Abstract
Invariant natural killer T (iNKT) cells are a major subset of lymphocytes found in the liver. These cells mediate various functions, including hepatic injury, fibrogenesis, and carcinogenesis. However, the function of iNKT cells in liver regeneration remains unclear. In the present study, partial hepatectomy (PHx) was used to study liver regeneration. α-GalCer, a specific ligand for iNKT cells, was used to induce iNKT cell activation. After PHx, two strains of iNKT cell-deficient mice, CD1d−/− and Jα281−/− mice, showed normal liver regeneration. Injection of α-GalCer before or after PHx, which rapidly stimulated IFN-γ and IL-4 production by iNKT cells, markedly inhibited liver regeneration. In vitro treatment with IFN-γ inhibited hepatocyte proliferation. In agreement with this in vitro finding, genetic disruption of IFN-γ or its downstream signaling molecule signal transducer and activator of transcription (STAT) 1 significantly abolished the α-GalCer-mediated inhibition of liver regeneration. In vitro exposure toIL-4 did not affect hepatocyte proliferation, but surprisingly, genetic ablation of IL-4 or its downstream signaling molecule STAT6 partially eliminated the inhibitory effect of α-GalCer on liver regeneration. Further studies revealed that IL-4 contributed to α-GalCer-induced iNKT cell expansion and IFN-γ production, and thereby inhibiting liver regeneration.
Conclusions
iNKT cells play a minor role in controlling liver regeneration after PHx under healthy conditions. Activation of iNKT cells by α-GalCer induces the production of IFN-γ, which directly inhibits liver regeneration, and IL-4, which indirectly attenuates liver regeneration by stimulating iNKT cell expansion and IFN-γ production.
Keywords: partial hepatectomy, STAT1, STAT6, α-GalCer
Introduction
The liver has the remarkable capacity to regenerate after tissue loss or injury. Partial hepatectomy (PHx) has been widely used to study liver regeneration, which is controlled by the interaction of various growth factors, hormones, and cytokines.1–5 In addition, the liver contains a large number of innate immune cells, including Kupffer cells, natural killer (NK) cells, and NKT cells, that also participate in liver regeneration.1–6 Studies have shown that after PHx, Kupffer cells are activated and produce TNF-α and IL-6, thereby promoting liver regeneration.1–4 We have previously demonstrated that NK cell depletion slightly enhances PHx-induced liver regeneration,7 suggesting that NK cells may play a minor role in inhibiting liver regeneration after PHx. Recently, Graubardt et al.8 reported that hepatocyte proliferation was markedly reduced in Rag2/common gamma-deficient mice (which lack T, B, and NK cells) but not in Rag1-deficient mice (which lacking T and B cells but contain NK cells), and these authors concluded that NK cells played a role in promoting liver regeneration. This opinion should be carefully considered because the reduced liver regeneration in Rag2/common gamma-deficient mice may be due not only to the absence of NK cells but also to the lack of T and B cells, which interact with NK cells to promote liver regeneration.9 Furthermore, we have previously demonstrated that injection of polyinosinic-polycytidylic acid or infection with mouse cytomegalo virus strongly activates NK cell IFN-γ production and inhibits liver regeneration post-PHx.7 Besides of the NK cells, mouse liver lymphocytes are also enriched in NKT cells; however, the results of studies investigating the role of NKT cells in liver regeneration have been controversial.6
NKT cells are a heterogeneous group of T lymphocytes that recognize lipid antigens presented by the nonclassical MHC class I-like molecule CD1. NKT cells can be divided into two types: type I and type II.10 Type I NKT cells express an invariant T cell receptor (TCR) α chain and are also called classical or invariant NKT (iNKT) cells. These cells comprise 95% of liver NKT cells. Type II NKT cells express diverse TCRs and compriseless than 5% of liver NKT cells.6 iNKT cells can be activated by lipid antigens (e.g., α-Galactosylceramide [α-GalCer]) or cytokines (e.g., IL-12). The hallmark of α-GalCer-mediated iNKT cell activation is the rapid production of both Th1- and Th2-type cytokines (IFN-γ and IL-4, respectively).10 IFN-γ binds to IFNGR1 and IFNGR2, which are ubiquitously expressed, and these receptors in turn predominantly activate signal transducer and activator of transcription 1 (STAT1) and to a lesser extent, other STATs. Activated STAT1 translocates into the nucleus and acts as a transcription factor, inducing the transcription of genes that induce liver injury, attenuate liver regeneration, and inhibit viral replication in the liver.11, 12 The functions of IL-4 are primarily mediated by activation of STAT6 and to a lesser degree, other STATs.13 In contrast to the function of STAT1, the function of STAT6 is less clear.
Accumulating evidence suggests that the functions of iNKT cells in the pathogenesis of liver disease are complex and that these cells likely play diverse roles, particularly given the existence of multiple types of NKT cells and their production of a large number of cytokines, chemokines, and other mediators.6, 14, 15 The results of studies investigating the role of iNKT cells in liver regeneration are controversial. A previous study revealed that treatment of mice with IL-12 or α-GalCer after PHx induced NKT cell activation and exacerbated liver injury during liver regeneration, but surprisingly, the effects of the NKT cells on liver regeneration were not examined.16 Nakashima et al.17 reported that mice treated with α-GalCerat 36 h after PHx showed enhanced hepatocyte mitosis at 44h post-PHx; however, it was not clear whether injection of α-GalCerat earlier time points after PHx affected liver regeneration. A more recent study demonstrated that NKT cells were activated in hepatitis B virus transgenic mice, and depletion of both NKT and NK cells enhanced liver regeneration post-PHx; however, depletion of NK cells alone had no effect.18 In contrast, Hosoya et al. reported that depletion of both NK and NKT cells reduced liver regeneration after PHx in wild-type (WT) mice.19 We have previously demonstrated that liver regeneration in NKT cell-deficient mice (CD1d−/− and β2 microglobulin−/− mice) was comparable to that in WT mice post-PHx,7 consistent with the results obtained from Hosoya et al.19 In the present study, we further demonstrated that Jα281−/− mice, which are specifically deficient in iNKT cells, had normal liver regeneration after PHx. These findings suggest that in the PHx model, iNKT cells may play a minor role in liver regeneration under normal conditions. Furthermore, we examined the effects of α-GalCer-mediated iNKT cell activation on liver regeneration after PHx. The results clearly indicate that α-GalCer treatment before or after PHx activates iNKT cells and inhibits liver regeneration via both IFN-γ- and IL-4-dependent mechanisms.
Materials and Methods
Animals
Eight- to ten-week-old male mice were used in the present study. C57BL/6J, IFN-γ−/−, IL-4−/−, STAT6−/−, and CD1d−/− mice on a C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, ME). STAT1−/− mice were originally purchased from Taconic (Hudson, NY) and backcrossed onto a C57BL/6J background for at least 11 generations. Mice deficient in Vα14 NKT cells (Jα281−/− mice) were kindly provided by Dr. Taniguchi (Yokohama, Kanagawa, Japan). All animals were maintained in accordance with the National Institutes of Health guidelines, and all animal experiments were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
PHx and treatment with iNKT cell activators
The PHx procedure was performed as previously described.20 Briefly, mice were anesthetized by inhalation of isoflurane (2%). Under aseptic conditions, the median and left lateral lobes of the liver were ligated at their stem and excised. For the sham operation, the peritoneum of anesthetized mice was opened, and the liver was manipulated but not resected. The animals were euthanized by decapitation at the indicated times following surgery. Mortality was less than 5% and was not associated with a particular genotype.
Mice were injected with α-GalCer at different time points before or after 70% PHx. α-GalCer (KRN7000) was purchased from Alexis Biochemicals Corporation (San Diego, CA).α-GalCer was dissolved in 0.5% polysorbate-20 [Tween-20] and diluted in phosphate-buffered saline (PBS). Mice were injected intravenously with α-GalCer (2μg/200μL in PBS per mouse) at different time points before or after surgery according to the experimental design.
Statistical Analysis
Data are expressed as the means ± SEM for each group. Student’s t test was used to compare values obtained from two groups. To compare values obtained from three or more groups, 1-factor analysis of variance (ANOVA) followed by Tukey’s post hoc test was performed using GraphPad Prism software (version 5.0a; GraphPad Software, Inc, La Jolla, CA). Statistical significance was considered at P<0.05.
Results
Activation of iNKT cells by α-GalCer inhibits liver regeneration after PHx
In a previous study, we demonstrated that iNKT cell-deficient and WT mice had comparable liver regeneration after PHx, suggesting that iNKT cells may play a minor role in PHx-induced liver regeneration under normal conditions.7 To further define the role of iNKT cells in liver regeneration, we examined the effects of α-GalCer-mediated iNKT cell activation on liver regeneration. As hepatocyte injury can lead to compensatory liver regeneration, we first determined whether α-GalCer treatment exacerbated liver injury in the PHx model. As shown in supplemental Fig. 1A, after α-GalCer injection with or without PHx, none of the mice showed obvious adverse phenotypes or died. Serum ALT levels were comparable between the PHx groups treated with or without α-GalCer (data not shown). Histologic examination of the livers revealed no obvious hepatocyte necrosis in any of the groups; however, the α-GalCer-treated mice showed an increased number of inflammatory foci, indicating that NKT cells were activated by α-GalCer (supplemental Fig. 1B).
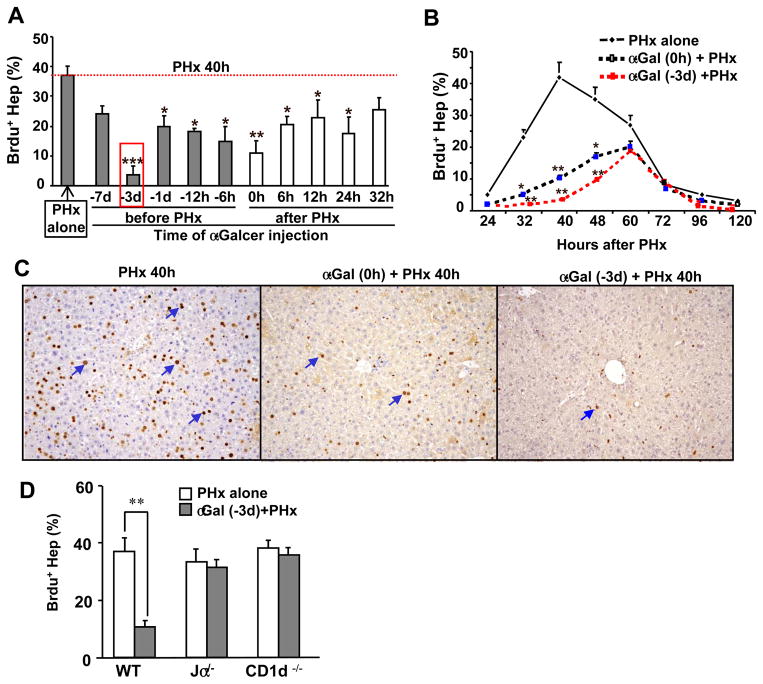
To explore whether iNKT cell activation affected liver regeneration, mice were treated with α-GalCer at various time points before or after PHx, and hepatocyte BrdU incorporation was determined at 40 h post-PHx. As shown in Fig. 1A, compared with PHx alone, α-GalCer treatment 1–3d before PHx and 0–24 h after PHx significantly decreased the peak of hepatocyte proliferation at 40h after PHx. This inhibition was most significant in the groups injected with α-GalCer 3 d before surgery or immediately (0h) after surgery. We selected these two time points for α-GalCer injection to examine the effect of iNKT cell activation on the time course of liver regeneration post-PHx. As illustrated in Figs. 1B–C, the peak of BrdU staining in hepatocytes was delayed to 60 h in the α-GalCer-treated PHx groups, and the staining was much lower than the peak of BrdU staining observed at 48 h in the PHx alone group. In addition, the number of BrdU+ hepatocytes was markedly lower at many time points in the α-GalCer-treated PHx groups compared with the PHx alone group.
Fig. 1. Activation of iNKT cells by α-GalCer inhibits PHx-induced liver regeneration.
(A) Mice were treated with α-GalCer before or after PHx. All mice were euthanized 40h post PHx, and liver tissues were collected. BrdU was injected 2 h prior to euthanizing mice. The red rectangle indicates the greatest inhibition of hepatocyte proliferation. (B) Mice were treated with α-GalCer 3 d prior to PHx or concurrently (0 h) with PHx and euthanized at various time points post-PHx. BrdU was injected 2 h prior to euthanizing mice. (C) Representative BrdU immunohistochemical staining from panel B. (D) WT and NKT cell knockout mice were treated with or without α-GalCer for 3 d, and then PHx was performed. BrdU incorporation was determined 40 h post-PHx. The BrdU-positive hepatocytes in panels A, B, and D were counted in 5 high-power fields (100×), and the results are expressed as a percentage of total hepatocytes. The blue arrows in panel C indicate representative BrdU+ hepatocytes. Data represent the means ± SEM (n=6–10 mice). *P<0.05, **P<0.01, and ***P<0.001 compared with PHx alone.
To further confirm the contribution of activated iNKT cells in inhibiting hepatocyte proliferation after PHx, CD1d−/− and Jα18−/− mice (deficient in iNKT cells) were used. As illustrated in Fig. 1D, liver regeneration in iNKT cell-deficient mice (both Jα18−/− and CD1d−/− mice) was similar to that observed in WT mice post-PHx, consistent with previous findings.7, 19 Treatment with α-GalCer markedly inhibited liver regeneration in WT mice, but not in Jα18−/− or CD1d−/− mice, suggesting that the α-GalCer-mediated inhibition of liver regeneration is due to the activation of iNKT cells.
Effects of iNKT cell stimulation on the activation of cytokines and their downstream STAT signaling molecules post-PHx
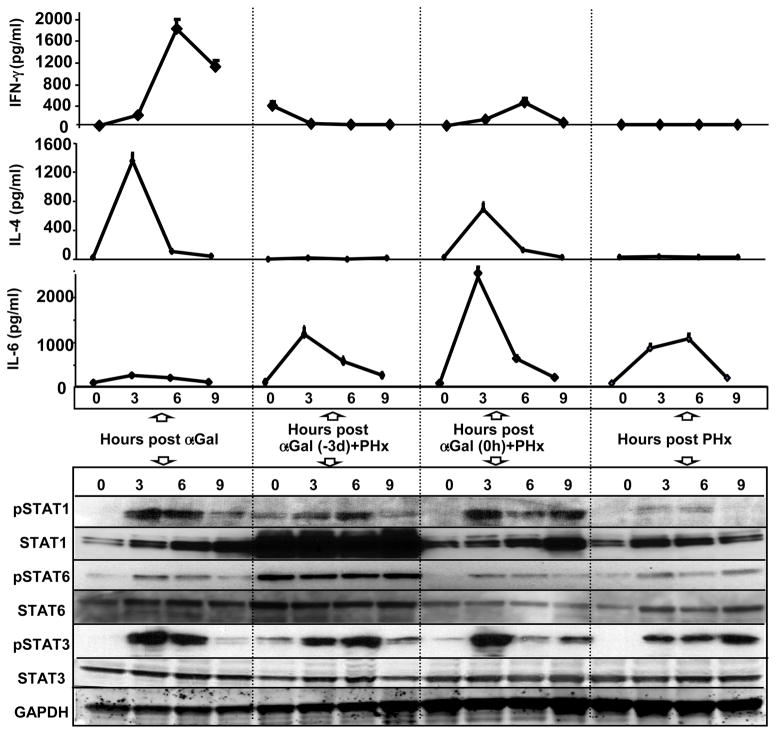
To examine the mechanisms underlying the α-GalCer-mediated inhibition of liver regeneration, we analyzed the production of cytokines and their related signaling pathways. We measured serum levels of IFN-γ and IL-4, the two major cytokines produced by activated iNKT cells,10 and IL-6, a cytokine that plays an important role in liver regeneration,1 and examined the activation of their downstream signaling pathways by western blot analysis. As illustrated in Fig. 2, serum IFN-γ levels were markedly increased in the α-GalCer alone group, and they were also increased, but to a lesser extent, in the α-GalCer (0h)+PHx group. Serum IFN-γ levels were not significantly elevated in the PHx alone and PHx+α-GalCer (−3d) groups, although slight elevation at 0h was observed in the latter group. Consistent with the serum levels of IFN-γ, activation of its downstream signaling molecule, STAT1 (pSTAT1), was detected in the livers of mice in the α-GalCer and α-GalCer (0h)+PHx groups. Weak pSTAT1 was also detected in the PHx alone and α-GalCer (−3d)+PHx groups. Expression of hepatic STAT1 protein was up regulated in the PHx alone, α-GalCer (0h), and α-GalCer (0 h)+PHx groups post-PHx, whereas basal levels (0h) of hepatic STAT1 protein were high and remained high after PHx in the α-GalCer (−3d)+PHx group.
Fig. 2. Activation of cytokines and their corresponding downstream signaling pathways post-α-GalCer and/or post-PHx.
Micewere treated with α-GalCer alone, 3 d prior to PHx [α-GalCer (−3 d)+PHx], concurrently with PHx [α-GalCer (0 h)+PHx], or PHx alone and euthanized at the indicated time points. Serum was collected to measure cytokine levels, and liver tissues were collected for western blot analysis. Data for serum cytokine levels represent the means ± SEM (n=4–8 mice). Representative immunoblot analyses of STAT proteins are shown from 3 independent experiments.
Similar to IFN-γ, serum IL-4 levels were also markedly increased in the α-GalCer alone group and increased to a lesser extent in the α-GalCer (0h)+PHx group, but IL-4 levels were not elevated in the PHx alone and α-GalCer (−3d) + PHx groups. Activation of STAT6 (pSTAT6), the primary downstream signaling molecule of IL-4,13 was weakly detected in the livers of mice in the α-GalCer alone, PHx alone, and α-GalCer (0 h)+PHx groups. Surprisingly, pSTAT6 was highly expressed in the livers of mice from the α-GalCer (−3 d)+PHx group both basally and after PHx.
Serum IL-6 levels were elevated in all groups with the highest levels observed in the α-GalCer (0 h)+PHx group followed by the α-GalCer (−3 d)+PHx, PHx alone, and α-GalCer alone groups. Moreover, activation of hepatic STAT3 was detected in all groups after surgery or injection.
IFN-γ and STAT1 partially contribute to the inhibitory effect of α-GalCer on PHx-induced liver regeneration
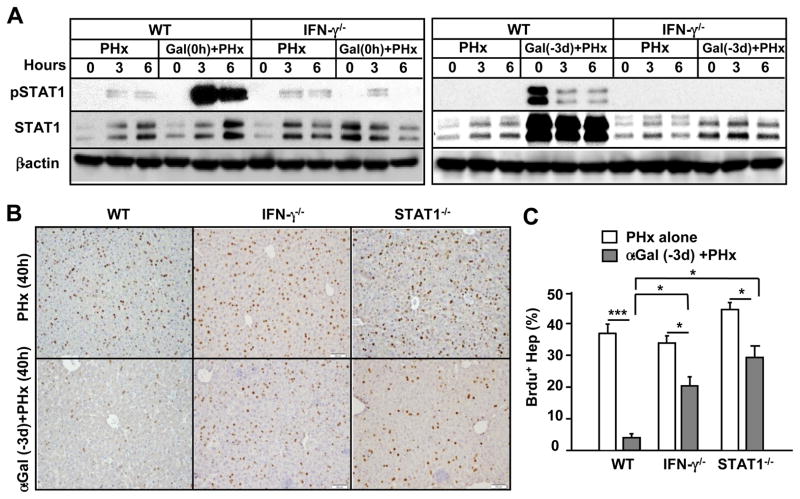
To examine whether upregulation of the STAT1 protein was mediated through IFN-γ, we compared hepatic STAT1 protein from WT and IFN-γ−/− mice in the PHx group treated with or without α-GalCer. As illustrated in Fig. 3A, in WT mice, hepatic pSTAT1 was highly activated post-α-GalCer (0 h)+PHx treatment (left panel) and weakly activated post-α-GalCer (−3 d)+PHx treatment. In addition, levels of hepatic STAT1 protein were comparable between the PHx alone and α-GalCer (0 h)+PHx groups (right panel), but hepatic STAT1 protein was markedly upregulated in the α-GalCer (−3 d)+PHx group (left panel). Levels of pSTAT1 and STAT1 protein in α-GalCer+PHx were diminished in IFN-γ−/− mice.
Fig. 3. The inhibitory effect of α-GalCer on PHx-induced liver regeneration is partially diminished in IFN-γ−/− and STAT1−/− mice.
(A) Western blot analysis of liver extracts from PHx mice treated with or without α-GalCer. (B) Representative images of BrdU immunostaining from PHx mice treated with or without α-GalCer. (C) The total number of BrdU+ hepatocytes is presented. Values represent the means ± SEM (n=8–10). *P<0.05, ***P<0.001
The IFN-γ/STAT1 signaling pathway plays an important role in inhibiting liver regeneration.21, 22 To determine whether this pathway was responsible for the α-GalCer-mediated inhibition of liver regeneration, we used both IFN-γ−/− and STAT1−/− mice. As illustrated in Fig. 3B, α-GalCer treatment 3 days prior to PHx resulted in a 90% inhibition of liver regeneration in WT mice, but only 40% inhibition was observed in both strains of knockout mice.
IL-4 and STAT6 partially contribute to the inhibitory effect of α-GalCer on PHx-induced liver regeneration
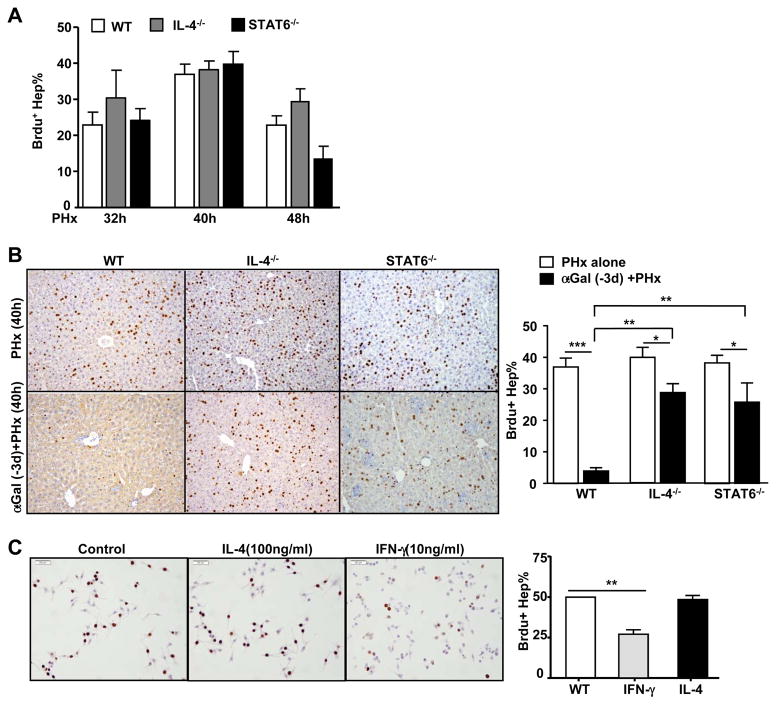
As blocking the IFN-γ/STAT1 pathway only partially reversed the inhibitory effect of α-GalCer on PHx-induced liver regeneration, we speculated that additional factors contributed to the inhibitory effect of activated iNKT cells on liver regeneration. Because IL-4 production is a hallmark of iNKT cell activation,10 we investigated the role of the IL-4/STAT6 pathway in α-GalCer-mediated PHx-initiated liver regeneration in IL-4−/− and STAT6−/− mice. As shown in Fig. 4A, both strains of mice had normal liver regeneration compared with WT mice. Interestingly, α-GalCer treatment 3 d prior to PHx resulted in a marked inhibition of liver regeneration in WT mice, but this inhibition was partially diminished in IL-4−/− and STAT6−/− mice (Fig. 4B).
Fig. 4. The inhibitory effect of α-GalCer on PHx-induced liver regeneration is partially diminished in IL-4−/− and STAT6−/− mice.
(A) WT, IL-4−/−, and STAT6−/− mice were subjected to PHx, and liver regeneration was determined by BrdU incorporation. (B) Representative images of BrdU immunostaining from PHx mice treated with or without α-GalCer. The total number of BrdU+ hepatocytes is presented in the right panel. (C) Representative images of BrdU immunostaining from cultured AML12 cells treated with or without cytokines for 24h. The percentage of BrdU+ cells is presented in the right panel. Valuesin panels A–C represent the means ± SEM (n=8–10). *P<0.05, ** P<0.01; ***P<0.001
These data suggest that both IFN-γ and IL-4 contribute to the α-GalCer-mediated inhibition of liver regeneration. Next, we examined whether these cytokines directly inhibited hepatocyte proliferation in an in vitro culture model. As illustrated in Fig. 4C, treatment with IFN-γ markedly inhibited proliferation of AML12 cells (a mouse hepatocyte cell line), whereas treatment with IL-4 had no effect. This result suggests that IFN-γ inhibits liver regeneration by directly suppressing hepatocyte proliferation, whereas IL-4 attenuates liver regeneration via an indirect mechanism.
IL-4 contributes to α-GalCer-induced iNKT cell proliferation and survival in a positive feedback loop: in vivo and in vitro evidence
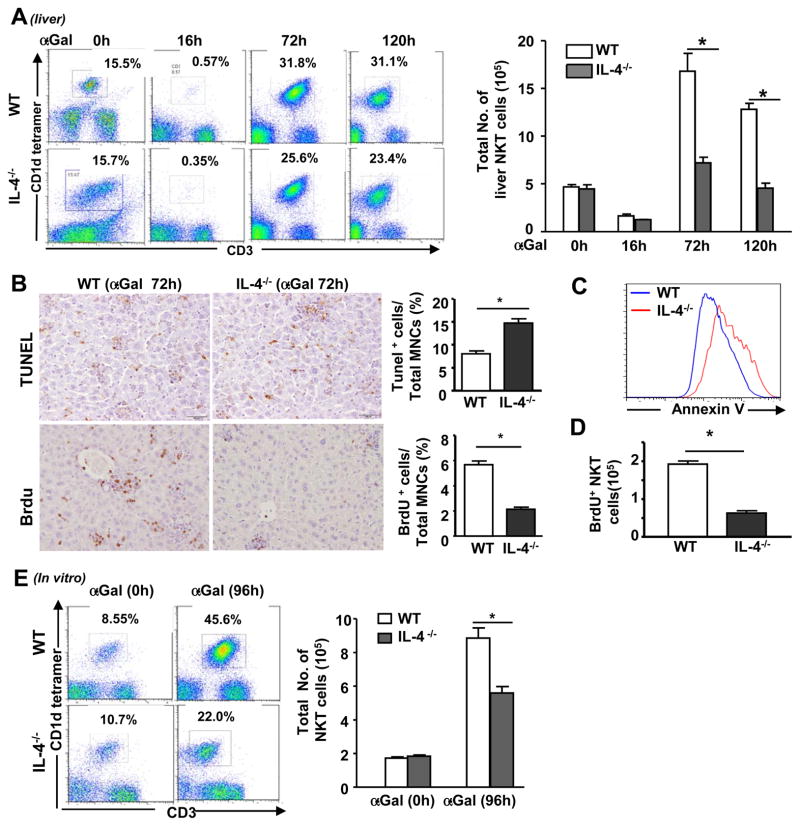
To further clarify the mechanism by which IL-4 contributes to the inhibitory effect of α-GalCer on PHx-induced liver regeneration, we determined the effect of IL-4 on iNKT cell proliferation in the liver and spleen of IL-4−/− and WT mice in vivo and in vitro after challenge with α-GalCer. As shown in Fig. 5A, the percentage and total number of iNKT cells were markedly reduced in both WT and IL-4−/− mice 16 h after α-GalCer administration, but these values increased 72 and 120 h post-α-GalCer injection. This increase was much lower in IL-4−/− mice compared with WT mice. Immunohistochemical examination revealed a greater number of TUNEL+ and fewer BrdU+ lymphocytes in the livers of IL-4−/− mice 72 h post-α-GalCer administration compared with WT mice (Fig. 5B). Flow cytometric analysis showed that a higher number of liver iNKT cells from IL-4−/− mice underwent apoptosis (Annexin V staining) (Fig. 5C), but fewer iNKT cells from these mice proliferated (BrdU+iNKT) compared with iNKT cells from WT mice 72 h post-α-GalCer challenge (Fig. 5D).
Fig. 5. IL-4−/− mice are resistant to α-GalCer-induced hepatic iNKT expansion in vivo and in vitro due to reduced proliferation and enhanced apoptosis.
(A) WT and IL-4−/− mice were treated with a single dose of α-GalCer and euthanized at different time points. Liver MNCs were analyzed by flow cytometry using an anti-CD3 antibody and CD1d tetramer. (B) Liver tissues from α-GalCer-treated mice were stained with TUNEL to assess cell apoptosis and anti-BrdU to determine cell proliferation. Representative images are shown on the left. The percentage of TUNEL+ or BrdU+ cells was quantified and is shown on the right. (C, D) Liver MNCs were isolated from α-GalCer-treated WT and IL-4−/− mice and subjected to flow cytometric analysis using an anti-CD3 antibody, CD1d tetramer, and Annexin V (C) or an anti-CD3 antibody, CD1d tetramer, and anti-BrdU antibody (D). (E) Liver MNCs were cultured in vitro with or without α-GalCer for 96 h and then analyzed by flow cytometry using an anti-CD3 antibody and CD1d tetramer. The total number of NKT cells in the livers of α-GalCer-treated WT and STAT6−/− mice was calculated and shown on the right. *P<0.05.
In vitro experiments revealed that treatment of liver mononuclear cells (MNCs) from WT mice with α-GalCer stimulated iNKT cell expansion, as the percentage and total number of NKT cells increased. This expansion was much lower in hepatic MNCs from IL-4−/− mice (Fig. 5E).
Finally, as shown in supporting Fig. 2A, compared with WT mice, STAT6−/− mice had less iNKT cell expansion in the liver at 72h post-α-GalCer administration, suggesting that STAT6is required for α-GalCer-induced iNKT cell accumulation.
The data in supporting Figs. 3A–B also suggested that IL-4 was required for α-GalCer-induced iNKT cell expansion in the spleen as demonstrated by the lower spleen index, lower percentage of iNKT cells, and lower number of iNKT cells in the spleens of IL-4−/− mice compared with WT mice. The lower number of iNKT cells maybe partly due to the enhanced spleen iNKT cell apoptosis in IL-4−/− mice (Supporting Fig. 3C). In vitro experiments showed that incubation of spleen cells with α-GalCer resulted in a significant increase in the percentage of iNKT cells 96 h post-culture, and this percentage was much lower in IL-4−/− mice than in WT mice post-α-GalCer incubation (Supporting Fig. 3D). In addition, STAT6−/− mice also had a lower spleen index and lower number of spleen iNKT cells after α-GalCer treatment compared with WT mice (Supporting Fig. 4).
These data suggest that IL-4 and STAT6 promote iNKT expansion. To understand the underlying mechanisms, we investigated the expression of several cell cycle arrest-related and pro-apoptotic genes in isolated iNKT cells. We observed that α-GalCer treatment markedly upregulated the expression of survivin and Bcl-2 in iNKT cells from WT mice. This upregulation was diminished in iNKT cells from α-GalCer-treated IL-4−/− and STAT6−/− mice (supporting Fig. 5).
IL-4 is required for the α-GalCer-induced production of IFN-γ in vivo and in vitro
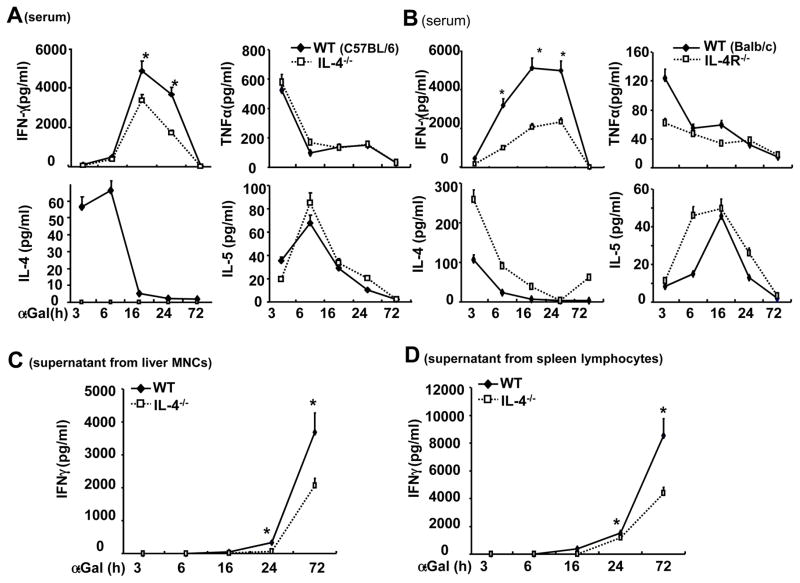
As illustrated in Figs. 6A–B, α-GalCer treatment induced high levels of serum IFN-γ with peak levels detected at 16 h in WT mice, and this elevation was much lower in IL-4−/− and IL-4R−/− mice. As expected, α-GalCer treatment induced enhanced levels of IL-4 in WT mice, but not IL-4−/− mice; IL-4 levels were higher in IL-4R−/− mice than in WT mice after α-GalCer administration. The higher levels of serum IL-4 in IL-4R−/− mice may due to lacking of cytokine-receptor binding in these mice. Serum levels of TNF-α and IL-5 were comparable between WT and IL-4−/− mice. Serum TNF-α levels were lower and serum IL-5 levels were higher in IL-4R−/− mice than in WT mice at early time points after α-GalCer injection. Furthermore, the in vitro experiments in Figs. 6C–D showed that the IFN-γ levels produced by spleen or liver MNCs from IL-4−/− mice in response to in vitro α-GalCer stimulation were lower than those from WT mice.
Fig. 6. IL-4 contributes to α-GalCer-induced iNKT cell production of IFN-γ in vivo and in vitro.
(A, B) IL-4−/− and IL-4R−/− mice and their corresponding WT control mice were treated with α-GalCer for various time points, and serum cytokines were measured. (C, D) Liver MNCs or spleen lymphocytes were stimulated with α-GalCer in vitro, supernatants were collected, and IFN-γ levels were measured. *P<0.05 compared with corresponding WT controls.
Discussion
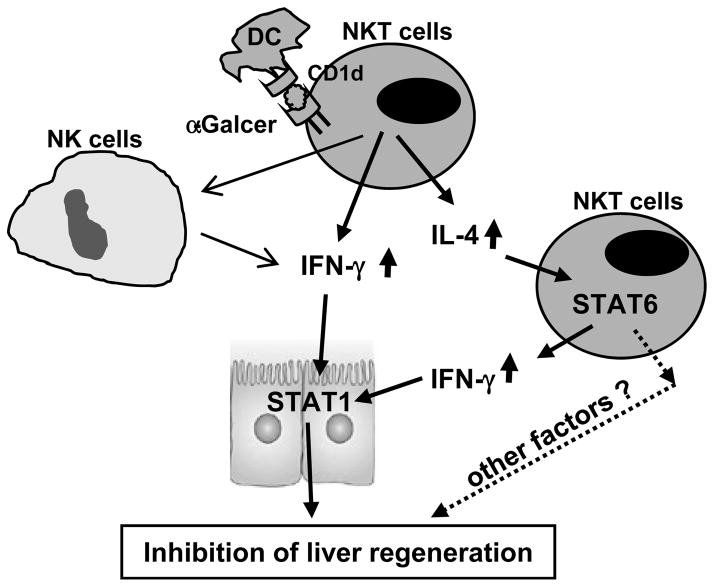
The lymphocytes present in the liver are enriched in iNKT cells, which play an important role in regulating liver injury, inflammation, fibrosis, and tumorigenesis.6, 14, 15, 23 In the present study, we provide several lines of evidence suggesting that iNKT cells have only a minor role in regulating liver regeneration after PHx under healthy conditions but that activation of iNKT cells by α-GalCer markedly inhibits PHx-induced liver regeneration by both IFN-γ- and IL-4-dependent mechanisms. IFN-γ directly inhibits hepatocyte proliferation and liver regeneration via the activation of STAT1; IL-4 has no direct effect on hepatocyte proliferation and survival, but this cytokine indirectly inhibits liver regeneration by promoting iNKT cell expansion and IFN-γ production (Fig. 7).
Fig. 7. The mechanisms underlying α-GalCer-mediated inhibition of liver regeneration.
Antigen-presenting cells present lipid antigen (α-GalCer) to iNKT cells via the CD1d molecule, resulting in iNKT cell activation and production of IFN-γ and IL-4. IFN-γ directly inhibits hepatocyte proliferation and liver regeneration via activation of STAT1. IL-4 indirectly inhibits liver regeneration by promoting iNKT cell expansion and IFN-γ production.
iNKT cells play a minimal role in controlling liver regeneration after PHx under healthy conditions
iNKT cells constitute 20–40% of liver lymphocytes in mice, and their numbers increase post-PHx.7, 24 Accumulating evidence suggests that iNKT cells play a minimal role in regulating liver regeneration under healthy conditions in the PHx model. Previous studies have revealed that several strains of iNKT cell-deficient mice (CD1d−/−, β2 microglobulin−/−, and Jα281−/− mice) have normal liver regeneration.7, 19 However, we cannot rule out the possibility that long-term adaptive changes may occur in these knockout strains. Second, although the number of liver iNKT cells is elevated after PHx,7, 24 these cells are only weakly activated or not activated at all, ashepatic expression of IFN-γ and IL-4 is only slightly elevated after PHx.7, 25, 26 Similarly, our studies showed that serum IFN-γ and IL-4 levels were not elevated post-PHx (Fig. 2). Third, the α-GalCer-mediated elevation of serum IFN-γ and IL-4 was markedly attenuated in the α-GalCer-treated PHx group compared to the PHx alone group (Fig. 2), suggesting that PHx suppresses iNKT cell function. This effect may be associated with PHx surgery-induced stress, which results in immune suppression. Because removal of 2/3 of the liver results in acute and strong regenerative responses with the rapid elevation of many growth factors (e.g., hepatocyte growth factor) and cytokines (e.g., IL-6), that initiate and promote liver regeneration,1–5 we believed that the accumulation of iNKT cells may be an epiphenomenon that does not play a major role in controlling liver regeneration in the PHx model under normal conditions.
IFN-γ contributes to the α-GalCer-mediated inhibition of liver regeneration post-PHx by directly inhibiting hepatocyte proliferation
Although iNKT cells play a minor role in controlling liver regeneration after PHx under healthy conditions, mice treated with the strong iNKT activator, α-GalCer, before or after PHx showed markedly attenuated liver regeneration. Injection of α-GalCer rapidly induced iNKT activation and production of both IFN-γ and IL-4. Serum levels of IFN-γ reached ~4,000 pg/ml at 16h post-α-GalCer.27 IFN-γ is one of most potent factors inhibiting hepatocyte proliferation via the activation of STAT1.12, 21 Thus, induction of IFN-γ likely contributes to the α-GalCer-mediated inhibition of liver regeneration. Indeed, the genetic ablation of either IFN-γ or STAT1 markedly abolished the inhibitory effect of α-GalCer on liver regeneration (Fig. 3).
The effects of α-GalCer on liver injury and regeneration in the PHx model have been previously examined. Ito et al.16 reported that treatment of mice with α-GalCer augmented liver injury during liver regeneration after PHx, but surprisingly, liver regeneration was not examined. Later, Nakashima et al.17 reported that mice treated with α-GalCer 36 h after PHx showed enhanced hepatocyte mitosis at 44 h post-PHx; however, we observed that treatment with α-GalCer at this late time point after PHx had no effect on liver regeneration. Surprisingly, Nakashima et al. only examined a single late time point. In the present study, we examined α-GalCer treatment at 10 time points before and after PHx and observed that treatment 1–3 d prior to PHx and 0–24 h after PHx markedly inhibited liver regeneration with the strongest inhibition observed when α-GalCer was injected 3 d before surgery. This result may reflect the fact that treatment with α-GalCer for 3 d induced extremely high levels of STAT1 protein (Figs. 2 and 3) and iNKT expansion27 in the liver, a process that likely contributed to the inhibition of liver regeneration.
IL-4 contributes to the α-GalCer-mediated inhibition of liver regeneration post-PHx by promoting iNKT expansion and IFN-γ production
IL-4 production is a hallmark of iNKT cell activation, and injection of α-GalCer significantly elevated serum levels of IL-4.10 In vitro exposure to IL-4 did not affect hepatocyte proliferation, but genetic ablation of IL-4 or STAT6 diminished the α-GalCer-mediated inhibition of liver regeneration (Fig. 4). These results indicate that IL-4/STAT6 contributes to the α-GalCer-mediated inhibition of liver regeneration via an indirect mechanism. Additional studies revealed that IL-4 was required for α-GalCer-mediated induction of iNKT cell expansion and IFN-γ production (Figs. 5–6 and supporting Fig. 3). Collectively, these results suggest that activation of iNKT cells by α-GalCer results in production of IL-4, which indirectly inhibits liver regeneration by stimulating iNKT cell expansion and IFN-γ production in a STAT6-dependent manner.
Two recent studies examined the role of IL-4 in liver regeneration. DeAngelis et al.25 reported that hepatic IL-4 expression was elevated post-PHx, and the secretion of IL-4 was controlled by the complement pathway through the recruitment of NKT cells. WT mice treated with an IL-4 neutralizing antibody or IL-4−/− mice had higher morbidity and mortality post-PHx. A more recent study from Goh et al.26 showed that eosinophils were responsible for the IL-4 production post-PHx and that IL-4/IL-13 double knockout mice had reduced liver regeneration post-PHx compared with WT mice. Surprisingly, liver regeneration was not reported in the studies by Goh et al.26 In contrast, in the present study, we demonstrated that IL-4−/− and STAT6−/− mice had normal liver regeneration after PHx, and no deaths occurred. The differences between these studies are not clear and may reflect the use of different surgical techniques, mouse strains, or research environments.
Clinical implications
α-GalCer has been tested in clinical trials for the treatment of viral hepatitis and liver cancer, but few beneficial effects have been observed.15, 28 Together with our previous studies, the current study of the effects of α-GalCer-mediated iNKT activation on liver injury and regeneration not only improves our understanding of the role of iNKT cells in the pathogenesis of liver diseases but also facilitates the development of iNKT activators for the treatment of liver disorders. For example, injection of α-GalCer activates iNKT cells, resulting in the production of IFN-γ and IL-4. IFN-γ is required for the anti-viral and anti-tumor effects of α-GalCer in vivo,29, 30 but it also protects against α-GalCer-induced liver injury27 and inhibits liver regeneration (as shown in this study). However, IL-4 production by iNKT cells has more detrimental effects, such as the impairment of anti-tumor and anti-viral effects, augmentation of liver injury,27 and impairment of liver regeneration (as shown in this study) by promoting iNKT cell expansion. Therefore, development of a ligand that activates iNKT cells to preferentially produce IFN-γ may have better therapeutic effects for viral hepatitis and liver cancer.
Supplementary Material
Acknowledgments
This work was supported in part through funding from the intramural program of NIAAA, NIH (B Gao), the Natural Science Foundation of China (S Yin, No. 81100311/H0318), and the New Century Excellent Talents in University, Ministry of Education of China (H Wang, No.NCET-13-0644).
List of Abbreviations
- α-GalCer
α-Galactosylceramide
- iNKT cells
invariant natural killer T cells
- IFN-γ
interferon-γ
- IL-4
interleukin 4
- PHx
partial hepatectomy
- STAT
signal transducer and activator of transcription
- WT
wild-type
References
- 1.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 3.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–4. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Fausto N. Involvement of the innate immune system in liver regeneration and injury. J Hepatol. 2006;45:347–9. doi: 10.1016/j.jhep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Van Sweringen HL, Sakai N, Tevar AD, et al. CXC chemokine signaling in the liver: impact on repair and regeneration. Hepatology. 2011;54:1445–53. doi: 10.1002/hep.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004;127:1525–39. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 8.Graubardt N, Fahrner R, Trochsler M, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology. 2013;57:1969–79. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 9.Besnard A, Julien B, Gonzales E, et al. Innate immunity, purinergic system, and liver regeneration: a trip in complexity. Hepatology. 2013;57:1688–90. doi: 10.1002/hep.26312. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 11.Gao B, Wang H, Lafdil F, et al. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57:430–41. doi: 10.1016/j.jhep.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horras CJ, Lamb CL, Mitchell KA. Regulation of hepatocyte fate by interferon-gamma. Cytokine Growth Factor Rev. 2011;22:35–43. doi: 10.1016/j.cytogfr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8:384–92. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santodomingo-Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev. 2011;10:793–800. doi: 10.1016/j.autrev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Duwaerts CC, Gregory SH. Targeting the diverse immunological functions expressed by hepatic NKT cells. Expert Opin Ther Targets. 2011;15:973–88. doi: 10.1517/14728222.2011.584874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito H, Ando K, Nakayama T, et al. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–24. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima H, Inui T, Habu Y, et al. Activation of mouse natural killer T cells accelerates liver regeneration after partial hepatectomy. Gastroenterology. 2006;131:1573–83. doi: 10.1053/j.gastro.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Zhang J, Sun R, et al. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology. 2007;45:1400–12. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- 19.Hosoya S, Ikejima K, Takeda K, et al. Innate immune responses involving natural killer and natural killer T cells promote liver regeneration after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G293–9. doi: 10.1152/ajpgi.00083.2012. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–70. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 21.Sun R, Park O, Horiguchi N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–66. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Park O, Lafdil F, et al. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology. 2010;51:1354–62. doi: 10.1002/hep.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Murphy BV, Kominsky DJ, Orlicky DJ, et al. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575–84. doi: 10.1002/hep.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minagawa M, Oya H, Yamamoto S, et al. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–15. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 25.DeAngelis RA, Markiewski MM, Kourtzelis I, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188:641–8. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goh YP, Henderson NC, Heredia JE, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Feng D, Park O, et al. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: Oppositely regulated by IL-4 and IFN-gamma. Hepatology. 2013 doi: 10.1002/hep.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneiders FL, Scheper RJ, von Blomberg BM, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–41. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Kakimi K, Guidotti LG, Koezuka Y, et al. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsumi T, Takehara T, Yamaguchi S, et al. Intrahepatic delivery of alpha-galactosylceramide-pulsed dendritic cells suppresses liver tumor. Hepatology. 2007;45:22–30. doi: 10.1002/hep.21447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.