Abstract
Active Achilles tendon kinesitherapy facilitates the functional recovery of a ruptured Achilles tendon. However, protein expression during the healing process remains a controversial issue. New Zealand rabbits, aged 14 weeks, underwent tenotomy followed immediately by Achilles tendon microsurgery to repair the Achilles tendon rupture. The tendon was then immobilized or subjected to postoperative early motion treatment (kinesitherapy). Mass spectrography results showed that after 14 days of motion treatment, 18 protein spots were differentially expressed, among which, 12 were up-regulated, consisting of gelsolin isoform b and neurite growth-related protein collapsing response mediator protein 2. Western blot analysis showed that gelsolin isoform b was up-regulated at days 7–21 of motion treatment. These findings suggest that active Achilles tendon kinesitherapy promotes the neurite regeneration of a ruptured Achilles tendon and gelsolin isoform b can be used as a biomarker for Achilles tendon healing after kinesitherapy.
Keywords: achilles tendon rupture, early, motion, functional exercise, exercise, Achilles tendon, healing, proteomics, marker, tissue repair
Research Highlights
-
(1)
Active Achilles tendon kinesitherapy can promote the neurite regeneration of a ruptured Achilles tendon.
-
(2)
Gelsolin isoform b likely participates in Achilles tendon healing.
-
(3)
Gelsolin isoform b can be used as a biomarker for Achilles tendon healing after kinesitherapy.
Abbreviations
CRMP2, collapsing response mediator protein 2; 2-DE, two-dimensional electrophoresis; 2D-PAGE, two-dimensional polyacrylamide gel electrophoresis; IPG: immobilized pH gradient
INTRODUCTION
The movements by the human body result from nerve and muscle contraction after coordination which is commanded by the brain. The fatigued human body likely responses to cerebral commands poorly and gives wrong muscle contraction modes, which increase the changes of injury. The Achilles tendon is the most commonly ruptured tendon in the human body[1]. Rupture of the Achilles tendon is a particularly common injury associated with sports[2,3,4] and also frequently occurs in elderly individuals[5,6]. Many factors likely lead to spontaneous rupture of the Achilles tendon, including congenital collagen abnormality, infectious disease, rheumaimmune systemic disease, endocrine disease, neural dysfunction, hormone level abnormality, decreased blood supply to the Achilles tendon in elderly individuals, Achilles tendon degeneration caused by hyperkinesias, use of steroid hormone or norfloxacin, high temperature and tendon calcification[1]. Injuries of the Achilles tendon easily lead to skin necrosis and defects as well as Achilles tendon exposure as a result of a few local surface soft tissues and special blood supply in this region[7]. Surgical repair is thought to provide better outcomes than conservative/non-surgical approaches[8,9]. Skin necrosis easily occurs on the surface of the Achilles tendon after anastomosis of closed ruptured Achilles tendons. There is no cutaneous artery passing through the leg back, but sural nerve nutrient vessels can provide blood supply to the full-length skin of the leg back by communicating with adjacent blood-supply systems. Flaps containing sural nerve nutrient vessels are often used to repair Achilles tendon rupture accompanied by soft tissue defects in the clinic[10]. The timing of tendon mobilization following surgery remains a controversial issue. On the one hand, although immobilization can provide quick recovery, on the other, it may induce the formation of adhesions between the tendon and tendon sheath, which increases local friction and may increase the risk of future injury during loading[11]. In an effort to shorten time to functional recovery, postoperative early motion (kinesitherapy) has been advocated by several research groups and consistently shows a shorter time to full recovery and better plantar flexion strength compared with immobilization until adequate healing has occurred[12,13].
Although early kinesitherapy improves time to functional recovery of the Achilles tendon, the mechanisms underlying these improvements compared with immobilization remain poorly understood[14,15]. Tendon repair is a complex and lengthy process that involves cell mobilization and proliferation, matrix secretion and collagen deposition, with changes in gene expression playing critical roles in these steps. However, few studies have attempted to determine the changes in gene expression during healing of a ruptured Achilles tendon. One study in rats showed that immobilization suppressed the expression of genes associated with tissue repair, including brain-derived neurotrophic factor, basic fibroblast growth factor, cyclooxygenase-1 and hypoxia-inducible factor-1α[13]. However, less is known about the expression of ultrastructural proteins. Therefore, in an effort to better understand the processes involved in tendon repair following rupture, we conducted the following study using New Zealand White rabbits, which underwent tenotomy followed by cast immobilization or early joint movement. Tendon tissue was subjected to proteomics analysis using two-dimensional polyacrylamide gel electrophoresis, mass spectrometry and western blotting to identify the effects of cast immobilization and early joint movement therapy on protein expression and identify the possible proteins that could be used as biomarkers for postoperative tissue repair as well as investigate the effects of active tendon kinesitherapy on neural regeneration of the Achilles tendon.
RESULTS
Quantitative analysis of rabbits
A total of 135 New Zealand rabbits were included in this study and randomized into three groups using a Table of Random Digits: normal control (n = 15), immobilization (n = 60; rupture of the Achilles tendon + cast immobilization), and early motion (n = 60; rupture of the Achilles tendon + early joint movement). Rabbits in each group would be excluded from the study if any of the following occurred: death; loosening of the plaster cast; infection; or a gap after surgery > 1.0 mm. Consequently, 13 rabbits from the normal control group, 47 rabbits from the immobilization group and 54 rabbits from the early motion group were included in the final analysis.
Two-dimensional electrophoresis (2-DE) comparison of proteins
2-DE maps of proteins indicated that most protein spots were concentrated in the pH 3.5–9 region (Figures 1A, B). A total of 574 and 572 protein spots were detected in Achilles tendon samples in the immobilization and early motion groups, respectively, of which 560 and 567 were matched with known proteins (Table 1). A total of 38 and 18 proteins were differentially expressed in the immobilization and early motion groups, with 24 and 13, respectively, being upregulated. Figures 1C and D show enlarged images of the 2-DE gels indicating the locations of the differentially expressed proteins.
Figure 1.
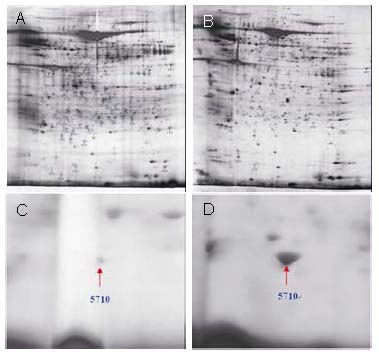
Two-dimensional electrophoresis maps (A and B) of proteins from Achilles tendon samples of rabbits in different groups at 14 days following Achilles tendon microsurgery.
Enlarged two-dimensional gels (C and D) reveal differentially expressed protein spots of 5710 in Achilles tendon samples of rabbits in immobilization (A, C) and early motion (B, D) groups.
Table 1.
Matching of protein spots in 2-dimensional polyacrylamide gels of Achilles tendon samples from rabbits in the immobilization and early motion groups

Identification of differentially expressed proteins
Differentially expressed proteins were identified in the immobilization and early motion groups (Tables 2 and 3). In the immobilization group, seven differentially expressed proteins were identified among 38 proteins compared with the early motion group. Two of 24 upregulated proteins exhibited 5-fold greater expression in the immobilization group than in the early motion group. In the early motion group, six of the 18 proteins were differentially expressed in the Achilles tendon. Six proteins showed 5-fold greater expression in the early motion group compared with 13 proteins in the immobilization group relative to the other groups.
Table 2.
Identification of differentially expressed proteins in Achilles tendon samples of rabbits in the immobilization group

Table 3.
Identification of differentially expressed proteins in Achilles tendon samples of rabbits in the early motion group

Some of the proteins identified in the early motion group are involved in various metabolism pathways and could play an important role in tissue healing, such as: myosin light chain 1, phosphoglycerate kinase (EC 2.7.2.3), F-actin capping protein subunit alpha 1, gi|45478150- LRRGT00155 protein, prolyl 4-hydroxylase, alpha I subunit isoform 2 precursor, and dihydropyrimidinase related protein-2 or collapsing response mediator protein 2 (CRMP2). The proteins with 5-fold greater expression included tetranectin precursor, chain A, crystal structure of bovine mitochondrial cytochrome Bc1 complex, gelsolin isoform b, type I, alpha 1, keratin protein and negative factor F. Figure 2 showed the identification results for peptide 5710 in the early motion group, which was identified as gelsolin isoform b. The unidentified spots were considered protein mixtures and were further separated and identified.
Figure 2.
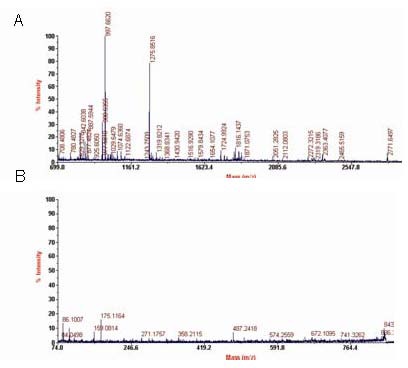
Identification of peptide 5710 in Achilles tendon samples of rabbits in the early motion group using peptide mass fingerprinting (A) and mass spectrometry sequence analysis (B).
The peptide was identified as gelsolin isoform b.
Western blot analysis
We selected one of the differentially expressed proteins, gelsolin isoform b (Figure 3), for further validation by western blotting because of its high GPS Explorer Protein confidence index (CI) and a paucity of research on this protein, as compared with the other proteins identified.
Figure 3.
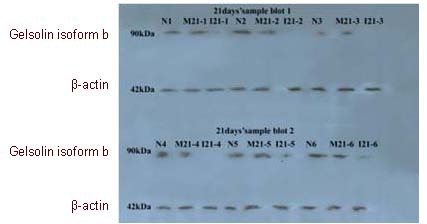
Western blot analysis of gelsolin proteins in three different groups at postoperative 21 days.
Gelsolin isoform b was further validated through western blot analysis. N: Normal control group; I: immobilization group; M: early motion group.
In Figure 4, the protein expression of gelsolin isoform b (relative absorbance) was compared using Student's t-test at each time point among the three groups. In the immobilization group, there was a significant difference between samples obtained at days 7 and 21 (P < 0.01), but not between those obtained at days 3 and 14. In the early motion group, there were significant differences between the samples obtained at day 3 and those obtained at days 7, 14 and 21 (P < 0.01), but not between the samples obtained at days 7, 14 and 21 (P > 0.05). These findings indicate significant differences between normal control and immobilization groups with regarding to increased expression of gelsolin isoform b. However, a significant difference in gelsolin isoform b expression existed between immobilization and early motion groups. Gelsolin isoform b expression was not significantly correlated with postoperative recovery time in either the immobilization group (Pearson's r = –0.885. P = 0.115) or in the early motion group (r = 0.713, P = 0.656). These findings are consistent with the results obtained by 2-DE.
Figure 4.
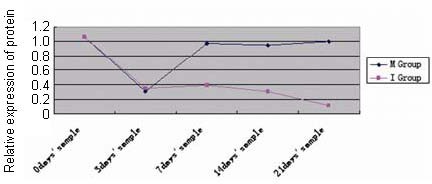
Gelsolin isoform b protein expression levels in the immobilization and early motion groups at days 3, 7, 14 and 21 after tenotomy, and in the normal control group (0 day's sample).
In the immobilization group, there was a significant difference between samples obtained at days 7 and 21 (P < 0.01), but not between those obtained at days 3 and 14. In the early motion group, there were significant differences between the samples obtained at day 3 and those obtained at days 7, 14 and 21 (P < 0.01), but not between the samples obtained at days 7, 14 and 21 (P > 0.05). Student's t-test was used.
I: Immobilization group; M: early motion group.
DISCUSSION
Proteomics research relies on large-scale, high-throughput separation and analysis technology[16,17,18,19,20,21]. In the present study, proteins were purified by 2-DE and two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) analysis, followed by identification of isolated protein using mass spectrometry and associated bio-informatics techniques. The 2D-PAGE technique can be used to compare differences between proteome samples, such as Achilles tendon samples between classical cast immobilization and early active exercise after Achilles tendon surgery, although the identification of hydrophobic proteins, extremely acid or alkaline proteins, high-molecular weight proteins and resolution of trace proteins remain difficult.
Using subproteomic technique, we found marked differences in protein expression profiles between rabbits treated by immobilization and those treated with early motion for 14 days after Achilles tendon reconstruction. Several proteins showing differential expression in the early motion group are of particular interest. Myosin light chain 1 functions as a regulatory light chain of myosin and does not bind to calcium; F-actin capping protein subunit alpha 1 regulates the polymerization state of actin; phosphoglycerate kinase generates ATP; while prolyl 4-hydroxylase and alpha I subunit isoform 2 precursor are key enzymes in collagen synthesis. Stress-mediated protein CRMP2 or dihydropyrimidinase related protein-2 is related to neural growth. In terms of the proteins showing 5-fold increases in expression, tetranectin precursor protein is involved in extracellular matrix organization, endocytosis, complement activation, pathogen recognition, and cell-cell interactions; chain A, a crystal structure of bovine mitochondrial cytochrome Bcl complex, is a predicted Zn-dependent peptidase; keratin is a cytoskeleton protein that physiologically regulates epithelial cell function; and negative factor F is involved in signal transduction. The gelsolin isoform b is an important actin-binding protein. In addition to their roles in actin filament remodeling, these proteins have some specific and apparently non-overlapping roles in several cellular processes, including gene expression regulation, cell regulation in cell movement, regulation of phagocytosis, neural growth, and programmed cell death.
Stress-mediated protein CRMP2 related to neural growth
CRMP2 or dihydropyrimidinase related protein-2 is a stress-reactive protein[22] that plays a key regulatory role[23] in establishing neural cell polarity. Meanwhile, CRMP-2 has various roles in regulating tubulin activity[24], transport to the growing axon[23,25,26], microtubule assembly and axonal extension[27,28].
The sound of the nervous system in the healing process is very important. If the healing tissue has the lack of nervous system innervations, then the tissue healing will be quite slow. The most appropriate example is spinal cord injury resulting in paraplegia patients with gluteal tissue susceptible to pressure sores, tissue necrosis and infection. Although given the perfect treatment, tissue healed always quite slow. In the present study, the rabbit Achilles tendon does not given postoperative early active kinesitherapy in the immobilization group, that is to say the Achilles tendon lacked active eccentric training at all during healing period. As in patients with spinal cord injury, the tissue heals quite slowly, so the same the Achilles tendon heals slowly in rabbit models. On the contrary, CRMP2 expressed uniquely in Achilles tendon tissue 14 days after Achilles tendon reconstruction in the rabbit models treated with early active motion, indicating that the healing Achilles tendon has won active eccentric training, and CRMP2 is involved in neural cell differentiation and axonal growth. Therefore, the neural network has recovered rapidly and systematically regulated the reorganization of healing Achilles tendon, thereupon, Achilles tendon heals smoothly.
The gelsolin isoform b of important actin-binding protein
Another one protein that showed marked differential expression in this study is gelsolin isoform b[29,30,31,32,33]. This protein is a member of the gelsolin protein superfamily, an important actin-binding protein, functions in assembly and disassembly of actin filaments; it controls actin organization by severing filaments, capping filament ends, blocking actin filaments, nucleating actin assembly, and gathering control of actin structures. Based on these known functions of gelsolin, and because few studies have investigated its role in tissue repair, we choose this protein for further validation by western blotting of Achilles tendon samples at several times after tenotomy and during the repair process.
The marked differences in protein expression between the early motion group and the immobilization group were likely due to the differences in postoperative therapy and a fact that early motion treatment influences tissue repair via mechanobiological mechanisms.
Identification of gelsolin isoform b
Western blotting analysis showed that, after surgery, there were dynamic changes in the expression of gelsolin protein in the Achilles tendon over time. In the immobilization group, gelsolin protein expression decreased significantly immediately after surgery and lowest expression was noted at day 21. The expression of gelsolin remained significantly lower in the immobilization group than in the normal control and early motion groups. On the other hand, although the expression of gelsolin decreased significantly immediately after surgery, the lowest level appeared at 3 days, and its expression returned to control level by day 7, and maintained the control level for the remainder of the study.
Collectively, these results indicate that the protein expression of gelsolin following tenotomy and surgical repair is regulated by joint movement. Furthermore, the expression of gelsolin is significantly disrupted by tissue injury and joint movement is necessary to allow its expression to recover to normal levels.
Mechanobiology during Achilles tendon healing
In clinical settings, Achilles tendon rupture is treated surgically and the limb is wrapped to provide some immobilization[34], and postoperative early motion treatment is typically controlled by the patients. Postoperative activities are generally limited to low-force, non-weight-bearing exercises, particularly for the first 3 days, followed by gradual loading, with up to 20% of body weight loading from day 3 to 1 week, and an increase to full weight-bearing loads over the following 2 weeks.
In the present study, the rabbits successfully underwent postoperative functional exercise, including centrifugal stress or eccentric training, which was controlled by self-conditioned reflex activities, such as freedom of movement and squat-stand up feeding. These functional exercises are similar to human movements. Because of pain and fear, the major activities in the first 3 days consist of non-weight-bearing, active movements, and a limited range of motion of the ankle. However, Achilles tendon repair must maintain the tension and micro-movement of the tendon. After about 3 days, the pain was slightly reduced and the rabbits were able to increase weight-bearing activity, which included standing up and squatting down. The active centrifugal stress may lead healing Achilles tendon to receive mechanical stimulation via mechanobiology mechanism. The mechanical stimulation can excite a series of stress-related proteins, such as gelsolin isoform b and CRMP2. Thus, it accelerates the healing of the Achilles tendon, maintain the function and promote the remolding of the Achilles tendon, thereby helping to restore the biomechanical properties of the Achilles tendon[14,15,34,35,36,37,38].
Limitations of the study
There are several limitations to this study. First, although we used proteomics analyses to identify differentially expressed proteins, we have not yet been able to identify some of the spots, some of which may be involved in the tissue repair response. Similarly, we can not validate the protein expression of other differentially expressed proteins, such as CRMP2. However, the ongoing research will be reported in the future. Second, the role of gelsolin isoform b and CRMP2 in the repair response could not be precisely ascertained in this study. For example, we are unable to determine whether gelsolin isoform b and CRMP2 are directly or indirectly involved in tissue repair, or whether it is simply a by-product of tissue repair processes. Further studies are needed to determine its role (if any) in tissue repair. Such studies may involve conditional knockout or gene silencing, as well as protein overexpression. Clinical studies are also needed to determine whether changes in gelsolin isoform b and CRMP2 expression are also apparent in humans.
Conclusions
The present study represents an extension of our own[14], [21], [34,35,36,37,38,39,40,41] and other studies[42,43,44,45,46,47,48,49,50,51]. We apply our novel surgical methods to perform animal experiments and then interpret the mechanisms how active kinesitherapy promotes the healing of the Achilles tendon.
Differentially expressed proteins were detected in rabbit Achilles tendon samples. Gelsolin and CRMP2 proteins could be a useful biomarker of tissue repair during postoperative early kinesitherapy. These results of this study provided further insight into the mechanobiological effects of postoperative early active eccentric training in the repair of a ruptured Achilles tendon. Further studies are needed to determine the functional role of these proteins in tissue repair and determine whether they could be used as a biomarker for tissue damage/repair.
MATERIALS AND METHODS
Design
Randomized, controlled animal experiments.
Time and setting
Animal models were prepared between January and June 2008 at Laboratory Animal Center of the First Teaching Hospital of Xinjiang Medical University, Urumqi, China.
Differential proteomics experiments and bioinformatics analysis were performed between June and December 2009 at the Center of Proteomics and Systems Biology, Institute of Biomedical Sciences, Fudan University, Shanghai, China.
Western blotting was performed between January and April 2010 at Institute of Biomedical Sciences, Tsinghua University, Beijing, China.
Materials
In total, 135 male, New-Zealand white rabbits, aged 14 weeks and weighing 2.5 ± 0.2 kg, were obtained from the Animal Center, First Teaching Hospital of Xinjiang Medical University (Urumqi, China).
Methods
Treatment
Rabbits in the immobilization and early motion groups underwent tenotomy followed immediately by Achilles tendon microsurgery. Tenotomy was performed 1.6 cm above the tendon insertion into the calcaneus of the unilateral Achilles surgery, as previously described[35]. The Achilles tendon was then repaired using the parachute-like (“Pa” bone) suture method[35]. All surgical procedures were done in an antiseptic manner in rabbits anesthetized with hypnotic induction and local anesthesia (lidocaine hydrochloride).
Postoperative rehabilitation
Rabbits in the immobilization group underwent postoperative cast immobilization with the knee joint maintained in flexion at 75° and the ankle joint in plantar flexion at 90° until sacrifice (Figure 5A)[35]. Rabbits in the early motion group received simulated and immediate postoperative early motion treatment by offering food and water; stretching exercises, centrifugal stress or eccentric training was considered to occur during alternative movements between standing up and squatting down Figure 5B)[35]. The movement frequency was approximately 150 ± 15 times/day until sacrifice.
Figure 5.

Postoperative cast immobilization (A) or postoperative early motion treatment (B) following Achilles tendon microsurgery.
Tissue collection and preparation
At days 3, 7, 14 and 21 after microsurgery, the rabbits were sacrificed (11–15 animals per group per time point) and tissue blocks (0.5 cm × 0.5 cm × 0.5 cm) were resected from the repaired Achilles tendon. Surrounding non-tendinous tissue was removed. The samples were thoroughly rinsed three times with 0.9% saline solution (4°C), and then snap-frozen in liquid nitrogen. These procedures were accomplished within 1 hour to ensure the healing period of the Achilles tendon.
The proteomics study was performed in the immobilization group and the early motion group at 14 days. Western blot analysis was performed in each group at each time point. Six samples were randomly selected from each group at each time point and tested in triplicate.
For protein extraction, the tissues were thawed, cut into small pieces with scissors, and rinsed with PBS to remove impurities. Samples (50 μg) were then crushed with a mortar, immersed in CSCP-chaps buffer (50 mM dithiothreitol, 0.1% phenylmethanesulfonyl fluoride, 7 M urea, and 2 M thiourea; Amersham Biosciences) and centrifuged at 4 400 × g for 45 minutes at 4°C. Protein concentrations were quantified using the Bradford method, as previously described[15] and cleaned using a Ready Pre 2-D Cleanup Kit (Bio-Rad Laboratories, Hercules, CA, USA).
2D-PAGE
For 2D-PAGE, protein samples obtained at 14 days after microsurgery were separated by isoelectric focusing according to the isoelectric point. Protein samples (400 μg) were loaded onto 18-cm immobilized pH gradient (IPG) strips with a non-linear pH range of 3–10 using in-gel rehydration (Amersham Pharmacia, Uppsala, Sweden). Each strip was overlaid with 2–3 mL mineral oil to prevent evaporation during rehydration. The IPG strips were then placed on a tray and isoelectric focusing was performed at 20°C using an IPG-Phor isoelectric system (Amersham Pharmacia). The initial voltage was set at 250 V and raised stepwise to 4 000 V to remove salt. Proteins were focused for 8 hours at 8 000 V. The IPG strips were removed and mineral oil was drained using wet filter paper. Immobilized proteins on the IPG strip were then run on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel (1 mm thick) and separated based on molecule weight using a Protean II electrophoresis system at 15 mA/gel (Bio-Rad Laboratories). The 2D gel was silver-stained for direct mass spectrometry[17,18].
The gels were scanned using a GS-800 molecular imaging system (Bio-Rad Laboratories), and PDQuest software (version 8.0; Bio-Rad Laboratories) was used for spot detection, spot matching, and quantitative intensity analysis. Gel images were normalized according to total expression in the analysis set, and differentially expressed proteins were defined by measuring density values of the protein spots. A 5-fold difference in protein spot abundance was used as the threshold.
Mass spectrometry analysis
Differentially expressed spots were excised from the gel using a spot cutter. In-gel digestion with trypsin (Promega, Madison, WI, USA) was performed to extract peptides, as previously described[18,19]. Each sample was suspended in 0.8 μL of 0.5 g/L matrix solution [α-EHCC-CHCA in acetonitrile/water (1:1, v/v) acidified with 0.1% (v/v) trifluoroacetic acid]. Then the mixture was immediately spotted onto a stainless steel MALDI target plate and allowed to dry and crystallize at room temperature. Mass spectrometry was performed using a 4700 Proteomics Analyzer (TOF/TOF™; Applied Biosystems, Foster, CA, USA) equipped with a 337 nm Nd: YAG laser. Peptide mass fingerprinting, combined with MS/MS queries, was performed using the MASCOT search engine (Matrix Science, London, UK) embedded into GPS-Explorer (V3.6, ABI) Software (Applied Biosystems/MDS SCIEX, Framingham, MA, USA) on the NCBInr database with the following settings: species, homo sapiens; mono-charged peptides; 50-ppm peptide mass accuracy; trypsin cleavage; 1 missed cleavage allowed; carbamidomethylation set as fixed modification; oxidation of methionines was allowed as variable modification; and the MS/MS fragment tolerance was set to 0.25 Da. Protein hits with MASCOT Protein score ≥ 68 and a GPS Explorer Protein confidence index (CI) ≥ 95% were used for further manual validation.
Validation of differentially expressed proteins by western blot analysis
Western blotting was performed three times to verify differentially expressed proteins at postoperative days 3, 7, 14 and 21. Six samples were randomly selected from each group at each time point. The selected proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. After washing and blocking of non-specific binding, the blocking fluid was removed and the membranes were incubated with 10 mL of rabbit anti-gelsolin isoform b antibody diluted 1:1 000 in PBS or rabbit anti-β-actin diluted 1:5 000 in PBS (both, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 12 hours (overnight) at 4°C. After washing, the membranes were incubated with anti-goat IgG horseradish peroxidase- conjugated secondary antibody (1:10 000) Bio Technology, Beijing, China) for 2 hours at room temperature. The membranes were washed with Tris-buffered saline containing Tween-20 once for 10 minutes and three times for 5 minutes each. The proteins were then visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA), followed by scanning and gray value analysis using Digital Image-Pro Plus software (version 4.1; Media Cybernetics, Bethesda, MD, USA). Relative protein expression was normalized to β-actin.
Statistical analysis
Statistical analyses were performed using Student's t-test (Stata version 10.0; StataCorp., College Station, TX, USA) to analyze data derived from 2D gels using six density values obtained for each protein spot. P < 0.05 was considered statistically significant. To further determine the correlation in gelsolin isoform b and the postoperative recovery time, Pearson's correlation analysis was performed in the immobilization and early motion therapy groups from day 0 to day 21. A P value < 0.05 was considered statistically significant.
Acknowledgments
We would like to express our thanks to Jin H, Yang PY, Yang FY and Zhou XW, Center of Proteomics and Systems Biology, Institute of Biomedical Sciences, Fudan University, Shanghai, China. We also thank Zhang YT, Zhang BY, Yin XL, Institutes of Biomedical Sciences, Tsinghua University, Beijing, for western blotting.
Footnotes
Funding: This study was financially supported by the National Natural Science Foundation of China, No. 30760256.
Conflicts of interest: None declared.
Ethical approval: The experimental protocols were approved by the Research Ethics Committee at Xinjiang Medical University, China.
(Edited by Song LP)
REFERENCES
- [1].Maffulli N, Ajis A, Longo UG, et al. Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12(4):583–96. doi: 10.1016/j.fcl.2007.07.007. vi. [DOI] [PubMed] [Google Scholar]
- [2].Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- [3].Parekh SG, Wray WH, 3rd, Brimmo O, et al. Epidemiology and outcomes of Achilles tendon ruptures in the National Football League. Foot Ankle Spec. 2009;2(6):283–386. doi: 10.1177/1938640009351138. [DOI] [PubMed] [Google Scholar]
- [4].Sankey RA, Brooks JH, Kemp SP, et al. The epidemiology of ankle injuries in professional rugby union players. Am J Sports Med. 2008;36(12):2415–2424. doi: 10.1177/0363546508322889. [DOI] [PubMed] [Google Scholar]
- [5].Cretnik A, Kosir R, Kosanović M. Incidence and outcome of operatively treated Achilles tendon rupture in the elderly. Foot Ankle Int. 2010;31(1):14–18. doi: 10.3113/FAI.2010.0014. [DOI] [PubMed] [Google Scholar]
- [6].Maffulli N, Waterston SW, Squair J, et al. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med. 1999;9(3):157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- [7].Maffulli N, Longo UG, Gougoulias N, et al. Ipsilateral free semitendinosus tendon graft transfer for reconstruction of chronic tears of the Achilles tendon. BMC Musculoskelet Disord. 2008;9:100. doi: 10.1186/1471-2474-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lynch RM. Achilles tendon rupture: surgical versus non-surgical treatment. Accid Emerg Nurs. 2004;12(3):149–158. doi: 10.1016/j.aaen.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [9].Molloy A, Wood EV. Complications of the treatment of Achilles tendon ruptures. Foot Ankle Clin. 2009;14(4):745–759. doi: 10.1016/j.fcl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [10].Chen XS, Chen JM, Xiao MM, et al. Modified sural neurocutaneous vascular flap based on single dominant perforator arising from peroneal artery for coverage of defects over achilles tendon. Zhonghua Zhengxing Waike Zazhi. 2012;28:22–25. [PubMed] [Google Scholar]
- [11].James R, Kesturu G, Balian G, et al. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- [12].Cetti R, Henriksen LO, Jacobsen KS. A new treatment of ruptured Achilles tendons. A prospective randomized study. Clin Orthop Relat Res. 1994;(308):155–165. [PubMed] [Google Scholar]
- [13].Troop RL, Losse GM, Lane JG, et al. Early motion after repair of Achilles tendon ruptures. Foot Ankle Int. 1995;16(11):705–709. doi: 10.1177/107110079501601106. [DOI] [PubMed] [Google Scholar]
- [14].Jielile J, Bai JP. Biomechanics during Achilles tendon healing. Chin J Clin Rehabilitative Tissue Eng Res. 2008;12(42):8352–8357. [Google Scholar]
- [15].Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [16].Bring D, Reno C, Renstrom P, et al. Prolonged immobilization compromises up-regulation of repair genes after tendon rupture in a rat model. Scand J Med Sci Sports. 2010;20(3):411–417. doi: 10.1111/j.1600-0838.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- [17].Sun QJ, Miao MY, Jia X, et al. Subproteomic analysis of the mitochondrial proteins in rats 24 h after partial hepatectomy. J Cell Biochem. 2008;105(1):176–184. doi: 10.1002/jcb.21811. [DOI] [PubMed] [Google Scholar]
- [18].Peter K. Proteomics unravels platelet function. Blood. 2010;115(20):4008–4009. doi: 10.1182/blood-2010-02-270298. [DOI] [PubMed] [Google Scholar]
- [19].Gharahdaghi F, Weinberg CR, Meagher DA, et al. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20(3):601–605. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [20].Welch GR, Clegg JS. From protoplasmic theory to cellular systems biology: a 150 year reflection. Am J Physiol Cell Physiol. 2010;298(6):C1280–1290. doi: 10.1152/ajpcell.00016.2010. [DOI] [PubMed] [Google Scholar]
- [21].Wu YF, Yin ZZ, Jielile J. Proteomics and its application in the studies of orthopedic disease. Chin J Med Postgrad. 2009;22(7):758–761. [Google Scholar]
- [22].Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48(6):881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- [23].Chae YC, Lee S, Heo K. Collapsin response mediator protein-2 regulates neurite formation by modulating tubulin GTPase activity. Cell Signal. 2009;21(12):1818–1826. doi: 10.1016/j.cellsig.2009.07.017. [DOI] [PubMed] [Google Scholar]
- [24].Kawano Y, Yoshimura T, Tsuboi D, et al. CRMP-2 is involved in Kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol. 2005;25(22):9920–9935. doi: 10.1128/MCB.25.22.9920-9935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fukata Y, Itoh TJ, Kimura T, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4(8):583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- [26].Kimura T, Watanabe H, Iwamatsu A, et al. Tubulin and CRMP-2 complex is transported via Kinesin-1. Neurochem. 2005;93(6):1371–1382. doi: 10.1111/j.1471-4159.2005.03063.x. [DOI] [PubMed] [Google Scholar]
- [27].Arimura N, Hattori A, Kimura T, et al. CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. J Neurochem. 2009;111(2):380–390. doi: 10.1111/j.1471-4159.2009.06317.x. [DOI] [PubMed] [Google Scholar]
- [28].Hamajima N, Matsuda K, Sakata S, et al. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene. 1996;180(1-2):157–163. doi: 10.1016/s0378-1119(96)00445-3. [DOI] [PubMed] [Google Scholar]
- [29].Litwin M, Mazur AJ, Nowak D, et al. Gelsolin in human colon adenocarcinoma cells with different metastatic potential. Acta Biochim Pol. 2009;56(4):739–743. [PubMed] [Google Scholar]
- [30].Mazzolai SL, Gauci C, Stergiopulos N, et al. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61(19-20):2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ohnishi M, Matsumoto T, Nagashio R, et al. Proteomics of tumor-specific proteins in cerebrospinal fluid of patients with astrocytoma: usefulness of gelsolin protein. Pathol Int. 2009;59(11):797–803. doi: 10.1111/j.1440-1827.2009.02447.x. [DOI] [PubMed] [Google Scholar]
- [32].Shoja MM, Ardalan MR, Tubbs RS, et al. Outcome of renal transplant in hereditary gelsolin amyloidosis. Am J Med Sci. 2009;337(5):370–372. doi: 10.1097/MAJ.0b013e3181a4199c. [DOI] [PubMed] [Google Scholar]
- [33].Solomon JP, Yonemoto IT, Murray AN, et al. The 8 and 5 kDa fragments of plasma gelsolin form amyloid fibrils by a nucleated polymerization mechanism, while the 68 kDa fragment is not amyloidogenic. Biochemistry. 2009;8(48):11370–11380. doi: 10.1021/bi901368e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jielile J, Bai JP, Wang Y. An experimental study and clinical application of parachute-like through calcaneus suture for repairing Achilles tendon rupture. Chin J Clin Rehab Tissue Eng Res. 2007;11(8):1419–1425. [Google Scholar]
- [35].Jielile J, Bai JP, Sabirhazi G, et al. Factors influencing the tensile strength of repaired Achilles tendon: a biomechanical experiment study. Clin Biomech (Bristol, Avon) 2010;25(8):789–795. doi: 10.1016/j.clinbiomech.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [36].Jielile J, Bai JP, Wang YJ. A biomechanical in vitro study of parachute-like and through-calcaneus suture combination for the repair of rabbit Achilles tendon rupture. J Med Biomech. 2004;19(2):108–111. [Google Scholar]
- [37].Jielile J, Bai JP. The development of research on the suture material to repair the Achilles tendon rupture. Chin J Med Biomech. 2003;18(1):62–65. [Google Scholar]
- [38].Tang B, Bai JP, Jielile J, et al. The serum difference protein mass spectrum expression under the mobilization and immobilization state after Achilles tendon of rabbits rupture repair. J Xinjiang Med Univ. 2009;32(4):463–465. [Google Scholar]
- [39].Jielile J. Development of suture method to repair Achilles tendon rupture. Chin J Gen Pract. 2003;12(1):49–53. [Google Scholar]
- [40].Jielile J, Bai JP. Progress in the clinical diagnosis of Achilles tendon rupture. Chin Gen Pract. 2004;3(9):823–825. [Google Scholar]
- [41].Tang B, Bai JP, Jielile J, et al. The histological effects of early mobilization in the healing after Achilles tendon of rabbits rupture repair. J Chin Physicians. 2010;12(7):19–22. [Google Scholar]
- [42].Aoki M, Ogiwara N, Ohta T, et al. Early active motion and weight bearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794–800. doi: 10.1177/03635465980260061001. [DOI] [PubMed] [Google Scholar]
- [43].Grewal R, Saw SC, Varitimidus S, et al. Evaluation of passive and active rehabilitation and of tendon repair for partial tendon lacerations after three weeks of healing in canines. Clin Biomech. 2006;21(8):804–809. doi: 10.1016/j.clinbiomech.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [44].Kerkhoffs GM, Struijs PA, Raaymakers EL, et al. Functional treatment after surgical repair of acute Achilles tendon rupture: wrap vs walking cast. Arch Orthop Trauma Surg. 2002;122(2):102–105. doi: 10.1007/s004020100312. [DOI] [PubMed] [Google Scholar]
- [45].Levy M, Velkes S, Goldstein J, et al. A method of repair for Achilles tendon ruptures without cast immobilization. Clin Orthop Relat Res. 1984;(187):199–204. [PubMed] [Google Scholar]
- [46].Lieberman JR, Lozman J, Czajka J, et al. Repair of Achilles tendon ruptures with Dacron vascular graft. Clin Orthop. 1988;(234):204–208. [PubMed] [Google Scholar]
- [47].Marti R, Weber BG. Achillessehnenruptur-funktionelle Nachbehandlung. Helvetica Chir Acta. 1974;4:293–296. [PubMed] [Google Scholar]
- [48].Motta P, Errichiello C, Pontini I. Achilles tendon rupture: a new technique for easy surgical repair and immediate movement of the ankle and foot. Am J Sports Med. 1997;25(2):172–176. doi: 10.1177/036354659702500205. [DOI] [PubMed] [Google Scholar]
- [49].Sorrenti SJ. Achilles tendon rupture: effect of early mobilization in rehabilitation after surgical repair. Foot Ankle Int. 2006;27(6):407–410. doi: 10.1177/107110070602700603. [DOI] [PubMed] [Google Scholar]
- [50].Speck M, Klaue K. Early full weight bearing and functional treatment after surgical repair of acute Achilles tendon rupture. Am J Sports Med. 1998;26(6):789–793. doi: 10.1177/03635465980260060901. [DOI] [PubMed] [Google Scholar]
- [51].Yotsumoto T, Miyamoto W, Uchio Y. Novel approach to repair of acute Achilles tendon rupture: early recovery without postoperative fixation or orthosis. Am J Sports Med. 2010;38(2):287–292. doi: 10.1177/0363546509351557. [DOI] [PubMed] [Google Scholar]


