Abstract
Introduction
In registration trials, triple therapy with telaprevir (TVR), pegylated-interferon (IFN), and ribavirin (RBV) achieved sustained virological response (SVR) rates between 64–75%, but the clinical effectiveness and economic burdens of this treatment in real-world practice remain to be determined.
Methods
Records of 147 patients who initiated TVR-based triple therapy at the Mount Sinai Medical Center (5/2011–12/2011) were reviewed. Direct medical costs for pre-treatment, on-treatment, and post-treatment care were calculated using data from Medicare reimbursement databases, RED Book, and Healthcare Cost and Utilization Project database. Costs are presented in 2012 US dollars. SVR (undetectable HCV RNA 24 weeks after the end-of-treatment) was determined on an intention-to-treat basis. Cost-per-SVR was calculated by dividing the median cost by the SVR rate.
Results
Median age of the 147 patients was 56 years [interquartile range (IQR) = 51 – 61], 68% were male, 19% were black, 11% had HIV/HCV co-infection, 36% had advanced fibrosis/cirrhosis (FIB-4 scores ≥ 3.25), 44% achieved an SVR. The total cost of care was $11.56 million. Median cost of care was $83,721 per patient (IQR=$66,652– $98,102). The median cost-per-SVR was $189,338 (IQR=$150,735 – $221,860). Total costs were TVR (61%), IFN (24%), RBV (4%), adverse event management (8%), professional fees (2%), and laboratory tests (1%).
Conclusions
TVR and IFN accounted for 85% of costs. Pharmaceutical prices and the low (44%) SVR rate, in this real-world study, were major contributors to the high cost-per-SVR.
Keywords: HCV, costs, telaprevir, SVR, real-world
Introduction
Hepatitis C virus (HCV) is a major public health threat. There are about 180 million HCV-infected people worldwide, the estimated number in the United States ranges from 2.7 to 4 million (1–4). HCV infection causes a slowly progressive disease in most patients and can lead to liver cirrhosis, hepatocellular carcinoma (HCC), liver failure, and death (5). The average age of the HCV-infected population is increasing and the extent of liver disease is increasing along with it, intensifying the urgency of finding and implementing effective treatments. By 2030, 45% of the HCV-infected persons in the US are projected to have liver cirrhosis (6). A recent study raises concern that mortality among HCV-infected persons may be increasing (4).
It is important to identify the most clinically- and cost-effective strategy for reducing the burden of HCV-related liver disease. HCV-positive patients have higher healthcare costs than HCV-negative patients (7–9). Costs increase as liver disease worsens (10). Estimated mean annual healthcare-related costs are approximately $17,000 for patients without liver cirrhosis and $60,000 for those with end-stage liver disease (10). Without dramatic changes in disease management, total healthcare costs are projected to peak in 2024 at $9.1 billion, with treatment of decompensated cirrhosis accounting for 46% (11).
The aim of HCV treatment is to interrupt disease progression and potentially allow some repair to occur. Successful treatment results in a sustained virologic response (SVR), which has historically been defined as the absence of HCV viral RNA 24 weeks after the end-of-treatment (EOT). Patients who achieve an SVR have lower rates of all-cause mortality, liver-related mortality, liver decompensation, HCC, and cirrhosis than patients who are non-responders (12–16). These benefits have been demonstrated most clearly for patients with advanced disease (18); however, because patients with liver cirrhosis who achieve an SVR remain at elevated risk for the development of HCC, Koh and colleagues have postulated that the greatest benefit from SVR may be in non-cirrhotic patients (17). These findings strongly suggest that an SVR improves health and reduces health care costs. However, the magnitude of the savings is uncertain and dependent on factors that are changing over time, such as the health status of the HCV-positive population and the clinical effectiveness and cost of antiviral therapy. The surprising similarity of long term clinical outcomes of patients who relapse after achieving an EOT and patients who remain HCV viral load undetectable contributes to the uncertainty (18, 19) and underscores the need for information about the clinical and economic significance of treatment.
Before May 2011, the standard treatment for genotype 1 HCV was 48 weeks of dual therapy with pegylated-interferon (IFN) and ribavirin (RBV). This treatment had SVR rates of 35–45% in phase III clinical trials (20, 21) at a cost of about $70,364 per SVR (22). In 2011, telaprevir (TVR), a first generation direct acting antiviral drug (DAA) targeting the HCV NS3/4A protease, received FDA approval for use in genotype 1 HCV in combination with IFN/RBV (triple therapy). Triple therapy achieved SVR rates between 64–75% in the phase III clinical trials (23–25). At these success rates, HCV triple therapy was considered cost-effective (26–30); however, real-world data about SVR rates and adverse events (AEs) are needed to reach final conclusions. Severe adverse events (AEs) (23–25) and several deaths have been reported (31, 32), raising safety concerns. The phase III trials enrolled relatively few blacks, patients with advanced fibrosis or cirrhosis, or patients above the age of 65 years (23–25), yet many people in these groups need care and wish to be treated. Despite the approval of newer agents in the United States and Europe, telaprevir remains the standard of care in other countries around the world, such as Australia (33, 34).
This investigation addresses the need for additional information about outcomes and costs of TVR-based triple therapy. To our knowledge, this is the first report of outcomes and direct medical costs of this regimen in real-world practice.
Methods
Study design and patients
Study subjects were identified using a combination of traditional and enhanced-IT methods. In one case-finding method, health care providers at the Mount Sinai Medical Center compiled lists of patients with chronic genotype 1 HCV infection who initiated TVR-based triple therapy between 5/2011 and 12/2011. In the other, patients were identified by querying the Mount Sinai Data Warehouse (MSDW), a database that integrates multiple electronic health record platforms. The automated process generated a list of patients whose record included the ICD-9 code 070.54 and a TVR prescription between May and December 2011. The lists generated by the two methods were inspected and disparities were resolved by examining medical records, yielding a cohort of 147 case patients. The combination of these two methods ensured that all patients receiving at least one dose of TVR were included. Patients with a previous liver transplant were excluded. Most patients received standard TVR-based triple therapy, with 750 mg of TVR three times a day for 12 weeks, and IFN and weight-based RBV for 48 weeks. Due to a known drug-drug interaction, HIV/HCV co-infected patients on efavirenz received 1,125 mg TVR three times a day. Patients were eligible 24 weeks of response-guided therapy (RGT) if they were non-cirrhotic, treatment-naive or relapsers to dual therapy, HCV mono-infected, and had an undetectable HCV viral load at weeks 4 and 12. No patient was treated for more than 48 weeks. Adverse events (AEs) were managed by health care providers according to clinical judgment. Generally, the dose of RBV was reduced when hemoglobin dropped below 10 g/dL and simultaneously, a request for authorization of erythropoeitin-α (EPO) use was submitted. Some patients, however, received EPO prior to a RBV dose reduction.
Data on demographics, HCV kinetics, clinical laboratory tests, office visits, medications, AE management, and other aspects of medical care were collected at baseline and other key time points, typically at weeks 4, 12, 24, 48 during treatment, and at weeks 12 and 24 post-treatment. Outcomes of prior dual therapy were extracted from laboratory reports and clinical notes and coded as follows: Patients with undetectable HCV RNA at the EOT who later had detectable HCV RNA were coded as “relapsers”, those whose HCV RNA remained detectable throughout treatment were coded as “non-responders” and those who were not able to complete prior therapy due to the adverse events and side effects were coded as “intolerant.”
HCV viral load was measured using a real-time polymerase chain reaction assay (Roche Cobas Ampliprep Cobas Taqman version 2.0). HCV viral load below the lower limit of detection (18 IU/mL) was coded as “undetectable”. Virologic failure (VF) was defined as a viral load >1000 IU/mL between weeks 4–24 or above the lower limit of detection after week 24. The FIB-4 score was used to estimate the extent of liver fibrosis (35–37), with a value ≥ 3.25 indicating advanced fibrosis/cirrhosis. The SVR rate was determined on an intention-to-treat basis. Undetectable HCV RNA was imputed for missing time points if HCV RNA was undetectable before and after. The study was conducted in accordance with the Helsinki agreement, with approval of the Mount Sinai Institutional Review Board (GCO10-0032).
Use of resources and costs
Pre-treatment costs included clinical laboratory tests, imaging, and office visits. On-treatment costs included HCV medications, AE management, clinical laboratory tests, and office visits. Post-treatment costs included clinical laboratory tests, post-treatment AE management, and office visits.
Table S1 lists the costs of HCV medications, AE pharmaceuticals and biologics, hospitalizations, emergency room visits, office visits, and clinical laboratory tests. The wholesale acquisition costs (WAC) of HCV medications were obtained from the Red Book in 2012. Hospitalizations and emergency room visits were classified by ICD-9 codes, which were used to estimate charges based on the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS), 2010 and the Nationwide Emergency Department Sample (NEDS), 2008. Hospitalizations charges were converted to costs by multiplying charges by 0.38 which was the nationwide average cost-to-charge ratio for the hospitalizations observed (36, 38). ER costs were approximated by multiplying ER charges by 0.27 which was the nationwide average Medicare payment-to-charge ratio (36, 38). Cost of care included HCV medications (TVR, IFN, and RBV), adverse event management, and professional fees, and clinical laboratory tests. All costs were expressed in 2012 US dollars. Cost-per-SVR was calculated by dividing the median cost by the SVR rate.
Sensitivity analysis
Univariable sensitivity analyses were conducted to determine the impact of the SVR rate and TVR and IFN prices on the cost-per-SVR. The rate of SVR was varied over a range of 20–75% and the TVR and IFN prices were varied over a range of 80–120% of the WAC. To assess the impact of time of treatment initiation on SVR, we evenly divided the cohort into two groups and analyzed them separately for SVR rates and cost of care.
Statistical analysis
Costs are presented as the median and interquartile range (IQR). In univariable analysis, t-tests were used for normally distributed continuous variables and Mann-Whitney U-tests for non-normally distributed variables. Chi-square or Fisher exact tests were used for categorical variables. A p-value below 0.05 was considered significant. SPSS (Chicago, IL. Version 22) was used for statistical analysis.
Results
Baseline characteristics of the 147 patients on TVR-based triple therapy
Table 1 shows the characteristics for the study group at baseline. The median age was 56 years (IQR: 51–61 years), 100 (68%) were male, 28 (19%) were black, 16 (11%) were HIV-positive. The median FIB-4 score was 2.52 (IQR: 1.77–4.26): 35% of the patients had a score ≥3.25, indicating advanced fibrosis/cirrhosis (METAVIR F3-F4) (35). The majority (73%) had received dual therapy in the past: 68 (46%) were non-responders, 29 (20%) were relapsers, and 10 (7%) were IFN intolerant.
Table 1.
Baseline characteristics of the study group
| Continuous variables: median (IQR) Categorical variables: n (%) |
|
|---|---|
| Demographics | |
| N | 147 |
| Male sex | 100 (68%) |
| Age, median (IQR) | 56 (51–61) |
| Black | 28 (19%) |
| Diabetes | 25 (17%) |
| BMI, kg/m2 | 26.9 (24.5 – 29.7) |
| Advanced fibrosis/cirrhosis | 52 (35%) |
| HIV/HCV co-infection | 16 (11%) |
| HCV treatment related characteristics | |
| IL28b | |
| CC | 9 (6%) |
| CT | 33 (22%) |
| TT | 10 (7%) |
| Unknown | 95 (65%) |
| Previous response | |
| Naïve | 40 (27%) |
| Relapser | 29 (20%) |
| Non-responder | 68 (46%) |
| Intolerant | 10 (7%) |
| Log (HCV) viral load | 6.35 (5.90 – 6.74) |
| Sub-genotype | |
| 1a | 76 (52%) |
| 1b | 39 (27%) |
| Unknown | 32 (22%) |
| Undetectable HIV viral load | 8/16 (50%) |
| Laboratory tests | |
| Platelets, ×103/uL | 160 (113 – 202) |
| Hemoglobin, g/dL | 14.2 (13.2 – 15.2) |
| Albumin, g/dL | 4.3 (4.0 – 4.50) |
| AST, U/L | 61 (39 – 102) |
| ALT, U/L | 67 (44 – 108) |
Outcomes
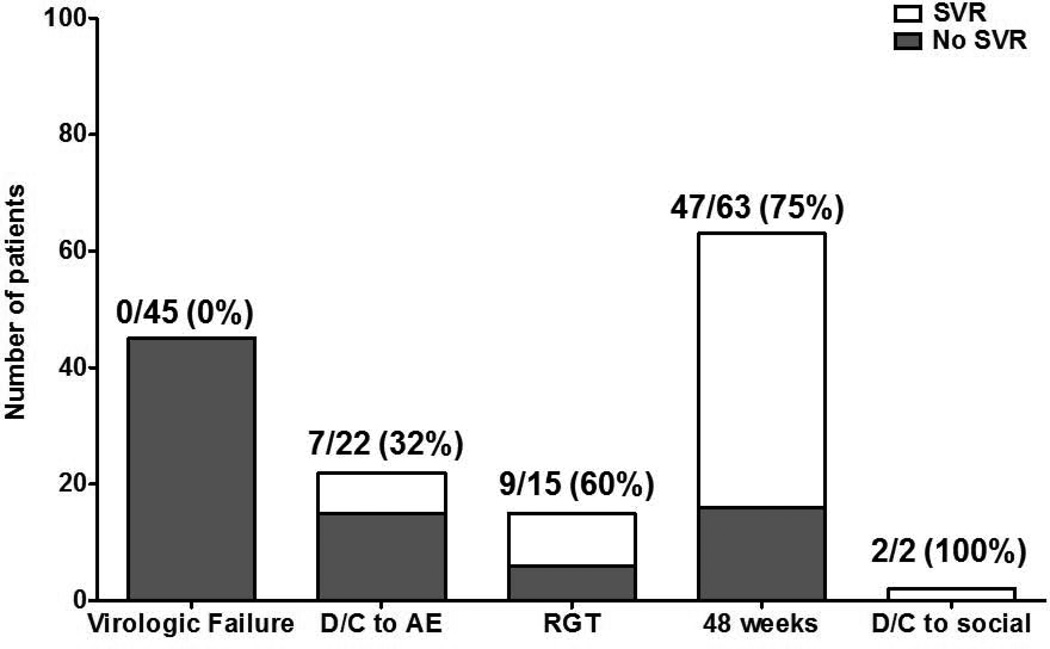
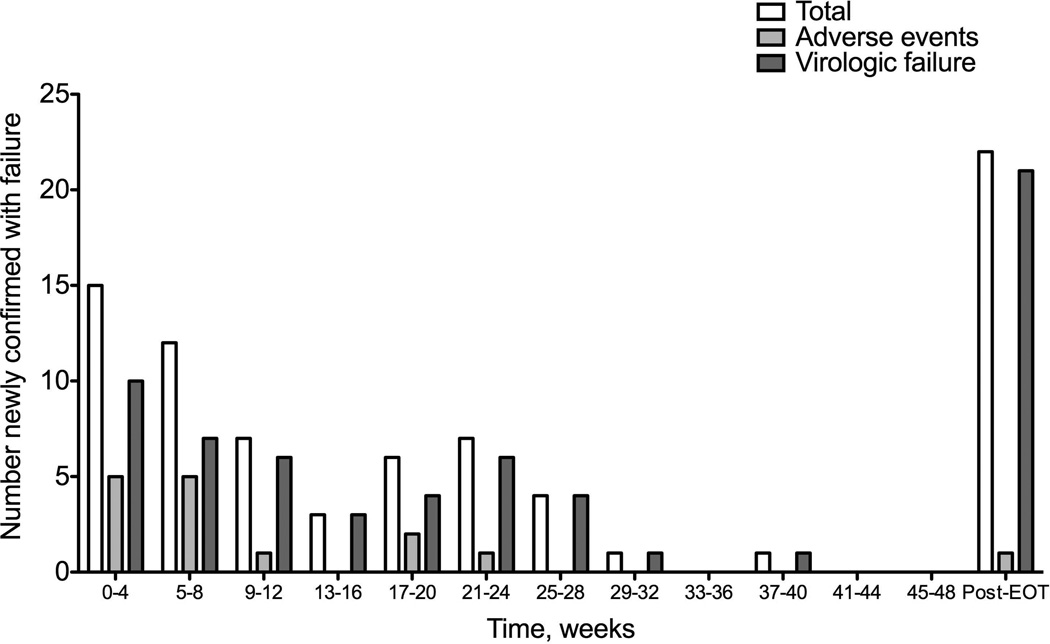
Sixty-five (44%) patients achieved an SVR. Sixty-nine (47%) stopped treatment before completing the planned regimen and the majority (87%) of these patients failed treatment. Twenty-two patients (15%) stopped during the first 12 weeks. Among the 82 (56%) patients who did not achieve an SVR, 42 had an inadequate virological response and terminated treatment according to stopping rules, 15 discontinued early due to AEs, 18 relapsed after the end-of-treatment, and 7 were lost to follow up. Figure 1 shows the SVR rates of patients in various subgroups: those who experienced virologic failure (0/45=0%), discontinued due to adverse events (7/22 =32%), completed RGT (9/15=60%), completed 48 weeks of treatment (47/63=75%) and discontinued due to social reasons (2/2=100%). Figure 2 shows the times during treatment when patients had confirmed evidence of treatment failure separated into those who discontinued due to virologic failure or adverse events.
Figure 1.
Treatment outcomes of the study group categorized by SVR and failure to achieve SVR. VF, virologic failure; D/C, discontinue; AE, adverse event; RGT, response-guided therapy.
Figure 2.
Number of patients that discontinued treatment due to virologic failure or adverse events (AE) with confirmed failure.
A comparison of SVR rates between various subgroups can be seen in Table S2. The SVR rate was higher in whites than in blacks, 50% vs. 21% (p<0.01) and it was higher in patients who completed the planned treatment than in patients who discontinued treatment early due to AEs or for social reasons, 72% vs 38% (p<0.01). SVR rates did not differ significantly by gender, previous treatment response, completion of standard treatment or RGT, HIV/HCV co-infection, and FIB-4 score below and above 3.25.
Cost of care
The total cost of care for all 147 patients was $11.56 million. Of this, the SVR group (n=65) accounted for $6.32 million (55%) and the non-SVR group (n=82) accounted for $5.24 million (45%). The median cost of care per patient was $83,721 (Table 2). Of the subgroups analyzed, the cost per patient was highest for patients completing 48 weeks of treatment ($99,357) and lowest for patients who discontinued treatment early due to adverse events ($51,778). The total costs for HCV medications, AE management, fees for professional services, and clinical laboratory tests are listed in Table 3.
Table 2.
Estimated median cost of SVR
| Outcome | Median cost per patient (IQR) |
SVR rate |
Cost per SVRa |
|---|---|---|---|
| TOTAL | $83,721 ($66,652 – $98,102) | 44% | $189,338 ($150,735 – $221,860) |
| Discontinued early for virologic failure | $65,905 ($33,294 – $75,573) | 0% | --b |
| Discontinued early for side effects | $51,778 ($29,902 – $72,388) | 32% | $162,731 ($93,978 – $227,506) |
| Completed RGT – 24 weeks | $75,321 ($75,321 – $84,771) | 60% | $125,535 ($125,535 – $141,285) |
| Completed 48 weeks | $99,357 ($94,358 – $111,042) | 75% | $133,181 ($126,480 – $148,844) |
| Discontinued early for social reasons | $84,758 ($84,758–$84,758) | 100% | $84,546 ($84,758–$84,758) |
Cost per SVR = [Median cost per patient × (1/SVR rate)]
Can not be calculated.
Table 3.
Cost of HCV medications, adverse events, laboratory fees, and physician fees by outcome, in millions
| Total | SVR | No SVR | |
|---|---|---|---|
| Telaprevir | $7.07 | $3.51 | $3.56 |
| Pegylated-interferon | $2.78 | $1.71 | $1.07 |
| Ribavirin | $0.45 | $0.28 | $0.17 |
| Adverse event management | $0.88 | $0.61 | $0.27 |
| Laboratory/Imaging Fees | $0.15 | $0.08 | $0.07 |
| Physician fees | $0.23 | $0.13 | $0.10 |
| Total | $11.56 | $6.32 | $5.24 |
HCV medications were the largest component of costs, totaling $10.30 million for the 147 patients. Table 4 shows the per-patient cost of HCV medications. The median cost per patient of these medications was $74,419, of which $55,274 was for TVR, $17,110 was for IFN, and $2,771 was for RBV. TVR, IFN, and RBV accounted for 69%, 27%, and 4% of medication costs, respectively.
Table 4.
Per patient cost of HCV medications.
| Median cost (IQR) | Median cost of patients with SVR (IQR) |
Median cost of patients without SVR (IQR) |
|
|---|---|---|---|
| All HCV medications | $74,419 ($64,110 – $90,618) | $90,618 ($81,782 – $90,618) | $68,528 ($32,055 – $75,707) |
| Telaprevir | $55,274 ($55,274 – $55,274) | $55,274 ($55,274 – $55,274) | $55,274 ($27,637 – $55,274) |
| Pegylated-interferon | $17,110 ($7,605 – $30,418) | $30,418 ($22,814 – $30,418) | $11,407 ($3,802 – $17,585) |
| Ribavirin | $2,771 ($1,232 – $4,926) | $4,926 ($3695 – $4926) | $1,847 ($616 – $2848) |
AE management costs totaled $0.88 million, 8% of the total cost of care. Eighty-three patients (56% of the cohort) had costs for AE management, which included medications/biologics and blood, hospitalizations, and emergency room visits. These costs were higher in the SVR group (Total: $611,049; Median: $10,500) than in the non-SVR group (Total: $266,561; Median: $4,829), p<0.01, as expected because of the longer duration of treatment in the SVR group, and accompanying AEs. Table S3 shows the cost of medications and blood transfusions for adverse event management. Seventy-one patients received EPO, at a total cost of $701,893, 14 received filgrastim, at a total cost of $32,704, 13 had blood transfusions, at a total cost of $13,066. Table S4 and S5 show the costs of emergency room visits and hospitalizations grouped by ICD-9 code. Emergency room costs totaled $9,214, with 27% used to treat anemia. Hospitalization costs totaled $102,403, with 54% used to treat anemia, 14% to treat infections, and 14% to treat renal insufficiency. Professional fees accounted for $234,357, 2% total costs. Clinical laboratory tests and imaging accounted for $147,740, 1% of total costs.
Cost-per-SVR and sensitivity analyses
The low, 44% SVR rate drove the cost-per-SVR to $189,338. The cost-per-SVR was higher in groups with lower SVR rates (Table 2). The cost-per-SVR was examined in various subgroups (Table 5). It was higher in previously treated patients than treatment naïve patients (p<0.01), in blacks than in non-blacks (p<0.01), in patients with genotype 1a HCV than in patients with genotype 1b HCV (p<0.01), in patients with HCV mono-infection than in patients with HIV/HCV co-infection (p=0.02), and in patients with a FIB-4 score ≥3.25 than in patients with FIB-4 <3.25 (p<0.01). Cost-per-SVR could not be calculated for relapsers because all of them failed therapy. It is important to keep in mind, however, that relapsers contributed disproportionately to the median cost-per-SVR of the study group, underscoring the importance of preventing relapse.
Table 5.
Cost of care and cost per SVR subgroup analysis
| Cost of care | p-value | SVR rate |
Cost per SVR | p-value | |
|---|---|---|---|---|---|
| Naïve | $75,579 ($47,313 – $98,187) | 0.18 | 0.48 | $159,113 ($99,607 – $206,709) | <0.01 |
| Previously Treated | $85,821 ($69,476 – $98,558) | 0.43 | $199,630 ($161,609 – 229,259) | ||
| Male | $84,240 ($68,160 – $96,927) | 0.80 | 0.44 | $191,454 ($154,909 – $220,289) | 0.52 |
| Female | $80,175 ($52,924 – $98,761) | 0.45 | $179,362 ($118,397 – $220,943) | ||
| Black | $68,754 ($23,569 – $94,107) | 0.01 | 0.21 | $327,398 ($112,234 – $448,130) | <0.01 |
| White | $82,821 ($72,108 – $98,729) | 0.5 | $171,642 ($144,215 – $197,457) | ||
| HCV genotype 1a | $79,863 ($60,377 – $96,590) | 0.93 | 0.33 | $242,746 ($183,518 – $293,586) | <0.01 |
| HCV genotype 1b | $94,107 ($55,836 – $98,558) | 0.56 | $166,857 ($99,000 – $174,749) | ||
| HCV/HIV co–infection | $81,857 ($66,156 – $97,645) | 0.39 | 0.56 | $163,412 ($126,091 – $178,335) | 0.03 |
| HCV mono-infection | $91,511 ($70,611 – $99,868) | 0.43 | $191,525 ($154,788 – $228,462) | ||
| FIB-4 >3.25 | $94,358 ($69,476 – $103,283) | 0.07 | 0.36 | $262,107 ($192,988 – $286,898) | <0.01 |
| FIB-4<3.25 | $80,130 ($65,905 – $94,107) | 0.49 | $163,864 ($134,775 – $192,448) |
In one-way sensitivity analyses, the median cost-per-SVR ranged from $418,059 to $111,482 across SVR rates of 20% to 75%, keeping costs of medications constant. The cost-per-SVR ranged from $164,090 to $214,092 across TVR prices ±20% of the WAC price at an SVR rate of 44% ; and it ranged from $181,118 to $195,970 across IFN prices ±20% of the WAC at an SVR rate of 44% (39).
To see if outcomes improved as providers gained experience with TVR, data on patients initiating treatment during the first half of the study were compared to those of patients initiating treatment during the second half; however, no significant differences were found for SVR rates (42% versus 47%, p=0.57), duration of treatment (25 weeks versus 30 weeks, p=0.32), or cost-per-SVR ($190,151 vs. $184,076, p=0.28), There was a non-significant trend in the percentage of patients completing therapy from 45% and 60% (p=0.08).
Discussion
This study reports the first data about the relationship between the clinical effectiveness and the costs of TVR-based triple therapy in real-world clinical practice. Our four major findings were (1) the SVR rate was 44%, (2) the median cost-per-SVR was $189,338 (3) TVR and IFN were the most important components of costs, accounting for about 85% of the total, and (4) 56% of patients had AEs that required management. Including HCV medications, AE costs, professional fees, and clinical laboratory tests, the median cost of care per patient was $83,721. The high cost-per-SVR was driven by the costs of TVR and IFN and the low effectiveness of treatment. Virologic failure and side effects caused early discontinuation in many patients, with only 53% of the patients completing therapy.
The cost-per-SVR in this study was more than double the projection by Thorlund and colleagues (40), who estimated a cost-per-SVR for TVR-based therapy of $74,380 – $76,370 for previously treated and untreated patients. Their projection was based on data from clinical trials and used an SVR rate between 70–90%. The discontinuation rate in our cohort was higher and the SVR rate was lower than the values they used, accounting for the disparity.
Effectiveness in clinical practice is typically lower than the efficacy achieved under the tightly controlled conditions of a trial, with the difference attributed to several factors, including the inclusion of a broader range of patients, older patients, patients with complex medical conditions, and patients who may be less adherent to treatment and other health promoting practices than those in clinical trials. Our data enable the development of models based on real-world experience with a cohort of patients closely resembling the HCV-positive population in the United States. Within the US population with chronic HCV, 39.5% are estimated to have METAVIR F3-F4 fibrosis (5), 22% are black (41), and 5% are over the age of 65 years (42). In our cohort, 35% had F3-F4 fibrosis (based on the FIB-4 score), 19% were black, 8% were over the age of 65 years, and 11% had HCV/HIV co-infection. In contrast, in clinical trials, 31% of patients had advanced fibrosis/cirrhosis, 9% were black, and none were HCV/HIV co-infected (23–25).
The cost-per-SVR in our study was strongly impacted by the cost of TVR and the SVR rate. This is consistent with two separate studies investigating the cost-effectiveness of TVR in naïve and previously treated patients conducted by Camma and colleagues (26, 39). They found that the incremental cost-effectiveness ratio of TVR triple therapy versus dual therapy was highly sensitive to the cost of TVR and the likelihood of SVR (26, 39). Other studies project that all-oral therapies for HCV will be cost-effective compared to current triple therapy regimens (43, 44).
Two new agents, simeprevir and sofosbuvir, were recently approved for HCV therapy. The pharmaceutical cost of simeprevir and sofosbuvir are greater than TVR and boceprevir; however, costs-per-SVR are expected to be lower, primarily due to higher SVR rates. Table S6 shows the expected cost of medications and the expected cost per SVR using each regimen. In comparison to the cost of TVR-based triple therapy, which ranges from $72,946 to $90,618, the costs of currently approved multi-drug regimens that contain simeprevir or sofosbuvir are substantially higher. The costs of these new regimens range from $84,024 to $170,472. At $150,000 per SVR, $480 billion will be required to induce an SVR in the estimated 3.2 million people in the US who have chronic HCV infection. This is 3% of the annual gross domestic product.
To fully assess the cost-benefit ratio of various regimens, it will be important to gain a better understanding of the long term impact on health and health care costs conferred by an SVR. A recent study provides a useful starting point. Manos and colleagues compared health care utilization costs before and after HCV IFN/RBV dual therapy and found that the adjusted difference in annual total mean costs between the SVR and non-SVR groups was $2,648 (95% CI, $737-$4,560) over a 5-year period. More effective therapies are expected to yield greater economic savings. IFN/RBV dual therapy selects for patients with specific baseline characteristics. On average, patients who achieve an SVR are younger and in better health than those who do not (45). They are less likely to be black, to have HIV co-infection, and they are more likely to have a favorable ILB28B genotype (46–49). Next generation therapies are expected to allow nearly all patients to achieve an SVR, not a selected subset. When this occurs, the economic benefits of SVR may be much greater than reported by Manos et al. Treatment will be especially beneficial if SVR leads to a long term reduction in one or more of the co-morbid conditions that are prevalent in HCV-positive population (50).
Our study has several strengths and some limitations. An independent group conducted a similar study and had identical results; the cost per patient was $83,376 and the cost per SVR was $183,428 (51). Our SVR rate, which was lower than observed in the clinical trials, has been reported in other studies, such as the CUPIC and TARGET (52, 53). As mentioned above, the cohort was racially diverse and included patients with a spectrum of liver disease and a wide range of ages. Cost estimates were based on events recorded in the medical record, rather than on group averages, which are often used to estimate health care utilization costs. However, AEs may have been under-reported in the medical record, leading to an under-estimate of AE-associated costs. Treatment costs covered by the patients, such as costs of over-the-counter medications and transportation, and the personal burdens of treatment, such as reduced productivity at work and reduced quality of life, were not included and may have been substantial. WAC prices were used instead of average wholesale prices, which may have under-estimated medication costs. Seven patients (5% of the cohort) were lost to follow up, potentially causing the SVR rate to be a slight underestimate in our intention-to-treat analysis (if one or more of these patients achieved an SVR). Since cost-to-charge ratios for emergency room visits were not available, emergency room costs were approximated by multiplying charges by medicare payment to charge ratios. Few patients were candidates for RGT and this may have increased costs. Finally, the entire cohort received TVR-based triple therapy and we are thus unable to directly compare the cost-per-SVR to alternative therapies.
In summary: Our analysis of TVR-based triple therapy in real-world practice showed that this intervention is less effective and more costly than projected. The SVR rate was 44% and the cost-per-SVR was almost $190,000 US. Our study holds important information for other countries continuing to use telaprevir (33, 34). This study provides data that will be valuable for future cost comparisons and highlights the importance of investigating new regimens outside formal clinical trials.
Supplementary Material
Acknowledgments
Sources of Funding: Supported in part by grants from Gilead Sciences, NIH DA031095, DK090317.
Valérie Martel-Laferrière was supported by 2011 AMMI Canada/Pfizer post-residency fellowship and 2012 Grant of the CHUM Foundation.
Abbreviations
- HCV
Hepatitis C Virus
- TVR
telaprevir
- PEG
pegylated-interferon
- RBV
ribavirin
- dual therapy
pegylated-interferon and ribavirin
- HCC
hepato-cellular carcinoma
- SVR
sustained virologic response
- AE
adverse events
- ER
emergency room
- AHRQ
Agency for Healthcare Research and Quality
- WAC
wholesale acquisition cost
- EPO
erythropoetin-α
- VF
virologic failure
Footnotes
Financial Disclosures:
Kian Bichoupan is a paid consultant of Gilead Sciences and Janssen Pharmaceuticals
Valerie Martel-Laferriere has no conflicts of interest.
David Sachs has no conflicts of interest.
Michel Ng serves on advisory boards for companies that include: Gilead, Janssen, and Abbott and is a paid lecturer for Boehringer Ingelheim.
Emily A. Schonfeld has no conflicts of interest.
Alexis Pappas has no conflicts of interest.
James Crismale has no conflicts of interest.
Alicia Stivala has no conflicts of interest.
Viktoriya Khaitova has no conflicts of interest.
Donald Gardenier has no conflicts of interest.
Michael Linderman has no conflicts of interest.
Ponni V. Perumalswami has no conflicts of interest.
Thomas Schiano is a paid lecturer, consultant, paid lecturer, and a participant in the data-safety monitoring board of companies that include Bristol-Myers Squibb/Sanofi-aventis partnership, Novartis, Pfizer Inc., and Salix Pharmaceuticals, Inc.
Joseph A. Odin has no conflicts of interest.
Lawrence Liu has no conflicts of interest.
Alan J. Moskowitz has no conflicts of interest.
Douglas T. Dieterich serves as a paid lecturer, consultant and is a member on scientific advisory boards of companies which either develop or assess medicines used for the treatment of viral hepatitis. These companies include Gilead Sciences, Boehringer Ingelheim, Novartis, Vertex Pharmaceuticals, Achillion, Tibotec, Idenix, Merck, Kadmon, Bayer Healthcare, Genentech and Hoffman-La Roche, Inc., and Bristol-Myers Squibb.
Dr. Andrea D. Branch is a paid consultant for Gilead Sciences, Inc. and Janssen Scientific Affairs, LLC
Contributor Information
Kian Bichoupan, Email: kian.bichoupan@mssm.edu.
Valerie Martel-Laferriere, Email: vmlaferriere@gmail.com.
David Sachs, Email: david.sachs@mssm.edu.
Michel Ng, Email: michel.ng@mountsinai.org.
Emily A. Schonfeld, Email: eschonfe@gmail.com.
Alexis Pappas, Email: alexis.pappas@gmail.com.
James Crismale, Email: jim.crismale@gmail.com.
Alicia Stivala, Email: aliciastivala@gmail.com.
Viktoriya Khaitova, Email: Viktoriya.khaitova@mountsinai.org.
Donald Gardenier, Email: Donald.gardenier@mountsinai.org.
Michael Linderman, Email: Michael.linderman@mssm.edu.
Ponni V. Perumalswami, Email: ponni.perumalswami@mountsinai.org.
Thomas D. Schiano, Email: Thomas.schiano@mountsinai.org.
Joseph A. Odin, Email: joseph.odin@mountsinai.org.
Lawrence Liu, Email: Lawrence.liu@mountsinai.org.
Alan J. Moskowitz, Email: alan.moskowitz@mountsinai.org.
Douglas T. Dieterich, Email: douglas.dieterich@mountsinai.org.
Andrea D. Branch, Email: andrea.branch@mssm.edu.
REFERENCES
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 4.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic Hepatitis C Virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of Internal Medicine. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521, e511–e516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 7.McCombs JS, Yuan Y, Shin J, Saab S. Economic burden associated with patients diagnosed with hepatitis C. Clin Ther. 2011;33:1268–1280. doi: 10.1016/j.clinthera.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 8.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17:531–546. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45:e17–e24. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608–618. e605. doi: 10.1053/j.gastro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camma C, Giunta M, Andreone P, Craxi A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593–602. doi: 10.1016/s0168-8278(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288. 288, e281. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 14.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 15.Camma C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–342. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 16.Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069–2076. doi: 10.1002/hep.23528. [DOI] [PubMed] [Google Scholar]
- 17.Koh C, Heller T, Haynes-Williams V, Hara K, Zhao X, Feld JJ, Kleiner DE, et al. Long-term outcome of chronic hepatitis C after sustained virological response to interferon-based therapy. Aliment Pharmacol Ther. 2013;37:887–894. doi: 10.1111/apt.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez SM, Foucher J, Combis JM, Metivier S, Brunetto M, Capron D, Bourliere M, et al. Longitudinal liver stiffness assessment in patients with chronic hepatitis C undergoing antiviral therapy. PLoS One. 2012;7:e47715. doi: 10.1371/journal.pone.0047715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenguer J, Alvarez-Pellicer J, Carrero A, Von Wichmann MA, Lopez-Aldeguer J, Mallolas J, Galindo MJ, et al. Clinical effects of viral relapse after interferon plus ribavirin in patients co-infected with human immunodeficiency virus and hepatitis C virus. J Hepatol. 2013;58:1104–1112. doi: 10.1016/j.jhep.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 21.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 22.YC J, T A, A K, MD D, SB H. Interferon-Based Therapies for Hepatitis C: Utilization, Costs, and Outcomes. The American Journal of Pharmacy Benefits. 2012;5:25–33. [Google Scholar]
- 23.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 24.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 26.Camma C, Petta S, Cabibbo G, Ruggeri M, Enea M, Bruno R, Capursi V, et al. Cost-effectiveness of boceprevir or telaprevir for previously treated patients with genotype 1 chronic hepatitis C. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Chan K, Lai MN, Groessl EJ, Hanchate AD, Wong JB, Clark JA, Asch SM, et al. Cost-Effectiveness Analysis of Direct-Acting Antiviral Therapy for Treatment-Naive Patients with Chronic Hepatitis C Genotype 1 Infection in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Elbasha EH, Chhatwal J, Ferrante SA, El Khoury AC, Laires PA. Cost-effectiveness analysis of boceprevir for the treatment of chronic hepatitis C virus genotype 1 infection in Portugal. Appl Health Econ Health Policy. 2013;11:65–78. doi: 10.1007/s40258-012-0007-8. [DOI] [PubMed] [Google Scholar]
- 29.Cure S, Bianic F, Gavart S, Curtis S, Lee S, Dusheiko G. Cost-effectiveness of telaprevir in combination with pegylated interferon alpha and ribavirin in previously untreated chronic hepatitis C genotype 1 patients. J Med Econ. 2014;17:65–76. doi: 10.3111/13696998.2013.860033. [DOI] [PubMed] [Google Scholar]
- 30.Blazquez-Perez A, San Miguel R, Mar J. Cost-effectiveness analysis of triple therapy with protease inhibitors in treatment-naive hepatitis C patients. Pharmacoeconomics. 2013;31:919–931. doi: 10.1007/s40273-013-0080-3. [DOI] [PubMed] [Google Scholar]
- 31.Hezode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 32.FDA. Incivek (telaprevir) In Combination with Drugs Peginterferon Alfa and Ribavirin (Incivek combination treatment): Drug Safety Communication - Serious Skin Reactions. 2012
- 33.Holmes J, Thompson A, Bell S. Hepatitis C--an update. Aust Fam Physician. 2013;42:452–456. [PubMed] [Google Scholar]
- 34.van der Meer AJ, Wedemeyer H, Feld JJ, Hansen BE, Manns MP, Zeuzem S, Janssen HL. Is there sufficient evidence to recommend antiviral therapy in hepatitis C? J Hepatol. 2014;60:191–196. doi: 10.1016/j.jhep.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 35.Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijayadeva V, Boscarino JA, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57:240–246. doi: 10.1093/cid/cit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mummadi RR, Petersen JR, Xiao SY, Snyder N. Role of simple biomarkers in predicting fibrosis progression in HCV infection. World J Gastroenterol. 2010;16:5710–5715. doi: 10.3748/wjg.v16.i45.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 38.HCUP Databases. Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2006–2011. www.hcup-us.ahrq.gov/databases.jsp. . In. [PubMed] [Google Scholar]
- 39.Camma C, Petta S, Enea M, Bruno R, Bronte F, Capursi V, Cicchetti A, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56:850–860. doi: 10.1002/hep.25734. [DOI] [PubMed] [Google Scholar]
- 40.Thorlund K, Druyts E, El Khoury AC, Mills EJ. Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection. Clinicoecon Outcomes Res. 2012;4:349–359. doi: 10.2147/CEOR.S37205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 42.Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, Luma HN, et al. The changing epidemiology of hepatitis C virus infection in the United States: National health and nutrition examination survey 2001 through 2010. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20:847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 44.Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Zhuang L, Yang L, Liu C, Lu Y, Xu Q, Chen X, et al. Efficacy and safety of peginterferon plus ribavirin for patients aged >/= 65 years with chronic hepatitis C: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2013 doi: 10.1016/j.clinre.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Xu HX, Wang LJ, Liu XX, Mahato RI, Zhao YR. Meta-analysis: IL28B polymorphisms predict sustained viral response in HCV patients treated with pegylated interferon-alpha and ribavirin. Aliment Pharmacol Ther. 2012;36:91–103. doi: 10.1111/j.1365-2036.2012.05131.x. [DOI] [PubMed] [Google Scholar]
- 47.Rangnekar AS, Fontana RJ. Meta-analysis: IL-28B genotype and sustained viral clearance in HCV genotype 1 patients. Aliment Pharmacol Ther. 2012;36:104–114. doi: 10.1111/j.1365-2036.2012.05145.x. [DOI] [PubMed] [Google Scholar]
- 48.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, Morand P, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 50.Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis. 2012;12:86. doi: 10.1186/1471-2334-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sethi N, Vong A, Firdoos S, Rourke M, Afdhal NH. Cost Effectiveness of HCV triple therapy with protease inhibitors; the true cost of SVR. 64th Annual Meeting of the American Association for the Study of Liver; Washington DC. 2013. [Google Scholar]
- 52.Hezode C, Fontaine H, Dufour JF, Dominique G, Larrey D, Zoulim F, De Ledinghen V, et al. Efficacy and safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotics according to the age. Data from the CUPIC cohort (ANRS CO20); 64th Annual Meeting of the American Association for the Study of Liver; 2013; Washington, DC. 2013. [Google Scholar]
- 53.Di Bisceglie AM, Kuo A, Rustgi V, Sulkowski MS, Sterling RK, Stewart T, Fried MW, et al. Virologic Outcomes and Adherence to Treatment Algorithms in a Longitudinal Study of Patients with Chronic Hepatitis C Treated with Boceprevir (BOC) or Telaprevir (TVR) in the United States (HCV-TARGET). 64th Annual Meeting of the American Association for the Study of Liver; Washington, DC. 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.