1 to ≥3 APS-I-type organ-specific autoantibodies 5
| Number of autoantibodies | APS-I n=38 | Thymoma | LOMG n= 63 | EOMG n= 55 | |
|---|---|---|---|---|---|
| with MG n=114 | no MG n=7 | ||||
| None | 8 | 57*** | 57 | 89*** | 82*** |
| 1 | 29 | 21 | 29 | 11 | 15 |
| 2 | 11 | 16 | 14 | 0 | 4 |
| ≥ 3 | 52 | 6 | 0 | 0 | 0 |
p < 0.0001 when comparing all thymoma patients (± MG) with MG patients without thymoma (EOMG+LOMG) using χ2 test.
p < 0.0001 when comparing all thymoma patients (± MG) with MG patients without thymoma (EOMG+LOMG) using χ2 test.
In the serial samples available from these patients, the autoAbs showed minor variations at different time-points (Supplemental Fig. 1); some of them might reflect changes in immuno-suppressive drug doses, though similar variations occur in APS-I where these drugs are used only rarely (35).The autoAbs in the thymoma patients showed no significant correlations with MG status or thymoma histology (where the proportions of thymocytes/TECs varied greatly). WHO typing was available for 71 MG thymomas; we found ≥1 APS-I-like autoAb in 2 of the 12 (17%) patients with Types A or AB versus 20 of the 56 (36%) with Types B2 and B3 (p = 0.20). Type B1 is the only subtype reported to express AIRE (in ~50%)(32). Among 4 such patients, P5 had high-index autoAbs against 21OH and 17OH (Table II), but, unfortunately, we did not have access to his thymoma.
In the LOMG, EOMG and ocular MG subgroups, by contrast, none of these autoAbs were more common than in the controls, even in P11 who had several APS-I-like manifestations (Table II). Since the above clinical and serologic associations thus appear to be with the thymomas rather than the MG, although we had only 7 non-MG thymoma patients, we group all the thymoma patients together from here on.
MG-specific autoAbs in APS-I patients
None of the APS-I patients had detectable autoAbs against either AChR (0/38 versus 114/114 of the MG/thymoma patients; p < 0.001) or titin (p < 0.001; not shown). Nor did any of them have any obvious MG-like neurologic features.
Transcript levels for AIRE and TSAgs in thymomas and adjacent thymic remnants
To test the hypothesis that these autoAbs correlate with decreased AIRE and TSAg expression, we assayed AIRE and 16 TSAg transcripts in the available thymoma, remnant or control thymus blocks. To adjust for content of any TEC subtype (36, 37), which varies greatly between thymomas (17, 34), we normalized the qPCR by the keratin-8 (KRT8) signals; these correlated strongly, but inversely, with the thymocyte content estimated when the tumors were first processed (34) (not shown). They were broadly consistent in the duplicate blocks available from 5 thymomas and one remnant, which have each therefore been averaged.
Relative AIRE transcript levels were low in almost all thymoma subtypes, but there were large individual differences (Fig 2A). Values were high in one of the two available type B1 thymomas, in line with previous reports (31, 32). As expected, AIRE expression was much lower in the thymomas than in the adjacent autologous thymic remnants in all 5 available pairs (one was non-MG; Fig 2B).
Data from these paired thymomas/thymic remnants also illustrate the variability between different patients and different TSAgs (Table V). TDRD6 and H+/K+ ATPase transcript values were lower in most of the thymomas than in the remnants, as expected. In contrast, the steroidogenic enzyme transcripts mostly showed similar or even higher values in the thymomas.
The 26 thymomas (including 2 non-MGs) showed the most striking variability in TSAg transcripts, even when AIRE expression was very low (black circles in Fig 3). Values for AADC, H+/K+ ATPase and AChR-α clearly followed AIRE’s expression pattern; highest in control thymi, intermediate in remnants, and low in thymomas (Fig 3). We also noted significantly lower values for insulin, HDC and TDRD6 in thymomas (p < 0.01; Kruskal-Wallis test), and for GAD65 when compared with the pooled control and remnant thymi (p < 0.01; Mann-Whitney test) (Fig 3).
Surprisingly, even when AIRE values were very low, we found higher TSAg transcript values per epithelial cell in many thymomas [numbers with z-scores >3 are shown in brackets] than in any of the control thymi (Fig 3) for:- 21OH [10], 17OH [1], TG [3], TPO [5], TH [1], HDC [2], TDRD6 [2], IA-2 [1], SOX9 [11] and TPH-1 [3] (Supplemental Table III). Thus, these TsAgs appear AIRE-independent, despite their frequent recognition by autoAbs in thymoma and APS-I patients (Fig 1, Table III). Transcript values showed no obvious correlations with MG status or thymoma histology.
Correlating APS-I type features and autoAbs with TSAg transcript values in thymomas
For manifestations that could be correlated with TSAg transcripts, there were only 6 informative patients (Supplemental Table III). In the two with alopecia (P6 and P7), TH transcript levels were similar or even higher than in control thymi (z-scores 13.4 and 0.8). In 3 of the 4 patients with autoimmune thyroid disease (P1, P13 and P14), it presented later than their MG and their thymomas. Interestingly, two of them gave elevated transcript values for TPO (z-scores 2.3 and 3.4), the target that correlates best with autoimmune thyroiditis, whereas values were low for both TPO and TG in P15, whose thyroiditis had presented several years earlier.
The autoAbs likewise failed to show consistent negative correlations with TSAg transcript values. In 14/26 patients, we detected a total of 31 autoAbs against informative targets (excluding AChR) (Supplemental Table III). Transcript values gave positive z-scores in 14 of these 31 instances (for 21OH, TPO and SOX9 they were >3 in >5 instances). Even though AIRE values overlapped the controls in P19 and P20, together they had autoAbs to 5 TSAgs, despite positive z-scores for 3 of these TSAgs (and AChR-α). Conversely, AIRE was very low in P21, but she had adrenal autoAbs despite z-scores >2.5 for 17OH and 21OH.
Overall, these findings provide no clear support for general TSAg under-expression in thymomas as the main cause of the associated autoimmunity.
Discussion
This wide-ranging study unexpectedly shows APS-I-typical organ-specific autoAbs in over 40% of thymoma patients, and APS-I-typical clinical manifestations in 8%, but often not in the same individuals. It confirms and extends an earlier observation of ‘thymocopying’ of APS-I development in a patient with a type B thymoma (27). Despite having no AIRE mutations, some thymoma patients developed major APS-I manifestations like AI, CMC or even asplenia, but at much lower frequencies than in APS-I. However, APS-I patients showed no clinical or serologic signs of MG. Surprisingly too, while some of the autoAgs targeted in thymoma patients showed the expected under-expression in their AIRE-deficient tumors, many did not – including several adrenocortical, gonadal and neuro-ectodermal targets, some of which are considered APSI-specific. Thus, our results highlight differences as well as similarities in pathogenesis in these two syndromes, and question current hypotheses that lack of AIRE-regulated TSAg expression in thymomas is the major cause of the unusually biased autoimmunity.
Clinical and serologic parallels implicating thymic aberrations in autoimmunization
The overlapping autoAb reactivities were largely confined to patients with thymomas rather than LOMG or EOMG, even though thymoma and LOMG patients are well known to share several other autoAbs, especially against internal muscle autoAgs (18), type I IFNs and/ or IL-12 (25). Thymoma patients without MG are very hard to collect; though their numbers here are too small to exclude differences in prevalences of APS-I-type autoAbs or manifestations definitively, the present results – and the contrasts with LOMG – suggest that the thymoma alone – rather than the associated MG – is responsible for this serological and clinical overlap. Thymic aberrations are further implicated by the type I IFN-, IL-17- and IL-22-neutralizing autoAbs – not only by their prevalence in both APS-I and thymoma patients but also by their rarity in numerous other autoimmune diseases, even where peripheral type I IFNs, dendritic or Th17/ Th22 cells are involved in pathogenesis (14-15, 25, 39). Conversely, most of the ‘standard autoAbs’ commonly found in sporadic and systemic autoimmune diseases are uncommon in thymoma patients (22, 32, 40, 41).
Thymic aberrations are also implicated in autoimmunization by our observations in patients with the 22q11.2 deletion/Di George Syndrome, who have primary thymus aplasia. About 15% have similar autoAbs against SCC, 17OH and 21OH, again with no signs of AI or POI (42, 43). Interestingly too, MG has been reported in rare patients with hypomorphic RAG1 mutations who also have disorganized AIRE-deficient thymi (44), and/ or IFN-α autoAbs (45), which again implicates aberrant AIRE-deficient thymopoiesis in autoimmunization in these disparate syndromes.
Clinical and immunologic differences between APS-I and thymoma patients
It is widely accepted that the autoimmune endocrine tissue damage in APS-I is T-cell-mediated (46). Whereas autoAbs against AChR are directly pathogenic in MG, those against intracellular targets are valuable as diagnostic markers. In APS-I, the autoAbs against steroidogenic enzymes correlate well with AI and POI, against TH with alopecia (47), and against-TPH-1 with malabsorption (7); curiously, GAD65 autoAbs correlate with malabsorption in APS-I instead of type 1 diabetes (7).
Although the APS-I-typical manifestations appear to be uncoupled from the autoAbs in our thymoma patients, both were probably under-estimated here for several clinical reasons. There are often delays of many years between detection of autoAbs and onset of the corresponding clinical feature in APS-I (35). Similarly, further manifestations appeared ≥15 years after initial presentation in several thymoma patients (eg, P1-P3, P8 and P9; Tables II and Supplemental Table SIII), but they might have been missed in many others where pre-thymomectomy follow-up was much shorter, as possibly in a previous study (where only 28 patients were tested for autoAbs (32)). Asplenia might well have escaped notice since it requires imaging. Moreover, the immunosuppressive therapy often used for their MG may have repressed autoimmune T-cell-mediated tissue destruction, and their glucocorticoid treatment might have compensated for unsuspected AI. There may also be genetic differences in responsiveness to certain TSAgs.
If there is some true uncoupling of autoimmunization of the B-cell and pathogenic T-cell responses in patients with thymomas, it might reflect several immunologic differences from APS-I. Normally, thymic AIRE plays its key roles when the T-cell repertoire is being established in the fetus and infant. Indeed, its deletion in mice after post-natal day 3 does not cause autoimmunity (48); nor does thymectomy, even in children. Whereas APS-I patients have genomic AIRE mutations from conception, the AIRE-deficiency arises only in the tumors (31, 32) and decades later in life in thymoma patients. Second, APS-I patients also lack functional AIRE expression in spleen and lymph nodes, where it could play important roles in maintaining peripheral tolerance (49, 50). Those checkpoints presumably remain intact in patients with thymomas, and may be sufficiently potent to restrain some of the potentially autoaggressive T-cells exported by these tumors, again making the observed parallels seem the more remarkable.
Moreover, most thymomas show additional aberrations not to be expected in APS-I thymi (which are not available for study). These might also contribute to loss of tolerance, as they include absence of HLA-class II on the neoplastic TECs and of muscle-like thymic myoid cells (17, 34). Interestingly, in both thymomas and Aire−/− mouse thymi, there are few (if any) Hassall’s corpuscles, around which AIRE+ TECs are normally frequent and FoxP3+ regulatory T cells (Tregs) are positively selected (17, 51-53). There are also changes in Tregs in both syndromes (52, 54-57).
These clinical factors, plus the late onset of AIRE-deficiency localized in aberrant thymoma microenvironments, probably contribute substantially to the apparent differences from APS-I.
TSAg transcripts in thymomas
Currently favored hypotheses assume that minimal or undetectable TSAg expression by the AIRE-deficient neoplastic TECs (31, 32) is the main cause of the associated autoimmunity. While it is much easier to test TSAgs for Aire-dependence in Aire−/− mouse TECs (3, 58) than in humans, their AIRE-deficient thymomas seem a practical alternative. Although we recognize that their TSAg transcript values alone may not fully reflect protein expression levels, other options were not available to us.
We also recognize that other biologic factors might have affected the AIRE and TSAg signals we detected. For example, thymoma TECs apparently derive from progenitors with combined cortical and medullary markers (34); the normal counterparts of these progenitors are rare, and have scarcely been studied. Since thymoma TECs appear clonal (59), AIRE and TSAg expression might vary between tumors, as they both do between individual mTECs (60, 61). They might vary within thymomas too, though that was not obvious in the available duplicate samples. Expression might also change as thymomas evolve over time/react to changing blood supply or hormonal influences (34), which might explain the correlations we noted with thymoma durations. Some of these issues might be clarified by testing single TEC from thymomas and pediatric thymi.
Nevertheless, some TSAgs were clearly under-expressed in thymomas, notably AChR-α, H+/K+ ATPase, AADC, insulin, GAD65, HDC and TDRD6. These data partially fit with previous reports of AIRE-dependent, but very variable, expression of insulin, AChR-α, IA-2 and H+/K+ ATPase (32, 33, 61, 62).
Notably, AChR-α transcript levels were high in some thymoma patients, implying that under-expression is not the sole cause of their MG. That also seems unlikely because none of our APS-I patients had MG or detectable autoAbs against AChR or titin. Other AChR subunits may be important targets too (63), and so may pre-existing peripheral tolerance to any of them.
In striking contrast, transcript values for 21OH, TPO and SOX9 were higher (with positive z-scores) in 40 - 65% of our thymomas than in the control thymi, even in tumors where AIRE transcripts were almost undetectable. Our data also suggest AIRE-independence of 17OH, SCC, TG, TH and TPH-1. Intriguingly, these include 4 of the 5 TSAgs most frequently recognized by autoAbs in the thymoma group (Fig.1; p < 0.05 in Table III). Likewise, AIRE-independence has been reported for TG, TPO and GAD67 in control TECs (61, 64), and for 17OH and 21OH in AIRE-negative thymomas (32) . Moreover, TG, steroidogenic enzymes and type I IFNs and Th17/Th22 cytokines are expressed by thymic cell types other than mTEC (14, 64, 65), and should therefore be available for negative selection even in the absence of AIRE, again suggesting that additional mechanisms are operating.
Towards a unifying hypothesis
Among APS-I-typical TSAgs, 21OH, 17OH, SCC and TPO stand out because of their apparent AIRE-independence and the significantly increased frequencies of autoAbs against the first three in thymoma patients. Moreover, these autoAbs – and others against IFNs, IL-17s and IL-22 – were among the first to appear in APS-I infants (66). By contrast, only autoAbs against AADC and GAD65 clearly conformed to current thinking. Overall, therefore, our data question the generality of current hypotheses that the similar AIRE-deficiency in APS-I and thymomas predisposes purely by impairing their expression of AIRE-dependent TSAgs/self-tolerance induction. We therefore propose that AIRE-deficiency might additionally create ‘dangerous’/Treg-deficient microenvironments where available AIRE-independent autoAgs bias selection, or even actively autoimmunize (33), as in paraneoplastic syndromes. To us and others (67), that explains these unusual early dominant responses more neatly than current hypotheses, which apply better to those against other TSAgs like GAD65. Once again, key evidence has come from observations in patients.
Our findings also have implications for patient management. They argue for reconsideration of thymectomy in APS-I children, to halt the continuing supply of autoaggressive T-cells. Further, in thymoma patients, subclinical adrenal insufficiency can mimic MG, with muscle weakness and fatigue (68). If not recognized, it could lead to an acute or even fatal Addisonian crisis.
Supplementary Material
Acknowledgements
We are very grateful to our many clinical colleagues, especially the late Prof J Newsom-Davis and Sr E Goodger, and the many patients, for their invaluable help; also Elisabeth Halvorsen for handling patient samples and coordinating the APS-I biobank; Ms. Laura Tomson and Maire Pihlap for excellent technical assistance with qPCR; Prof. R Klein for autoAb results on tissue sections.
The study was supported by grants from the Regional Health Authorities of Western Norway, the FP7 project Euradrenal (grant no. 201167), Bergen Medical Research Foundation, Estonian Research Agency grant IUT2-2, Center of Excellence of Translational Medicine of the Tartu University, Sir Jules Thorn Charitable Trust and the UK Medical Research Council.
The funding sources have not been involved in the study design, the execution of the study nor in the writing process.
List of Abbreviations
- AI
adrenal insufficiency
- AADC
aromatic L-amino acid decarboxylase
- AChR
acetylcholine receptor
- AIRE
Autoimmune Regulator
- APS-I
Autoimmune polyendocrine syndrome type I
- AutoAb
auto-antibody
- autoAg
autoantigen
- CMC
chronic mucocutaneous candidiasis
- EOMG
early-onset myasthenia gravis (onset before 45 years)
- GAD65
glutamic acid decarboxylase 65
- HP
hypoparathyroidism
- HC
healthy controls
- IA-2
insulinoma-associated tyrosine phosphatase-like protein
- KRT-8
keratin-8
- LOMG
late-onset MG (onset from 45 years and older)
- mTEC
medullary thymic epithelial cells
- NALP-5
NACHT leucine-rich-repeat protein 5
- POI
premature ovarian insufficiency
- SCC
side-chain cleavage enzyme
- 17OH
17-hydroxylase
- 21OH
21-hydroxylase
- TPH-1
tryptophan hydroxylase type 1
- TSAgs
tissue-specific autoantigens
Footnotes
The authors confirm no conflict of interest regarding this manuscript.
References
- 1.The Finnish-German APECED Consortium An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 2.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Husebye ES, Perheentupa J, Rautemaa R, Kampe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265:514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 5.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 6.Alimohammadi M, Bjorklund P, Hallgren A, Pontynen N, Szinnai G, Shikama N, Keller MP, Ekwall O, Kinkel SA, Husebye ES, Gustafsson J, Rorsman F, Peltonen L, Betterle C, Perheentupa J, Akerstrom G, Westin G, Scott HS, Hollander GA, Kampe O. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018–1028. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- 7.Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, Gustafsson J, Husebye ES, Perheentupa J, Gylling M, Manns MP, Rorsman F, Kampe O, Nilsson T. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89:557–562. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 8.Soderbergh A, Rorsman F, Halonen M, Ekwall O, Bjorses P, Kampe O, Husebye ES. Autoantibodies against aromatic L-amino acid decarboxylase identifies a subgroup of patients with Addison’s disease. J Clin Endocrinol Metab. 2000;85:460–463. doi: 10.1210/jcem.85.1.6266. [DOI] [PubMed] [Google Scholar]
- 9.Winqvist O, Gebre-Medhin G, Gustafsson J, Ritzen EM, Lundkvist O, Karlsson FA, Kampe O. Identification of the main gonadal autoantigens in patients with adrenal insufficiency and associated ovarian failure. J Clin Endocrinol Metab. 1995;80:1717–1723. doi: 10.1210/jcem.80.5.7745025. [DOI] [PubMed] [Google Scholar]
- 10.Winqvist O, Karlsson FA, Kampe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet. 1992;339:1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 11.Meager A, Visvalingam K, Peterson P, Moll K, Murumagi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y, Willcox N. Anti-Interferon Autoantibodies in Autoimmune Polyendocrinopathy Syndrome Type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meloni A, Furcas M, Cetani F, Marcocci C, Falorni A, Perniola R, Pura M, Boe Wolff AS, Husebye ES, Lilic D, Ryan KR, Gennery AR, Cant AJ, Abinun M, Spickett GP, Arkwright PD, Denning D, Costigan C, Dominguez M, McConnell V, Willcox N, Meager A. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2008;93:4389–4397. doi: 10.1210/jc.2008-0935. [DOI] [PubMed] [Google Scholar]
- 13.Oftedal BE, Boe Wolff AS, Bratland E, Kampe O, Perheentupa J, Myhre AG, Meager A, Purushothaman R, Ten S, Husebye ES. Radioimmunoassay for autoantibodies against interferon omega; its use in the diagnosis of autoimmune polyendocrine syndrome type I. Clin Immunol. 2008 Oct;129(1):163–9. doi: 10.1016/j.clim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aarli JA, Stefansson K, Marton LS, Wollmann RL. Patients with myasthenia gravis and thymoma have in their sera IgG autoantibodies against titin. Clin Exp Immunol. 1990;82:284–288. doi: 10.1111/j.1365-2249.1990.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx A, Willcox N, Leite MI, Chuang WY, Schalke B, Nix W, Strobel P. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43:413–427. doi: 10.3109/08916930903555935. [DOI] [PubMed] [Google Scholar]
- 18.Skeie GO, Aarli JA, Gilhus NE. Titin and ryanodine receptor antibodies in myasthenia gravis. Acta neurologica Scandinavica. Supplementum. 2006;183:19–23. doi: 10.1111/j.1600-0404.2006.00608.x. [DOI] [PubMed] [Google Scholar]
- 19.Vrolix K, Fraussen J, Molenaar PC, Losen M, Somers V, Stinissen P, De Baets MH, Martinez-Martinez P. The auto-antigen repertoire in myasthenia gravis. Autoimmunity. 2010;43:380–400. doi: 10.3109/08916930903518073. [DOI] [PubMed] [Google Scholar]
- 20.Beekman R, Kuks JB, Oosterhuis HJ. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol. 1997;244:112–118. doi: 10.1007/s004150050059. [DOI] [PubMed] [Google Scholar]
- 21.Gaman A, Gaman G, Bold A. Acquired aplastic anemia: correlation between etiology, pathophysiology, bone marrow histology and prognosis factors. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2009;50:669–674. [PubMed] [Google Scholar]
- 22.Oosterhuis HJ, Feltkamp TE, van Rossum AL, van den Berg-Loonen PM, Nijenhuis LE. HL-A antigens, autoantibody production, and associated diseases in thymoma patients, with and without myasthenia gravis. Ann N Y Acad Sci. 1976;274:468–474. doi: 10.1111/j.1749-6632.1976.tb47708.x. [DOI] [PubMed] [Google Scholar]
- 23.Souadjian JV, Enriquez P, Silverstein MN, Pepin JM. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Archives of internal medicine. 1974;134:374–379. [PubMed] [Google Scholar]
- 24.Hapnes L, Willcox N, Oftedal BE, Owe JF, Gilhus NE, Meager A, Husebye ES, Wolff AS. Radioligand-binding assay reveals distinct autoantibody preferences for type I interferons in APS I and myasthenia gravis subgroups. J Clin Immunol. 2012;32:230–237. doi: 10.1007/s10875-011-9617-4. [DOI] [PubMed] [Google Scholar]
- 25.Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, Willcox N. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132:128–136. doi: 10.1046/j.1365-2249.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karner J, Meager A, Laan M, Maslovskaja J, Pihlap M, Remm A, Juronen E, Wolff AS, Husebye ES, Podkrajsek KT, Bratanic N, Battelino T, Willcox N, Peterson P, Kisand K. Anti-cytokine autoantibodies suggest pathogenetic links with autoimmune regulator deficiency in humans and mice. Clin Exp Immunol. 2013;171:263–272. doi: 10.1111/cei.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng MH, Fan U, Grewal N, Barnes M, Mehta A, Taylor S, Husebye ES, Murphy EJ, Anderson MS. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med. 2010;362:764–766. doi: 10.1056/NEJMc0909510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherbaum WA, Schumm F, Maisch B, Muller C, Fateh-Moghadam A, Fluchter SH, Seif FJ, Bottazzo GF, Berg PA. Myasthenia gravis: overlap with ‘polyendocrine’ autoimmunity. Klinische Wochenschrift. 1983;61:509–515. doi: 10.1007/BF01488718. [DOI] [PubMed] [Google Scholar]
- 29.Seker M, Gozu HI, Oven Ustaalioglu BB, Sonmez B, Erkal FY, Kocak M, Barisik NO, Orbay E, Sargin M, Sargin H, Boru UT, Yaylaci M. Myasthenia gravis and autoimmune Addison disease in a patient with thymoma. Clinical lung cancer. 2009;10:367–370. doi: 10.3816/CLC.2009.n.051. [DOI] [PubMed] [Google Scholar]
- 30.Qiao J, Zhou G, Ding Y, Zhu D, Fang H. Multiple paraneoplastic syndromes: myasthenia gravis, vitiligo, alopecia areata, and oral lichen planus associated with thymoma. Journal of the neurological sciences. 2011;308:177–179. doi: 10.1016/j.jns.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Scarpino S, Di Napoli A, Stoppacciaro A, Antonelli M, Pilozzi E, Chiarle R, Palestro G, Marino M, Facciolo F, Rendina EA, Webster KE, Kinkel SA, Scott HS, Ruco L. Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas. Clin Exp Immunol. 2007;149:504–512. doi: 10.1111/j.1365-2249.2007.03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strobel P, Murumagi A, Klein R, Luster M, Lahti M, Krohn K, Schalke B, Nix W, Gold R, Rieckmann P, Toyka K, Burek C, Rosenwald A, Muller-Hermelink HK, Pujoll-Borrell R, Meager A, Willcox N, Peterson P, Marx A. Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1) The Journal of pathology. 2007;211:563–571. doi: 10.1002/path.2141. [DOI] [PubMed] [Google Scholar]
- 33.Kisand K, Lilic D, Casanova JL, Peterson P, Meager A, Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol. 2011;41:1517–1527. doi: 10.1002/eji.201041253. [DOI] [PubMed] [Google Scholar]
- 34.Willcox N, Schluep M, Ritter MA, Schuurman HJ, Newsom-Davis J, Christensson B. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? Am J Pathol. 1987;127:447–460. [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff AS, Erichsen MM, Meager A, Magitta NF, Myhre AG, Bollerslev J, Fougner KJ, Lima K, Knappskog PM, Husebye ES. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J Clin Endocrinol Metab. 2007;92:595–603. doi: 10.1210/jc.2006-1873. [DOI] [PubMed] [Google Scholar]
- 36.Masunaga A, Sugawara I, Nakamura H, Yoshitake T, Itoyama S. Cytokeratin expression in normal human thymus at different ages. Pathology international. 1997;47:842–847. doi: 10.1111/j.1440-1827.1997.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 37.Shezen E, Okon E, Ben-Hur H, Abramsky O. Cytokeratin expression in human thymus: immunohistochemical mapping. Cell and tissue research. 1995;279:221–231. doi: 10.1007/BF00300707. [DOI] [PubMed] [Google Scholar]
- 38.Oftedal BE, Kampe O, Meager A, Ahlgren KM, Lobell A, Husebye ES, Wolff AS. Measuring autoantibodies against IL-17F and IL-22 in autoimmune polyendocrine syndrome type I by radioligand binding assay using fusion proteins. Scand J Immunol. 2011;74:327–333. doi: 10.1111/j.1365-3083.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 39.Willcox N, Leite MI, Kadota Y, Jones M, Meager A, Subrahmanyam P, Dasgupta B, Morgan BP, Vincent A. Autoimmunizing mechanisms in thymoma and thymus. Ann N Y Acad Sci. 2008;1132:163–173. doi: 10.1196/annals.1405.021. [DOI] [PubMed] [Google Scholar]
- 40.Boonen A, Rennenberg R, van der Linden S. Thymoma-associated systemic lupus erythematosus, exacerbating after thymectomy. A case report and review of the literature. Rheumatology. 2000;39:1044–1046. doi: 10.1093/rheumatology/39.9.1044. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Marx A, Strobel P, Schalke B, Nix W, Willcox N. Autoimmune associations and autoantibody screening show focused recognition in patient subgroups with generalized myasthenia gravis. Human immunology. 2013;74:1184–1193. doi: 10.1016/j.humimm.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Erichsen MM, Lovas K, Skinningsrud B, Wolff AB, Undlien DE, Svartberg J, Fougner KJ, Berg TJ, Bollerslev J, Mella B, Carlson JA, Erlich H, Husebye ES. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94:4882–4890. doi: 10.1210/jc.2009-1368. [DOI] [PubMed] [Google Scholar]
- 43.Lima K, Abrahamsen TG, Wolff AB, Husebye E, Alimohammadi M, Kampe O, Folling I. Hypoparathyroidism and autoimmunity in the 22q11.2 deletion syndrome. Eur J Endocrinol. 2011;165:345–352. doi: 10.1530/EJE-10-1206. [DOI] [PubMed] [Google Scholar]
- 44.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, Douek DC, Pittaluga S, Poliani PL, Lee YN, Notarangelo LD, Wang L, Alt FW, Kang EM, Milner JD, Niemela JE, Fontana-Penn M, Sinal SH, Malech HL. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–1271. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen K, Wu W, Mathew D, Zhang Y, Browne SK, Rosen LB, McManus MP, Pulsipher MA, Yandell M, Bohnsack JF, Jorde LB, Notarangelo LD, Walter JE. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations. J Allergy Clin Immunol. 2014;133:880–882. e810. doi: 10.1016/j.jaci.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bratland E, Skinningsrud B, Undlien DE, Mozes E, Husebye ES. T cell responses to steroid cytochrome P450 21-hydroxylase in patients with autoimmune primary adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:5117–5124. doi: 10.1210/jc.2009-1115. [DOI] [PubMed] [Google Scholar]
- 47.Hedstrand H, Ekwall O, Haavik J, Landgren E, Betterle C, Perheentupa J, Gustafsson J, Husebye E, Rorsman F, Kampe O. Identification of tyrosine hydroxylase as an autoantigen in autoimmune polyendocrine syndrome type I. Biochem Biophys Res Commun. 2000;267:456–461. doi: 10.1006/bbrc.1999.1945. [DOI] [PubMed] [Google Scholar]
- 48.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardner JM, Metzger TC, McMahon EJ, Au-Yeung BB, Krawisz AK, Lu W, Price JD, Johannes KP, Satpathy AT, Murphy KM, Tarbell KV, Weiss A, Anderson MS. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4(+) T cells. Immunity. 2013;39:560–572. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kekalainen E, Miettinen A, Arstila TP. Does the deficiency of Aire in mice really resemble human APECED? Nat Rev Immunol. 2007;7:1. doi: 10.1038/nri2136-c1. [DOI] [PubMed] [Google Scholar]
- 52.Strobel P, Rosenwald A, Beyersdorf N, Kerkau T, Elert O, Murumagi A, Sillanpaa N, Peterson P, Hummel V, Rieckmann P, Burek C, Schalke B, Nix W, Kiefer R, Muller-Hermelink HK, Marx A. Selective loss of regulatory T cells in thymomas. Annals of neurology. 2004;56:901–904. doi: 10.1002/ana.20340. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Laan M, Bichele R, Kisand K, Scott HS, Peterson P. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Frontiers in immunology. 2012;3:19. doi: 10.3389/fimmu.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kekalainen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pontynen N, Talvensaari K, Perheentupa J, Miettinen A, Arstila TP. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 55.Laakso SM, Laurinolli TT, Rossi LH, Lehtoviita A, Sairanen H, Perheentupa J, Kekalainen E, Arstila TP. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. Journal of autoimmunity. 2010;35:351–357. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Ryan KR, Lawson CA, Lorenzi AR, Arkwright PD, Isaacs JD, Lilic D. CD4+CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Allergy Clin Immunol. 2005;116:1158–1159. doi: 10.1016/j.jaci.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 57.Wolff AS, Oftedal BE, Kisand K, Ersvaer E, Lima K, Husebye ES. Flow cytometry study of blood cell subtypes reflects autoimmune and inflammatory processes in autoimmune polyendocrine syndrome type I. Scand J Immunol. 2010;71:459–467. doi: 10.1111/j.1365-3083.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 58.Kyewski B, Klein L. A central role for central tolerance. Annual review of immunology. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 59.Inoue M, Starostik P, Zettl A, Strobel P, Schwarz S, Scaravilli F, Henry K, Willcox N, Muller-Hermelink HK, Marx A. Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res. 2003;63:3708–3715. [PubMed] [Google Scholar]
- 60.Derbinski J, Kyewski B. How thymic antigen presenting cells sample the body’s self-antigens. Current opinion in immunology. 2010;22:592–600. doi: 10.1016/j.coi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Taubert R, Schwendemann J, Kyewski B. Highly variable expression of tissue-restricted self-antigens in human thymus: implications for self-tolerance and autoimmunity. Eur J Immunol. 2007;37:838–848. doi: 10.1002/eji.200636962. [DOI] [PubMed] [Google Scholar]
- 62.Giraud M, Taubert R, Vandiedonck C, Ke X, Levi-Strauss M, Pagani F, Baralle FE, Eymard B, Tranchant C, Gajdos P, Vincent A, Willcox N, Beeson D, Kyewski B, Garchon HJ. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007;448:934–937. doi: 10.1038/nature06066. [DOI] [PubMed] [Google Scholar]
- 63.Maclennan CA, Vincent A, Marx A, Willcox N, Gilhus NE, Newsom-Davis J, Beeson D. Preferential expression of AChR epsilon-subunit in thymomas from patients with myasthenia gravis. J Neuroimmunol. 2008;201-202:28–32. doi: 10.1016/j.jneuroim.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Li B, Li J, Hsieh CS, Hale LP, Li YJ, Devlin BH, Markert ML. Characterization of cultured thymus tissue used for transplantation with emphasis on promiscuous expression of thyroid tissue-specific genes. Immunol Res. 2009;44:71–83. doi: 10.1007/s12026-008-8083-4. [DOI] [PubMed] [Google Scholar]
- 65.Pazirandeh A, Xue Y, Rafter I, Sjovall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:893–901. doi: 10.1096/fasebj.13.8.893. [DOI] [PubMed] [Google Scholar]
- 66.Wolff AS, Sarkadi AK, Marodi L, Karner J, Orlova E, Oftedal BE, Kisand K, Olah E, Meloni A, Myhre AG, Husebye ES, Motaghedi R, Perheentupa J, Peterson P, Willcox N, Meager A. Anti-cytokine autoantibodies preceding onset of autoimmune polyendocrine syndrome type I features in early childhood. J Clin Immunol. 2013;33:1341–1348. doi: 10.1007/s10875-013-9938-6. [DOI] [PubMed] [Google Scholar]
- 67.Arstila TP, Jarva H. Human APECED; a Sick Thymus Syndrome? Frontiers in immunology. 2013;4:313. doi: 10.3389/fimmu.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherbaum WA, Berg PA. Development of adrenocortical failure in non-Addisonian patients with antibodies to adrenal cortex. A clinical follow-up study. Clin Endocrinol (Oxf) 1982;16:345–352. doi: 10.1111/j.1365-2265.1982.tb00726.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.