Abstract
Objective
Insecticide resistance molecular markers can provide sensitive indicators of resistance development in Anopheles vector populations. Assaying these makers is of paramount importance in the resistance monitoring programme. We investigated the presence and distribution of knock-down resistance (kdr) mutations in Anopheles gambiae s.l. in Tanzania.
Methods
Indoor-resting Anopheles mosquitoes were collected from 10 sites and tested for insecticide resistance using the standard WHO protocol. Polymerase chain reaction-based molecular diagnostics were used to genotype mosquitoes and detect kdr mutations.
Results
The An. gambiae tested were resistance to lambdacyhalothrin in Muheza, Arumeru and Muleba. Out of 350 An. gambiae s.l. genotyped, 35% were An. gambiae s.s. and 65% An. arabiensis. L1014S and L1014F mutations were detected in both An. gambiae s.s. and An. arabiensis. L1014S point mutation was found at the allelic frequency of 4–33%, while L1014F was at the allelic frequency 6–41%. The L1014S mutation was much associated with An. gambiae s.s. (χ2 = 23.41; P < 0.0001) and L1014F associated with An. arabiensis (χ2 = 11.21; P = 0.0008). The occurrence of the L1014S allele was significantly associated with lambdacyhalothrin resistance mosquitoes (Fisher exact P < 0.001).
Conclusion
The observed co-occurrence of L1014S and L1014F mutations coupled with reports of insecticide resistance in the country suggest that pyrethroid resistance is becoming a widespread phenomenon among our malaria vector populations. The presence of L1014F mutation in this East African mosquito population indicates the spreading of this gene across Africa. The potential operational implications of these findings on malaria control need further exploration.
Objectif
Les marqueurs moléculaires de la résistance aux insecticides peuvent fournir des indicateurs sensibles du développement de la résistance dans les populations de vecteurs Anopheles. Le test de ces indicateurs est d'une importance énorme dans le programme de surveillance de la résistance. Nous avons étudié la présence et la répartition des mutations de résistance knockdown (kdr) chez Anopheles gambiae s.l. en Tanzanie.
Méthodes
Des anophèles d'intérieur, au repos ont été collectées dans 10 sites et testées pour la résistance aux insecticides en utilisant le protocole standard de l'OMS. Les diagnostics moléculaires basés sur la PCR ont été utilisés pour le génotypage des moustiques et la détection des génotypes kdr.
Résultats
Les An. gambiae testées étaient résistantes à la lambdacyhalothrine à Muheza, Arumeru et Muleba. Sur 350 An. gambiae s.l. génotypées, 35% étaient An. gambiae s.s. et 65% étaient An. arabiensis. Les mutations L1014S et L1014F ont été détectées à la fois chez An. gambiae s.s. et An. arabiensis. La mutation ponctuelle L1014S a été trouvée à la fréquence allélique de 4 à 33%, tandis que L1014F était à la fréquence allélique de 6 à 14%. La mutation L1014S a été fortement associée à An. gambiae s.s. (Chi carré = 23,41; P<0,0001) et L1014F était associée à An. arabiensis (chi carré = 11,21; P = 0,0008). L'allèle L1014S était significativement associé aux moustiques résistants à la lambdacyhalothrine (Fisher P exact <0,001).
Conclusion
La cooccurrence des mutations L1014S et L1014F couplées à des rapports sur la résistance aux insecticides suggèrent que la résistance aux pyréthrinoïdes est en train de devenir un phénomène répandu dans les populations de vecteurs du paludisme en Tanzanie. La présence de la mutation L1014F dans cette population de moustiques en Afrique de l'Est indique la propagation de ce gène à travers l'Afrique. L'investigation des implications opérationnelles potentielles de ces résultats sur le contrôle du paludisme devraient être approfondie.
Objetivo
Los marcadores moleculares de resistencia a insecticidas pueden ser indicadores sensibles del desarrollo de resistencias en las poblaciones de los vectores Anopheles. Evaluar dichos marcadores es crucial para los programas de monitorización de resistencias. Hemos investigado la presencia y la distribución de las mutaciones de resistencia knockdown (kdr) en Anopheles gambiae s.l. en Tanzania.
Métodos
Se recolectaron mosquitos Anopheles intradomiciliarios de 10 lugares diferentes y se evaluaron en busca de resistencia a insecticidas utilizando el protocolo estándar de la OMS. Mediante un diagnóstico molecular basado en la PCR se genotiparon los mosquitos y se detectaron los genotipos kdr.
Resultados
Los An. gambiae evaluados eran resistentes a lambdacialotrina en Muheza, Arumeru y Muleba. De 350 An. gambiae s.l. genotipados, 35% eran An. gambiae s.s. y 65% eran An. arabiensis. Se detectaron mutaciones L1014S y L1014F tanto en An. gambiae s.s. como en An. arabiensis. La mutación puntual L1014S se encontró con una frecuencia alélica de 4-33%, mientras que L1014F tenía una frecuencia alélica de 6-14%. La mutación L1014S estaba ampliamente asociada a An. gambiae s.s. (Chi-Cuadrado = 23.41; P < 0.0001) y la L1014F estaba asociada con An. arabiensis (Chi-Square = 11.21; P = 0.0008). El alelo L1014S estaba significativamente asociado con mosquitos resistentes a la lambdacialotrina (P < 0.001).
Conclusión
La simultaneidad de mutaciones de L1014S y L1014F junto con informes de resistencia a los insecticidas sugiere que la resistencia a piretroides se está convirtiendo en un fenómeno común entre las poblaciones del vector de la malaria en Tanzania. La presencia de la mutación L1014F en estas poblaciones del Este de África indican la diseminación del gen a lo largo del continente africano. Determinar las implicaciones potenciales a nivel operativo de estos hallazgos sobre el control de la malaria requiere de más estudios.
Keywords: kdr, L1014S, L1014F, insecticide resistance, Anopheles gambiae, Tanzania
Introduction
Malaria vector control programmes in Africa rely heavily on the use of pesticides for insecticide-treated nets (ITNs)/long-lasting insecticide-treated nets (LLINs) and for indoor residual spraying (IRS)(WHO 2012b). The use of these strategies is known to contribute in the reduction in malaria transmission (Lengeler 2002; Pluess et al. 2010). The effectiveness of the current vector control depends much on the susceptibility of the local malaria vectors to insecticides used (WHO 2012a). Four major classes of chemical insecticides (i.e. pyrethroids, organochlorines, organophosphates and carbamates) are the mainstay of these malaria vector control strategies (Najera & Zaim 2002; WHO 2006; Kelly-Hope et al. 2008). All of these four classes are recommended for IRS. Pyrethroids are the only class of insecticide currently recommended for use on ITNs/LLINs because of their irritant and fast-acting properties and their safety for humans (Zaim et al. 2000). These major classes of chemical insecticides are nerve poisons and either target acetylcholinesterase in the synapses or the voltage-gated sodium channel in the insect neurones. Pyrethroids and DDT are neurotoxins that act on the voltage-gated sodium channels by modifying their gating kinetics, resulting in the prolonged opening of individual channels leading to paralysis and death of the insect (Ranson et al. 2011).
Massive use of insecticides in agriculture (Yadouleton et al. 2010) and public health (Czeher et al. 2008; Trape et al. 2011) has resulted in increasing resistance among malaria vectors due to the selection pressure placed on resistance genes (Ranson et al. 2011). Reduced susceptibility of Anopheles mosquitoes to insecticides such as DDT (dichloro-diphenyl-trichloroethane), malathion, fenitrothion, propoxur and bendiocarb was first reported in 1950s (Brown 1958; Hamon et al. 1968). To date, resistance among Anopheles species to at least one of the four commonly used insecticide classes has been reported in 64 malaria-endemic countries worldwide, the vast majority reporting resistance to pyrethroids (WHO 2012a,2012b). Even in four insecticide classes available for IRS, resistance has been reported for all of them in some populations of Anopheles gambiae s.s (Ranson et al. 2009). The increasing resistance of malaria vectors to available insecticides especially pyrethroids, puts current global control efforts at risk.
The major mechanisms by which insects acquire resistance to insecticides are elevated levels of detoxifying enzymes (metabolic resistance) and target-site insensitivity (Hemingway & Ranson 2000; Ranson et al. 2011). Metabolic resistance to pyrethroids is mostly associated with increased cytochrome P450 activity (Berge et al. 1998; Vulule et al. 1999). Recent studies have reported overexpression of cytochrome P450 genes: CYP6M2, CYP6P3 and CYP6Z2 in pyrethroid-resistant populations of An. gambiae (Muller et al. 2007, 2008; Djouaka et al. 2008; Mitchell et al. 2012).
Target-site insensitivity in An. gambiae is associated with two distinct mutations in the S6 transmembrane segment of domain II of the para-type sodium channel at position 1014. The mutations result in either a leucine–phenylalanine (L1014F) (Martinez-Torres et al. 1998) or a leucine–serine (L1014S) substitution (Ranson et al. 2000). The former mutation, which leads to the substitution of a leucine (TTA) for phenylalanine (TTT), was first detected in populations of the Savanna chromosomal form and S molecular form of An. gambiae s.s. in coastal Ivory Coast (Elissa et al. 1993). This was later found to be widespread in West Africa and reported to be strongly associated with pyrethroid resistance in An. gambiae (Martinez-Torres et al. 1998; Chandre et al. 1999a). The latter kdr mutation, with the same amino acid substituting the leucine (TTA) for serine (TCA), was first described in East African An. gambiae s.s. (Ranson et al. 2000). Both types of kdr mutations have been linked with DDT and pyrethroid-resistant phenotypes in wild An. gambiae s.l. populations (Martinez-Torres et al. 1998; Kolaczinski et al. 2000; Ranson et al. 2000; Donnelly et al. 2009).
Several studies with limited geographical sampling have attempted to detail the distribution of kdr mutations in An. gambiae. Most have either screened for the L1014F allele in West African countries (Martinez-Torres et al. 1998; Chandre et al. 1999b; Awolola et al. 2005; Coetzee et al. 2006), or the L1014S mutation in East Africa (Ranson et al. 2000; Kawada et al. 2011; Mawejje et al. 2012; Protopopoff et al. 2013). However some studies have screened for the presence of both resistance alleles in several parts of Africa (Stump et al. 2004; Etang et al. 2006; Pinto et al. 2006; Verhaeghen et al. 2006; Awolola et al. 2007; Moreno et al. 2008). Studies have demonstrated the presence of L1014S point mutation in West Africa (Djegbe et al. 2011) and L1014F mutation in East Africa (Kulkarni et al. 2006), indicating that the two mutations does not follow the previously described geographical distribution. Although several studies have been carried out in Tanzania to investigate the insecticide resistance status of the malaria vectors (Kulkarni et al. 2006, 2007; Kabula et al. 2012; Protopopoff et al. 2013), there has been no detailed information on the presence and the distribution of both kdr mutations in the country. This is the first such study designed to investigate the presence and the distribution of the two kdr mutations (L1014F and L1014S) in local population of Anopheles gambiae s.l. of Tanzania.
Methods
Study sites
The study was a follow-up to the main insecticide resistance survey carried in 2011. This was carried out in 10 sentinel districts across Tanzania mainland (Figure1), namely Muheza, Handeni, Lushoto, Arumeru, Uyui, Kyela, Ilala, Muleba, Kilombero and Mvomero. Additionally, this study used mosquitoes (for molecular analysis) collected in the main insecticide resistance survey from Moshi, Dodoma, Magu and Babati whose results have been reported elsewhere (Kabula et al. 2013). The study districts were chosen to encompass previously described WHO-recommended criteria (Kabula et al. 2012, 2013). The detailed characteristics of these study districts are described elsewhere (Kabula et al. 2012, 2013) and are summarised in Table1.
Figure 1.
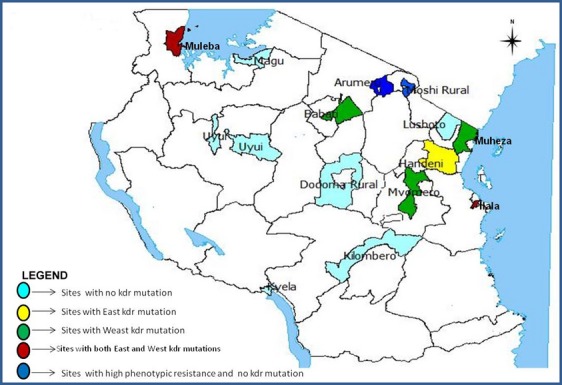
Map showing the geographical locations of the study sites and the distribution of East (L1014S) and West (L1014F) African knock-down resistance (kdr) mutations in Anopheles gambiae s.l. in Tanzania
Table 1.
Distribution of mosquitoes genotyped and characteristics of the study sites
| Region | Site | N | (N) identified as An. gambiae s.s. | (N) identified as An. arabiensis | (N) Resistant to Lambdacyhalothrin | (N) Susceptible to Lambdacyhalothrin | Agricultural Insecticide Pressure (H/L) in the site |
|---|---|---|---|---|---|---|---|
| Tanga | Handeni | 25 | 1 | 24 | 1 | 24 | For crop protection (L) |
| Dar es Salaam | Ilala | 25 | 9 | 16 | 3 | 22 | For horticulture and Industrial pollution/effluents (H) |
| Manyara | Babati | 25 | 12 | 13 | 0 | 25 | For cereals plantations (H) |
| Tanga | Muheza | 25 | 5 | 20 | 16 | 9 | For crop protection (L) |
| Kagera | Muleba | 25 | 21 | 4 | 15 | 10 | For coffee protection (H) |
| Morogoro | Mvomero | 25 | 0 | 25 | 0 | 25 | For cereal & sugarcane protection (H) |
| Kilimanjaro | Moshi | 25 | 0 | 25 | 25 | 0 | For coffee, cereal & sugarcane protection (H) |
| Arusha | Arumeru | 25 | 0 | 25 | 25 | 0 | For floriculture and coffee plantations (H) |
| Mwanza | Magu | 25 | 0 | 25 | 0 | 25 | For cotton protection (H) |
| Tanga | Lushoto | 25 | 25 | 0 | 0 | 25 | For horticulture (H) |
| Morogoro | Kilombero | 25 | 0 | 25 | 0 | 25 | For cereal & sugarcane protection (H) |
| Tabora | Uyui | 25 | 25 | 0 | 0 | 25 | For tobacco protection (L) |
| Mbeya | Kyela | 25 | 0 | 25 | 0 | 25 | For cereal & cocoa protection (H) |
| Dodoma | Dodoma Rural | 25 | 25 | 0 | 0 | 25 | For crop protection (L) |
(L/H): L – stands for low insecticide usage, H – stands for high insecticide usage; N = sample size.
Mosquito sampling
Adult female Anopheles mosquitoes for susceptibility testing and molecular characterisation of insecticide resistance were collected by the indoor-resting catch technique (WHO 1975) in June–July 2011. Indoor-resting catches were carried out between 0600 and 0900 h in all locations. Freshly blood-fed and unfed female Anopheles mosquitoes were collected. Captured mosquitoes were collected in paper cups and transported to a field laboratory for morphological identification (Gillies & Coetzee 1987) and susceptibility testing (WHO 1998). They were fed with 10% sugar solution embedded in cotton wool pads during transportation. In Tabora, Lushoto and Muleba, the number of adult Anopheles mosquitoes was not sufficient for the susceptibility test; therefore, larvae were collected and reared to adults under standard laboratory conditions (WHO 1975).
Insecticide susceptibility tests
The standard WHO susceptibility tests were conducted on field collected mosquitoes using test-kits and insecticide-impregnated filter papers supplied by the WHO (1975, 1998). Adult female Anopheles mosquitoes were exposed to 0.05% lambdacyhalothrin for 1 h. There were 4–9 replicates of 15–25 wild adult female mosquitoes per test. The controls were exposed to silicone oil impregnated paper. At this exposure time, the number of mosquitoes knocked down was recorded at 10, 15 20, 30, 40, 50 and at 60 min (WHO 1998, 2013). Mosquitoes were then transferred into the holding tube and fed on 10% (w/v) sugar solution for 24 h. Final mortality was scored after a 24-h holding. Insecticide susceptibility was classified according to the WHO criterion, which considers mortality of 98–100% and below 90% representative of susceptible and resistant populations, respectively, while the intermediates (90–97%) need further investigation (WHO 2013). Estimates for 50% knock-down time (KDT50) were assessed using log-probit analysis (Finney 1971).
Mosquito identification
Mosquitoes were identified to species based on morphological characteristics (Gillies & Coetzee 1987) and stored individually over silica gel for molecular identification and detection of kdr variants. Surviving mosquitoes from susceptibility tests were killed by exposure to ether fumes or by freezing at -20°C prior to morphological identification and storage. All lambdacyhalothrin-resistant mosquitoes were picked from each sentinel site for molecular species identification and kdr analysis. Stored mosquito samples that were previously exposed to lambdacyhalothrin in the 2011 main insecticide resistance survey (Kabula et al. 2013) from Magu, Babati, Moshi and Dodoma were also used in this molecular analysis. In sites where the number of resistant mosquitoes was less than 25 or 0, An. gambiae s.l. were picked at random to make up the total number of 25 per site (Table1). Genomic DNA was extracted from the whole mosquito of a proportion of females using standard methods (Collins et al. 1987) and amplified using specific diagnostic primers for An. gambiae s.l (Collins et al. 1987; Scott et al. 1993).
Detection of knock-down resistance (kdr) alleles in An. gambiae s.l
Mutations associated with knock-down resistance (i.e. L1014S and L1014F) to pyrethroids were assayed using the standard PCR assays (Martinez-Torres et al. 1998; Ranson et al. 2000). The PCR products were electrophoresed through 2% agarose gel with ethidium bromide stain and visualised under UV light. Successful reactions had a band of 285 bp. Additionally, there was a 210-bp band for wild-type susceptible and 188 bp for resistant allele (Figures2 and 3).
Figure 2.
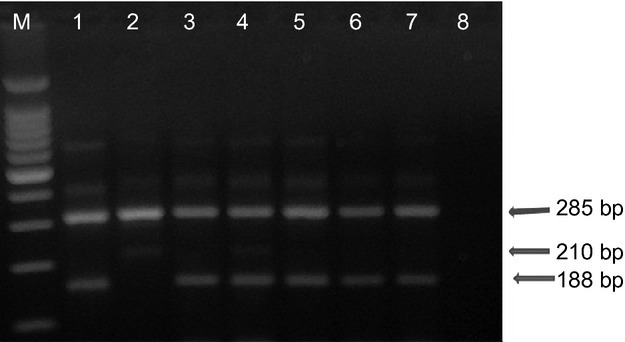
Gel electrophoresis of East African knock-down (L1014S) resistance assay. All successful reactions contain a band of 285 bp, a band of 210 bp indicates the susceptible (wild-type) allele and one of 188 bp the resistant allele. The first lane contains a 100-kb ladder marker, lane 1 is the control for the L1014S homozygous resistant, lane 2 is control for the L1014S homozygous susceptible. Lanes 3 and 5 are samples from Muleba. Lanes 4 and 6 are samples from Dar es Salaam (Ilala); lane 7, sample from Handeni; and lane 8, negative control.
Figure 3.
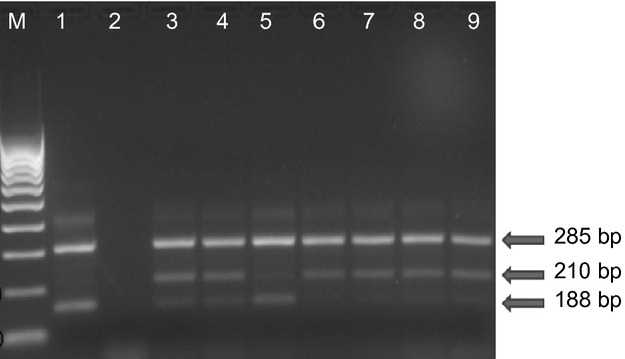
Gel electrophoresis of West African knock-down (L1014F) resistance assay. All successful reactions should contain a band of 285 bp, a band of 210 bp indicates the susceptible (wild-type) allele and one of 188 bp the resistant allele. The first lane contains a 100-kb ladder marker, lane 1 is the control for the L1014F homozygous resistant, lane 2 is a negative control, lanes 3–7 are samples from Muheza, Dar es Salaam (Ilala) and Muleba, respectively. Lanes 8 and 9 are samples from Babati (Magugu) and Mvomero respectively.
Results
Mean mortality rates of An. gambiae s.l. 24 h post-exposure (Figure4) ranged from 72% to 100%. Full susceptibility to lambdacyhalothrin was observed in Mvomero, Lushoto, Handeni, Kilombero, Kyela and Uyui (mortality of 98–100%). Resistance to lambdacyhalothrin was recorded in Muheza, Arumeru and Muleba (mortality of 83.5%, 72%, and 85%, respectively), while Dar es Salaam recorded reduced susceptibility (mortality of 96.7%).
Figure 4.
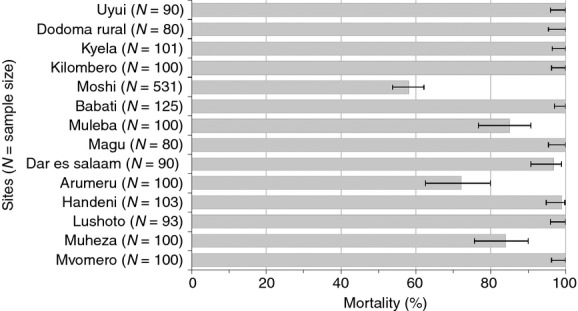
Mortality rates in field populations of Anopheles gambiae s.l. exposed to 0.05% lambdacyhalothrin for 60 min. 24-hmortalities <90% are indicative of resistance under WHO terminology and mortality of 90–97% indicates incipient resistance. N = number of mosquitoes exposed to lambdacyhalothrin. Mortality rates for Magu, Babati, Moshi and Dodoma were adapted from Kabula et al. (2013).
The median knock-down time (KDT50) of the wild mosquitoes ranged from 13.4 to 152.7 min. Highest KDT50 were recorded in Arumeru, Dar es Salaam and Muleba (KDT50 of 129, 42 and 39 min, respectively). The low KDT50 of 13.4 20.9, 21.2, 25, 27.7 and 31.9 min were recorded in Kyela, Muheza, Mvomero, Lushoto, Uyui, Kilombero and Handeni, respectively. The proportion of KDT50 of the wild populations to that of susceptible laboratory Kisumu mosquitoes known as resistance ratio (RR) was also calculated. Muleba, Dar es Salaam and Arumeru had the highest RRs. The KDT50 in these sites was between 2.6, 2.8 and 8.5 times than that of the control susceptible Kisumu strain, respectively.
A total of 1563 mosquitoes were morphologically identified as An. gambiae s.l. and tested for their susceptibility to lambdacyhalothrin. Of these, 350 (22% of the total morphologically identified mosquitoes) were identified to species level using PCR-based techniques. Of the 350, 123 (35.1%) were identified as An. gambiae s.s. and 227 (64.9%) as An. arabiensis (Table1). These 350 mosquitoes were also genotyped for kdr-east (L1014S) and kdr-west (L1014F) mutations. Of these, 341 were homozygous for the susceptible wild type and 9 were homozygous for L1014S genotype (Table2). When genotyped for L1014F, 317 were homozygous for the susceptible wild type and 33 were heterozygous (Table3). There was a significant difference in L1014S allele between lambdacyhalothrin-resistant and susceptible mosquitoes (Fisher exact P < 0.000001). However, there was no significant difference in L1014F allele between lambdacyhalothrin-resistant and susceptible mosquitoes (χ2=0.68; P = 0.409) (Table4). No L1014S allele was identified among lambdacyhalothrin susceptible (Table5).
Table 2.
Distribution of kdr-East (L1014S) mutation in An. gambiae s.s. and An arabiensis mosquitoes
|
Anopheles gambiae s.s. |
Anopheles arabiensis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype count |
Allelic frequency |
Genotype count |
Allelic frequency |
|||||||||
| Site | N | RR | RS | SS | R | S | N | RR | RS | SS | R | S |
| Handeni | 1 | 0 | 0 | 1 | 0.000 | 1.000 | 24 | 1 | 0 | 23 | 0.042 | 0.958 |
| Dar es Salaam | 9 | 3 | 0 | 6 | 0.333 | 0.667 | 16 | 0 | 0 | 16 | 0.000 | 1.000 |
| Babati | 12 | 0 | 0 | 12 | 0.000 | 1.000 | 13 | 0 | 0 | 13 | 0.000 | 1.000 |
| Muheza | 5 | 0 | 0 | 5 | 0.000 | 1.000 | 20 | 0 | 0 | 20 | 0.000 | 1.000 |
| Muleba | 21 | 5 | 0 | 16 | 0.238 | 0.762 | 4 | 0 | 0 | 4 | 0.000 | 1.000 |
| Mvomero | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Moshi | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Arumeru | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Magu | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Lushoto | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | * |
| Kilombero | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Uyui | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | * |
| Kyela | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Dodoma Rural | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | * |
RR, RS and SS are three possible kdr genotypes, where R represents the resistant L1014S allele and S represents the susceptible wild-type allele.
No member of a particular species were found in molecular identification, that is, all were identified as either An. gambiae s.s. or An. arabiensis.
Table 3.
Distribution of kdr-west (L1014F) mutation in An. gambiae s.s. and An arabiensis mosquitoes
|
Anopheles gambiae s.s. |
Anopheles arabiensis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype count |
Allelic frequency |
Genotype count |
Allelic frequency |
|||||||||
| Site | N | RR | RS | SS | R | S | N | RR | RS | SS | R | S |
| Handeni | 1 | 0 | 0 | 1 | 0.000 | 1.000 | 24 | 0 | 0 | 24 | 0.000 | 1.000 |
| Dar es Salaam | 9 | 0 | 0 | 9 | 0.000 | 1.000 | 16 | 0 | 13 | 3 | 0.406 | 0.594 |
| Babati | 12 | 0 | 0 | 12 | 0.000 | 1.000 | 13 | 0 | 3 | 10 | 0.115 | 0.885 |
| Muheza | 5 | 0 | 0 | 5 | 0.000 | 1.000 | 20 | 0 | 8 | 12 | 0.200 | 0.800 |
| Muleba | 21 | 0 | 3 | 18 | 0.071 | 0.929 | 4 | 0 | 3 | 1 | 0.375 | 0.625 |
| Mvomero | 0 | * | * | * | * | * | 25 | 0 | 3 | 22 | 0.060 | 0.940 |
| Moshi | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Arumeru | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Magu | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Lushoto | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | 1.000 |
| Kilombero | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Uyui | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | * |
| Kyela | 0 | * | * | * | * | * | 25 | 0 | 0 | 25 | 0.000 | 1.000 |
| Dodoma Rural | 25 | 0 | 0 | 25 | 0.000 | 1.000 | 0 | * | * | * | * | * |
RR, RS and SS are three possible kdr genotypes, where R represents the resistant L1014S allele and S represents the susceptible wild-type allele.
No member of a particular species were found in molecular identification, that is, all were identified as either An. gambiae s.s. or An. arabiensis.
Table 4.
Number of mosquitoes with kdr-east (L1014S) and kdr-west (L1014F) mutation genotypes among surviving (resistant) and dead (susceptible) mosquitoes after exposure to lambdacyhalothrin
|
kdr-east genotype |
kdr-west genotype |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | RR | RS | SS | Statistics | RR | RS | SS | Statistics | |
| Resistants (surviving) | 85 | 9 | 0 | 76 | Fisher's exact test P < 0.000001 | 0 | 10 | 75 | χ2 = 0.68; P = 0.409 |
| Susceptibles (dead) | 265 | 0 | 0 | 265 | 0 | 23 | 242 | ||
RR, RS and SS are three possible kdr genotypes, where R represents the resistant L1014S or L1014F allele and S represents the susceptible wild-type allele.
Table 5.
Number of mosquitoes with kdr-east (L1014S) and kdr-west (L1014F) genotypes among An. gambiae s.s. and An. arabiensis
|
kdr-east genotype |
kdr-west genotype |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | RR | RS | SS | Statistics | RR | RS | SS | Statistics | |
| An. gambiae s.s. | 123 | 8 | 0 | 115 | χ2 = 23.41; P < 0.0001 | 0 | 3 | 120 | χ2 = 11.21; P = 0.0008 |
| An. arabiensis | 227 | 1 | 0 | 226 | 0 | 30 | 197 | ||
RR, RS and SS are three possible kdr genotypes, where R represents the resistant L1014S or L1014F allele and S represents the susceptible wild-type allele.
The distribution of L1014S and L1014F mutations in An. gambiae s.s. and An. arabiensis in different parts of the country is shown in Tables2 and 3 and in Figure1. The L1014S mutation was detected in both An. gambiae s.s. and An. arabiensis. The L1014S mutation was found at the allelic frequency of 33.3% in Dar es Salaam (95% CI: 16–56%) and 23.8% in Muleba (95% CI: 13–38.5%) in An. gambiae s.s.; and 4.2% (95% CI: 1.1–13.9%) of An. arabiensis from Handeni. Similary, the L1014F point mutation was detected in both An. gambiae s.s. and An. arabiensis. The L1014F mutation was found in An. gambiae s.s. from Muleba at the allelic frequency of 7.1% (95% CI: 2.5–19%). This L1014F mutation was found in An. arabiensis at the allelic frequency of 40.6% in Dar es Salaam (95% CI:25.5–57.7%), 11.5% in Babati (95% CI:4–28.9%), 20% in Muheza (95% CI:10.5–34.8%), 37.5% in Muleba (95% CI:13.7–69.4%) and 6% in Mvomero (95% CI:2–16.2%). The L1014S and L1014F mutations occurred together in Muleba and Dar es Salaam (Figure1). Although the two kdr mutations appeared in both An. gambiae s.s. and An. arabiensis, the L1014F was much associated with An. arabiensis (χ2 = 11.21; P = 0.0008) while the L1014S was associated with An. gambiae s.s. (χ2 = 23.41; P < 0.0001) (Table5).
Discussion
Results from this study continued to demonstrate that the field population of An. gambiae s.l. are resistant to lambdacyhalothrin. Resistance of these malaria vectors to pyrethroids has previously been reported in Tanzania (Kabula et al. 2012, 2013; Protopopoff et al. 2013). The persistence of such resistance could be due the pressure created by the cumulative effect of insecticides used in malaria vector control and agriculture (Kabula et al. 2012, 2013). This study also reports the countrywide distribution of kdr mutations (L1014S and L1014F) in members of An. gambiae s.l. It reports the presence and wide distribution of the L1014S mutation in An. gambiae s.s. and An. arabiensis in Tanzania. It also further confirms the presence of L1014F point mutation in An. gambiae s.s. and An. arabiensis. The L1014S and L1014F mutations were detected in both An. gambiae s.s. and An. arabiensis. However, L1014S mutation was frequently found in An. gambiae s.s. while L1014F was frequently found in An. arabiensis. Presence of L1014F mutation at very low frequency in An. arabiensis had previously been reported in the country (Kulkarni et al. 2006) and in the neighbouring Kenya and Uganda (Stump et al. 2004; Kawada et al. 2011; Mawejje et al. 2012). The occurrence of both mutations in An. gambiae s.s. and An. arabiensis in this study may indicate that these mosquitoes have similar exposure to the sources which create selection pressure for knock-down resistance. The difference in their frequency of these mutations in the two members of An. gambiae s.l. may, however, be related to a different origin of the mutations in the two populations or linked to different ecological or behavioural characters between An. gambiae s.s. and An. arabiensis (Stump et al. 2004).
The L1014S mutation was detected in An. gambiae s.s. from Dar es Salaam (allelic frequency of 33%) and Muleba (allelic frequency of 24%) and in An. arabiensis from Handeni (allelic frequency of 4%). The L1014F mutation was found in An. gambiae s.s. from Muleba (allelic frequency of 7%) and in Anopheles arabiensis from Babati, Dar es Salaam, Muheza, Muleba and Mvomero. The L1014S and L1014F mutations co-occurred in Muleba and Dar es Salaam. The high frequency of kdr mutations in Muleba district, also previously reported (Protopopoff et al. 2013), may be a response to selection by recurrent IRS with lambdacyhalothrin since 2007, increased use of permethrin LLINs in association with the extensive usage of pesticides in coffee plantations. However, kdr has been reported in some areas with no IRS pressure in Burundi (Protopopoff et al. 2008) – which explains the occurrence of kdr in Handeni. Low insecticide usage in Handeni for agriculture may also play a role in the occurrence of kdr mutation. High frequency of kdr mutation in Dar es Salaam may be attributed to increased selection pressure resulting from industrial waste/pollutants, high LLINs use (Kabula et al. 2013) and extensive local use of insecticides for fumigation and agricultural (mainly horticulture) purposes. The high kdr frequency in Dar es Salaam is supported by the previous report of high level of DDT resistance(Kabula et al. 2012). Occurrence of kdr mutations in Muheza, Babati and Mvomero may be attributed to the high LLINs use and use of pyrethroids in agriculture.
Selection of knock-down resistance has been attributed mainly to the use of DDT and pyrethroids in agriculture and public health (Elissa et al. 1993; Stump et al. 2004). For example, the use of pyrethroids in malaria vector control interventions such as ITNs and IRS is known to create the selection of kdr alleles (Stump et al. 2004; Protopopoff et al. 2013). Similarly, domestic use of insecticides (e.g. fumigation) may play an important role in selection of knock-down resistance (Elissa et al. 1993), and this may be the case for urban settings such as Dar es Salaam.
The L1014S allele occurred significantly more often in lambdacyhalothrin phenotypically resistant-selected samples than in susceptible ones. Apart from the association found in this study, some sites which previously reported pyrethroid and DDT resistance (Kabula et al. 2012, 2013; Protopopoff et al. 2013) were found with kdr mutations (e.g. Muheza, Muleba). Such resistance to pyrethroids and DDT in An. gambiae is known to associate closely with both L1014S and L1014F mutations (Williamson et al. 1996; Martinez-Torres et al. 1998; Ranson et al. 2000, 2004; Reimer et al. 2008). However, the association was not found in the case of L1014F mutation and the pyrethroid-resistant phenotypes. Similarly, this study could not establish such associations in some sites (e.g. Mvomero and Babati) where kdr mutations were recorded without obvious phenotypic resistance to pyrethroids being observed. The absence of pyrethroid phenotypic resistance in Mvomero and Babati may be explained by the recessiveness of the kdr allele. Henceforth, the occurrence of the genes in heterozygous recessive form leads to their appearance at low frequencies in these two sites. This might explain the absence of phenotypic resistance to pyrethroids, as the conventional bioassay methods that measure phenotypic resistance cannot detect the heterozygous proportion of the population (Chandre et al. 2000). However, models of insecticide resistance show rapid increase in the frequency of resistance, especially when the frequency reaches levels as low as 0.1%, resulting in control failure (Roush & McKenzie 1987). Conversely, the presence of kdr mutation in Babati is strongly supported by KDT50 for lambdacyhalothrin. High values of KDT50 in the field mosquitoes gives early indication of the presence of kdr mutation (Chandre et al. 2000). A significant increase in knock-down time may be observed in some mosquito populations before any decrease in mortality, suggesting that knock-down time could also be a good indicator for the early detection of pyrethroid resistance (Chandre et al. 2000).
Mosquitoes from Moshi and Arumeru did not have kdr mutations despite having high levels of phenotypic pyrethroid resistance (Kabula et al. 2013). This suggests that other mechanisms are responsible for the observed phenotypic resistance in these sites. Possibly the main mechanisms involved in these sites might be biochemical resistance which had previously been reported in Moshi (Matowo et al. 2010). Even in the areas where the kdr mutations were found, the presence of other mechanisms cannot be ruled out. Both target-site insensitivity and metabolic resistance have been found in An. gambiae (Vulule et al. 1999; Stump et al. 2004; Mitchell et al. 2012). Therefore, there is a need to further investigate the presence and distribution of cytochrome P450-based metabolic resistance mechanisms in malaria vectors. Such information will help to explain the mechanism(s) of resistance responsible for the observed or even suspected resistance and thus facilitate planning for appropriate insecticide resistance management.
This study reports the countrywide distribution of L1014S and L1014F kdr mutations among members of An. gambiae s.l., and further confirms the presence of a typically West African L1014F kdr mutation in Tanzania. Therefore, we re-emphasise the need to test for both kdr mutations regardless of geographical location (Kulkarni et al. 2006). Sequencing analysis is required to provide further insights on the phylogenetic relations of the L1014F alleles found in East and West Africa. We also reported the presence and wide distribution of the L1014S mutation in An. gambiae s.s. and An. arabiensis in Tanzania. The presence of these kdr mutations in the mosquito populations has since been used as predictor for their resistance to DDT and pyrethroids (Ranson et al. 2004; Reimer et al. 2008). These findings coupled with previous reports on insecticide resistance in the country (Kabula et al. 2013; Protopopoff et al. 2013) suggest that pyrethroid resistance is a widespread phenomenon among our malaria vector populations.
The implications of high kdr frequency on the malaria vector control interventions such as ITNs and IRS are uncertain. However, studies in Benin showed some reduced effectiveness of LLINs and IRS in areas where An. gambiae have high kdr frequency (N'guessan et al. 2007; Asidi et al. 2012). Thus, the potential operational impact of insecticide resistance on the effectiveness of vector control interventions such as ITNs and IRS needs to be properly evaluated. Meanwhile, periodic monitoring of the frequency of both L1014S and L1014F mutations and phenotypic pyrethroid resistance in An. gambiae s.l. is essential for the rational and effective control of these vectors.
Acknowledgments
The authors are grateful to the data collection team and to the district and village authorities where samples were collected. The authors are also grateful to the anonymous reviewers for their valuable comments. The study was conducted by the National Institute for Medical Research in Tanzania. The work described was supported by: the malaria capacity development consortium (MCDC) through a PhD studentship scholarship to BK (MCDC is funded by The Wellcome Trust Grant Number WT084289MA); the Bill & Melinda Gates Foundation through WHO; and the U.S. President's Malaria Initiative through the U.S. Agency for International Development under the RTI International Tanzania Vector Control Scale-Up Project (621-A-00-10-00015-00). The opinions expressed in this paper are those of the authors and may not reflect the position of their employing organisations nor of their work's sources of funding.
References
- Asidi A, N'guessan R, Akogbeto M, Curtis C. Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin. Emerging Infectious Diseases. 2012;18:1101–1106. doi: 10.3201/eid1807.120218. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oyewole IO, Amajoh CN, et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Tropica. 2005;95:204–209. doi: 10.1016/j.actatropica.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Awolola TS, Oduola AO, Oyewole IO, et al. Dynamics of knockdown pyrethroid insecticide resistance alleles in a field population of Anopheles gambiae s.s. in southwestern Nigeria. Journal of Vector Borne Diseases. 2007;44:181–188. [PubMed] [Google Scholar]
- Berge JB, Feyereisen R. Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW. The insecticide-resistance problem: a review of developments in 1956 and 1957. Bulletin World Health Organization. 1958;18:309–321. [PMC free article] [PubMed] [Google Scholar]
- Chandre F, Darrier F, Manga L, et al. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bulletin of the World Health Organization. 1999a;77:231–234. [PMC free article] [PubMed] [Google Scholar]
- Chandre F, Manguin S, Brengues C, et al. Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999b;41:319–322. [PubMed] [Google Scholar]
- Chandre F, Darriet F, Duchon S, et al. Modifications of pyrethroid effects associated with kdr in Anopheles gambiae. Medical and Veterinary Entomology. 2000;14:81–88. doi: 10.1046/j.1365-2915.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Coetzee M, Wyk PV, Booman M, Koekemoer LL. Hunt RH. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bulletin of the Exotic Pathology Society. 2006;99:400–403. &. [PubMed] [Google Scholar]
- Collins FH, Mendez MA, Razmussen MO, Mehaffey PC, Besansky NJ. Finnerty V. A ribosomal RNA gene probe differentiates member species of Anopheles gambiae complex. The American Journal of Tropical Medicine and Hygiene. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. &. [DOI] [PubMed] [Google Scholar]
- Czeher C, Labbo R, Arzika I. Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malaria Journal. 2008;7:189. doi: 10.1186/1475-2875-7-189. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djegbe I, Boussari O, Sidick A, et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malaria Journal. 2011;10:261. doi: 10.1186/1475-2875-10-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka RF, Bakare AA, Coulibaly ON, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MJ, CorbeL V, Weetman D, Wilding CS, Williamson MS. Black WCT. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends in Parasitology. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. &. [DOI] [PubMed] [Google Scholar]
- Elissa N, Mouchet J, Riviere F, Meunier JY. Yao K. Resistance of Anopheles gambiae s.s. to pyrethroids in Cote d'Ivoire. Annales de La Societe Belge de Medecine Tropicale. 1993;73:291–294. &. [PubMed] [Google Scholar]
- Etang J, Fondjo E, Chandre F, et al. Short report: first report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. American Journal of Tropical Medicine and Hygiene. 2006;74:795–797. [PubMed] [Google Scholar]
- Finney JD. Probit Analysis. 3rd edn. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- Gillies MT. Coetzee M. A Supplement to the Anophelinae of Africa. South of the Sahara. Publications of the South African Institute for Medical Research. 1987;55:3–36. &. [Google Scholar]
- Hamon J, Subra R, Sales S. Coz J. Presence in the south western part of Upper Volta of a population of Anopheles gambiae resistant to DDT. Medicine Tropical (Mars) 1968;28:521–528. &. [PubMed] [Google Scholar]
- Hemingway J. Ranson H. Insecticide resistance in insect vectors of human disease. Annual Reviews Entomology. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. &. [DOI] [PubMed] [Google Scholar]
- Kabula B, Tungu P, Matowo J, et al. Susceptibility status of malaria vectors to insecticides commonly used for malaria control in Tanzania. Tropical Medicine and International Health. 2012;17:742–750. doi: 10.1111/j.1365-3156.2012.02986.x. [DOI] [PubMed] [Google Scholar]
- Kabula B, Tungu P, Malima R, et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Medical and Veterinary Entomology. 2013 doi: 10.1111/mve.12036. doi:10.1111/mve.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Futami K, Komagata O, et al. Distribution of a knockdown resistance mutation (L1014S) in Anopheles gambiae s.s. and Anopheles arabiensis in western and southern Kenya. PLoS ONE. 2011;6:e24323. doi: 10.1371/journal.pone.0024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L, Ranson H. Hemingway J. Lessons from the past:managing insecticide resistance in malaria control and eradication programmes. Lancet Infectious Diseases. 2008;8:387–389. doi: 10.1016/S1473-3099(08)70045-8. &. [DOI] [PubMed] [Google Scholar]
- Kolaczinski JH, Fanello C, Herve JP, Conway DJ, Carnevale P. Curtis CF. Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bulletin of Entomological Research. 2000;90:125–132. doi: 10.1017/s0007485300000237. &. [DOI] [PubMed] [Google Scholar]
- Kulkarni MA, Rowland M, Alifrangis M, et al. Occurrence of the leucine-to-phenylalanine knockdown resistance (kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method. Malaria Journal. 2006;5:56. doi: 10.1186/1475-2875-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni MA, Malima R, Mosha FW, et al. Efficacy of pyrethroid-treated nets against malaria vectors and nuisance-biting mosquitoes in Tanzania in areas with long-term insecticide-treated net use. Trop Med Int Health. 2007;12:1061–1073. doi: 10.1111/j.1365-3156.2007.01883.x. [DOI] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Review. Oxford: Update Software; 2002. [DOI] [PubMed] [Google Scholar]
- Martinez-Torres D, Chandre F, Williamson MS, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s. s. Insect Molecular Biology. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Matowo J, Kulkarni MA, Mosha FW, et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malaria Journal. 2010;9:193. doi: 10.1186/1475-2875-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D. Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Medical and Veterinary Entomology. 2012;27:276–283. doi: 10.1111/j.1365-2915.2012.01055.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Stevenson BJ, Muller P, et al. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6147–6152. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Vicente JL, Cano J, et al. Knockdown resistance mutations (kdr) and insecticide susceptibility to DDT and pyrethroids in Anopheles gambiae from Equatorial Guinea. Tropical Medicine and International Health. 2008;13:430–433. doi: 10.1111/j.1365-3156.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- Muller P, Donnelly MJ. Ranson H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. BMC Genomics. 2007;8:36. doi: 10.1186/1471-2164-8-36. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Warr E, Stevenson BJ, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genetics. 2008;4:e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najera JA. Zaim M. 2002. World Health Organization & Decision making criteria and procedures for the judicious use of insecticides, In vol. WHO/CDS/WHOPES/2002.5 Rev.1 Geneva.
- N'guessan R, Corbel V, Akogbeto M. Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases. 2007;13:199–206. doi: 10.3201/eid1302.060631. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J, Lynd A, Elissa N, et al. Co-occurrence of East and West African kdr mutations suggests high levels of resistance to pyrethroid insecticides in Anopheles gambiae from Libreville, Gabon. Medical and Veterinary Entomology. 2006;20:27–32. doi: 10.1111/j.1365-2915.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- Pluess B, Tanser FC, Lengeler C. Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Systematic Review. 2010:CD006657. doi: 10.1002/14651858.CD006657.pub2. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopoff N, Van Bortel W, Marcotty T, et al. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. American Journal of Tropical Medicine and Hygiene. 2008;79:12–18. [PubMed] [Google Scholar]
- Protopopoff N, Matowo J, Malima R, et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malaria Journal. 2013;12:149. doi: 10.1186/1475-2875-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J. Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Molecular Biology. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. &. [DOI] [PubMed] [Google Scholar]
- Ranson H, Paton MG, Jensen B, et al. Genetic mapping of genes conferring permethrin resistance in the malaria vector, Anopheles gambiae. Insect Molecular Biology. 2004;13:379–386. doi: 10.1111/j.0962-1075.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, Abdallah H, Badolo A, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malaria Journal. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N'guessan R, Lines J, Moiroux N, Nkuni Z. Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in Parasitology. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. &. [DOI] [PubMed] [Google Scholar]
- Reimer L, Fondjo E, Patchoke S, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. Journal of Medical Entomology. 2008;45:260–266. doi: 10.1603/0022-2585(2008)45[260:rbkmar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Roush RT. Mckenzie JA. Ecological genetics of insecticide and acaricide resistance. Annual Review of Entomology. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. &. [DOI] [PubMed] [Google Scholar]
- Scott JA, Brogdon WG. Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. &. [DOI] [PubMed] [Google Scholar]
- Stump AD, Atieli FK, Vulule JM. Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. American Journal Tropical Medicine Hygiene. 2004;70:591–596. &. [PubMed] [Google Scholar]
- Trape JF, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. The Lancet Infectious Diseases. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- Verhaeghen K, Bortel WV, Roelants P, Backeljau T. Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malaria Journal. 2006;5:16. doi: 10.1186/1475-2875-5-16. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, et al. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Medical and Veterinary Entomology. 1999;13:239–244. doi: 10.1046/j.1365-2915.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- WHO. Manual on Practical Entomology in Malaria. Geneva, Switzerland: Part II -Methods & Techniques WHO; 1975. [Google Scholar]
- WHO. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces: Report of the WHO Informal Consultation. Switzerland, Geneva: WHO; 1998. [Google Scholar]
- WHO. Pesticides and their Application for the Control of Vectors and Pests of Public Health Importance. Geneva: Switzerland, WHO; 2006. [Google Scholar]
- WHO. Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM) Geneva: Switzerland WHO-Global Malaria Programme; 2012a. [Google Scholar]
- WHO. 2012b. Geneva World Health Organization World Malaria Report 2012.
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. Switzerland, Geneva: World Health Organization; 2013. [Google Scholar]
- Williamson MS, Martinez-Torres D, Hick CA. Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Molecular and General Genetics. 1996;252:51–60. doi: 10.1007/BF02173204. &. [DOI] [PubMed] [Google Scholar]
- Yadouleton AW, Padonou G, Asidi A, et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malaria Journal. 2010;9:83. doi: 10.1186/1475-2875-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim M, Aitio A. Nakashima N. Safety of pyrethroid-treated mosquito nets. Medical and Veterinary Entomology. 2000;14:1–5. doi: 10.1046/j.1365-2915.2000.00211.x. &. [DOI] [PubMed] [Google Scholar]


