Abstract
OBJECTIVE:
To identify global research trends in the use of nerve conduits for peripheral nerve injury repair.
DATA RETRIEVAL:
Numerous basic and clinical studies on nerve conduits for peripheral nerve injury repair were performed between 2002–2011. We performed a bibliometric analysis of the institutions, authors, and hot topics in the field, from the Web of Science, using the key words peripheral nerve and conduit or tube.
SELECTION CRITERIA:
Inclusion criteria: peer-reviewed published articles on nerve conduits for peripheral nerve injury repair, indexed in the Web of Science; original research articles, reviews, meeting abstracts, proceedings papers, book chapters, editorial material, and news items. Exclusion criteria: articles requiring manual searching or telephone access; documents not published in the public domain; and several corrected papers.
MAIN OUTCOME MEASURES:
(a) Annual publication output; (b) publication type; (c) publication by research field; (d) publication by journal; (e) publication by funding agency; (f) publication by author; (g) publication by country and institution; (h) publications by institution in China; (i) most-cited papers.
RESULTS:
A total of 793 publications on the use of nerve conduits for peripheral nerve injury repair were retrieved from the Web of Science between 2002–2011. The number of publications gradually increased over the 10-year study period. Articles constituted the main type of publication. The most prolific journals were Biomaterials, Microsurgery, and Journal of Biomedical Materials Research Part A. The National Natural Science Foundation of China supported 27 papers, more than any other funding agency. Of the 793 publications, almost half came from American and Chinese authors and institutions.
CONCLUSION:
Nerve conduits have been studied extensively for peripheral nerve regeneration; however, many problems remain in this field, which are difficult for researchers to reach a consensus.
Keywords: nerve conduit, biomaterial, axon, neurotrophic factor, stem cell, extracellular matrix, peripheral nerve injury, peripheral nerve repair, degradation, biocompatibility, neural regeneration
Research Highlights
-
(1)
We performed a bibliometric analysis of studies published during 2002–2011 retrieved from the Web of Science on the use of nerve conduits for peripheral nerve injury repair.
-
(2)
We analyzed the publication year, type, research field, journal, funding agency, author, country, and institution.
-
(3)
We analyzed the institutions and authors depending on the number of publications. We especially analyzed the publication patterns of Chinese institutions and authors to provide information on the research status of this field in China.
INTRODUCTION
Peripheral nerve injury is a common clinical disease, and its incidence is much higher than central nervous system injury. Under suitable conditions, axons on the proximal side of the damaged peripheral nerve can regenerate the original nerve functions through sprouting regeneration[1,2]. Prior to this, the severed ends must re-aggregate so that the axonal growth cone can grow smoothly in right direction towards the endoneurial tube on the distal side, before it can reinnervate the target organs and re-dominate the intrinsical domain[3,4,5]. In the case of nerve tissue defects, direct anastomosis of the severed nerve can cause tension, which can result in hyperplasia of fibrous tissue near the anastomotic stoma, thereby seriously obstructing axonal growth. Thus, physicians mainly choose nerve autografting in the case of large defects to avoid anastomotic tension on the nerve and ensure successful axonal regeneration[4,5,6,7,8]. However, this method has some unavoidable disadvantages: additional surgeries are required to obtain a donor nerve; functional lesions occur at the donor site; there are limited sources of donor nerve; autografting cannot repair wide or severe neurological defects, especially in cases of brachial plexus injury[9,10,11]. For these reasons, researchers have been continually exploring the use of neural conduits to bridge nerve defects, so that one day they will be able to effectively replace autologous transplantation. The discovery and application of nerve conduits has had some success. Furthermore, as nerve conduits are fabricated from biological or synthetic materials, they do not require any donor tissue from other parts of the nervous system.
In this study, we analyzed the research trends in the use of nerve conduits for the repair of peripheral nerve injury, based on a bibliometric analysis of papers from the Web of Science during 2002–2011.
DATA SOURCES AND METHODOLOGY
Data retrieval
This study used bibliometric analyses to quantitatively and qualitatively investigate research trends in studies of nerve conduits for peripheral nerve injury repair. We searched the Web of Science, a research database of publications and citations selected and evaluated by the Institute for Scientific Information in Philadelphia, PA, USA, using the key words peripheral nerve and conduit or tube. We limited the period of publication from 2002–2011, and compiled a bibliography of all articles related to nerve conduits for peripheral nerve injury repair. All data were downloaded on August 12, 2012.
Inclusion criteria
The inclusion criteria were as follows: (1) published peer-reviewed articles on the use of nerve conduits for peripheral nerve injury repair, including original research articles, reviews, meeting abstracts, proceedings papers, book chapters, editorial material, and news items, which were indexed in the Web of Science; (2) published between 2002-2011; and (3) the citation database was the Science Citation Index Expanded.
Exclusion criteria
We excluded articles that required manual searching or telephone access, documents that were not published in the public domain, and several corrected papers from the total articles analyzed.
The outcomes of all articles referring to the use of nerve conduits for peripheral nerve injury repair were assessed using the following criteria: (a) annual publication output; (b) type of publication; (c) publication by research field; (d) publication by journal; (e) publication by funding agency; (f) publication by author; (g) publication by country and institution; (h) publication by institution in China; (i) most-cited papers.
RESULTS
Output by year of publications relating to nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011 (Figure 1)
Figure 1.
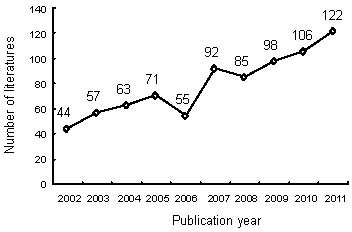
Output by year of publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011.
A total of 793 publications on nerve conduits for peripheral nerve injury repair were retrieved from Web of Science, 2002–2011. The number of relevant publications gradually increased over the 10-year study period, with 44 papers published and included in the Web of Science in 2002, increasing to 122 in 2011. Numbers of papers published slightly decreased in 2006 and 2008.
Different types of publications relating to nerve conduits for peripheral nerve injury repair during 2002–2011
Articles constituted the major type of publication relating to nerve conduits for the repair of peripheral nerve injury during this period (Figure 2), with 617 articles. The other types were proceedings papers, reviews, meeting abstracts, letters, book chapters, corrections, and editorial material.
Figure 2.
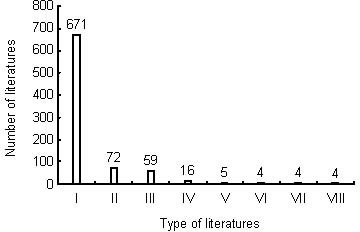
Types of publications on nerve conduits for peripheral nerve injury repair included in the Web of Science during 2002–2011.
I: Articles; II: proceedings papers; III: reviews; IV: meeting abstracts; V: letters; VI: book chapters; VII: corrections; VIII: editorial materials.
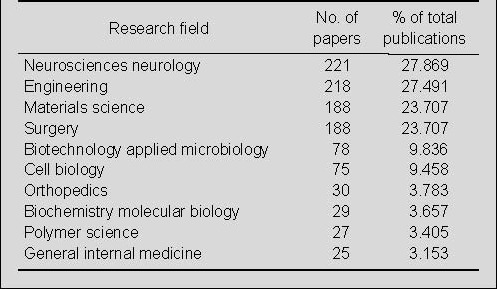
Distribution by research field of publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Among the research fields represented in publications relating to the use of nerve conduits for the repair of peripheral nerve injury in the Web of Science during 2002–2011, 221 papers were in the field of neuroscience/neurology. The second best-represented field, with 218 papers, was engineering. In the fields of materials science and surgery, 188 papers were published on the use of nerve conduits for the repair of peripheral nerve injury (Table 1).
Table 1.
Distribution by research field of publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
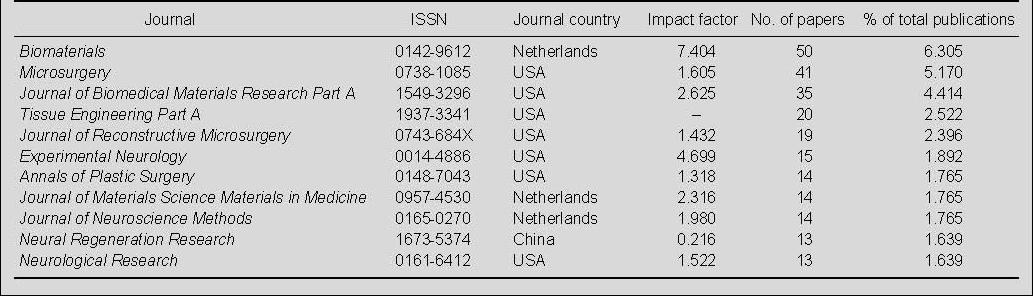
Output by journal of publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
In the period of interest, Biomaterials published 50 papers, followed by Microsurgery and Journal of Biomedical Materials Research Part A, which published 41 and 35 papers, respectively. The other eight top journals were Tissue Engineering Part A, Journal of Reconstructive Microsurgery, Experimental Neurology, Annals of Plastic Surgery, Journal of Materials Science Materials in Medicine, Journal of Neuroscience Methods, Neural Regeneration Research, and Neurological Research (Table 2).
Table 2.
Top 11 journals for publications on nerve conduits for peripheral nerve injury repair from 2002 to 2011
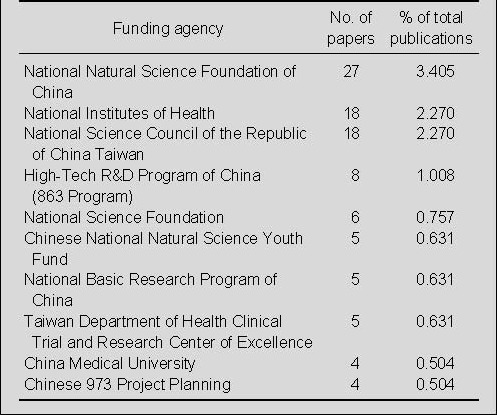
Distribution by funding agency for publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Among the publications, 27 articles were supported by the National Natural Science Foundation of China, and 18 articles each were supported by the National Institutes of Health, and the National Science Council of the Republic of China, Taiwan. Most of the funding agencies were in China (Table 3).
Table 3.
The top 10 funding agencies on nerve conduits for peripheral nerve injury repair from 2002 to 2011
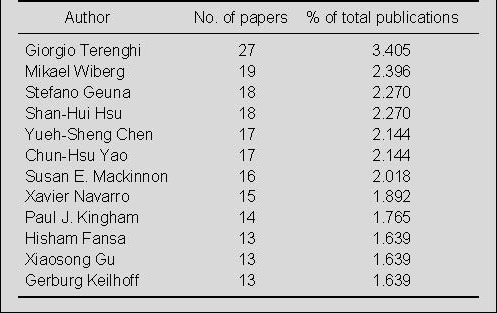
Distribution by author for publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Giorgio Terenghi published 27 papers (3.405%) on nerve conduits for the repair of peripheral nerve injury, which is much more than any other author (Table 4). Mikael Wiberg ranked second with 19 papers (2.396%), Stefano Geuna and Shan-Hui Hsu ranked third with 18 papers (2.27%).
Table 4.
Top 12 authors publishing papers on nerve conduits for peripheral nerve injury repair included in the Web of Science during 2002–2011
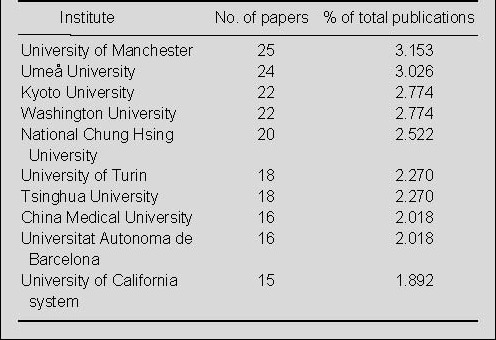
Output by country and institution of publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Analysis of the contributions of different countries/states to publications was based on journal articles in which the address and affiliation of at least one author were provided. A total of 793 articles were analyzed by country and institution. Most papers on nerve conduits for the repair of peripheral nerve injury were published in USA (206 papers), which was followed second by China (177 papers) (Figure 3). The University of Manchester, Umeå University, Kyoto University and Washington University were the most prolific research institutes (Table 5).
Figure 3.
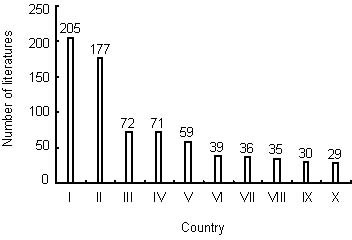
The top 12 countries publishing papers on nerve conduits for peripheral nerve injury repair during 2002–2011.
I: USA; II: China; III: Germany; IV: Japan; V: UK; VI: Italy; VII: Sweden; VIII: Canada; IX: Switzerland; X: Netherlands.
Table 5.
The top 10 institutes publishing papers on nerve conduits for peripheral nerve injury repair during 2002–2011
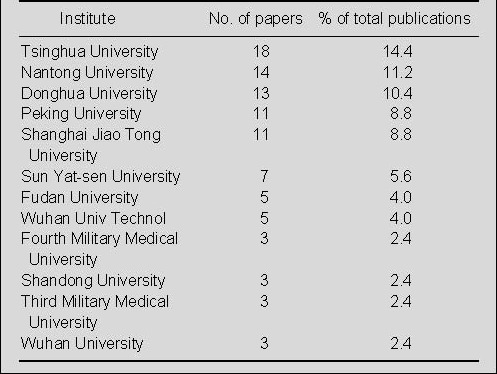
Distribution by institutes in China for publications on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Tsinghua University was the most prolific research institute in China for the publication of papers on nerve conduits for repair of peripheral nerve injury in the Web of Science during 2002–2011 (Table 6). Nantong University, Donghua University, Peking University, and Shanghai Jiao Tong University published more than 10 papers in this field.
Table 6.
The top 12 Chinese institutes publishing papers on nerve conduits for peripheral nerve injury repair during 2002–2011
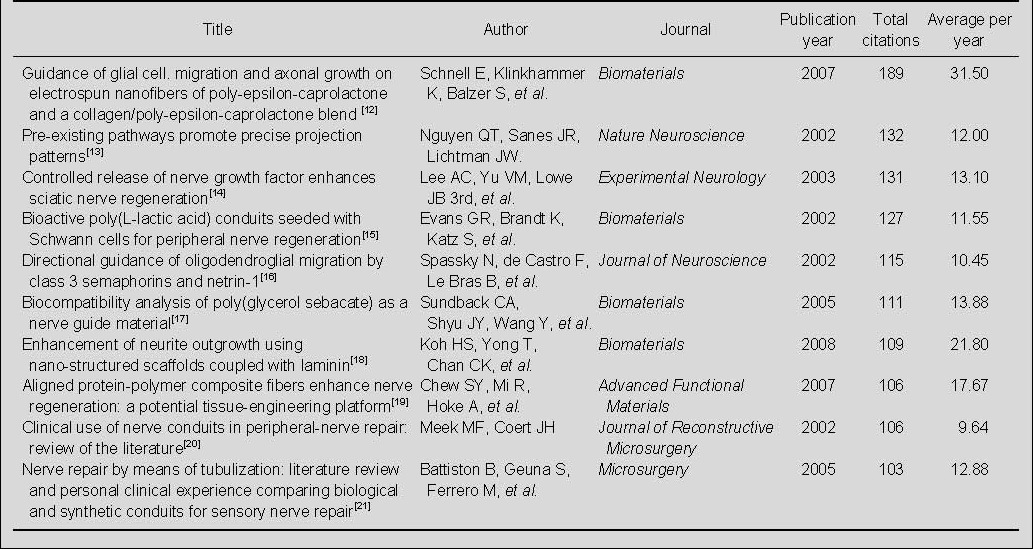
Highly cited papers on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
Of the 793 papers on nerve conduits for the repair of peripheral nerve injury cited in the Web of Science during 2002–2011, the 2007 paper, “Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly- epsilon-caprolactone blend”[12], published in Biomaterials, was the most cited paper, with 189 citations. Of the 10 most-cited papers, four were published in Biomaterials, and the remaining six were published in six different journals; four were published in 2002, and two each were published in the years 2003, 2005, 2007 and 2008 (Table 7).
Table 7.
The 10 top-cited papers on nerve conduits for peripheral nerve injury repair in the Web of Science during 2002–2011
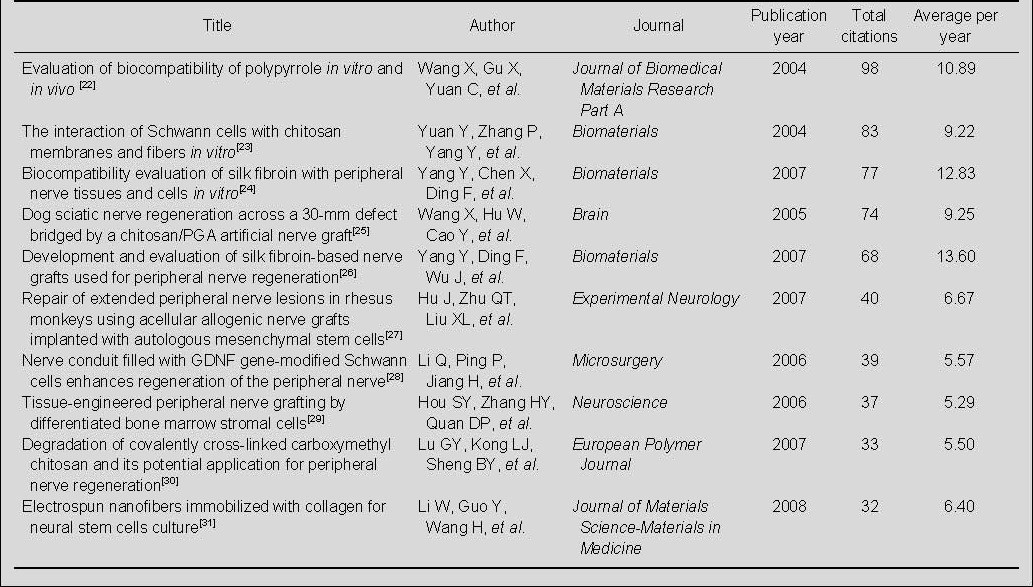
Highly cited papers on nerve conduits for peripheral nerve injury repair published by Chinese authors or institutions in the Web of Science during 2002–2011
A total of 177 papers on nerve conduits for the repair of peripheral nerve injury published by Chinese authors or institutions were indexed in the Web of Science during 2002–2011 (Table 8). The 2004 paper, “Evaluation of biocompatibility of polypyrrole in vitro and in vivo”[26], published by Journal of Biomedical Materials Research Part A was cited 98 times—more times than any other paper in this group. Of the 10 most-cited papers, three were published in Biomaterials, and the remaining seven were published in seven different journals; four were published in 2007, and two each were published in the years 2004, 2005, 2006, and 2008.
Table 8.
The 10 top-cited papers on nerve conduits for peripheral nerve injury repair from Chinese authors or institutions during 2002–2011
DISCUSSION
Our bibliometric analysis, based on the Web of Science, identified several research trends in studies of nerve conduits for the repair of peripheral nerve injury over the past decade. The number of publications gradually increased over the 10-year study period, and most were articles. The most prolific journals in this area were Biomaterials, Microsurgery, and Journal of Biomedical Materials Research Part A. Of the 793 publications retrieved from the Web of Science during 2002–2011, almost half came from American and Chinese authors and institutions.
To date, a great quantity of literature has been published on the application of nerve conduits for the repair of small nerve defects, with some success.
However, the application of nerve conduits for the repair of long segmental nerve defects has had poor results[32,33,34,35]. Although there were a large number of studies on nerve conduit materials, no single material has been shown to be superior to autografts in performance[36]. The reason for this could be the lack of an ideal structure and biological constituents to repair peripheral nerve defects, or the lack of extracellular matrix and Schwann cells, all of which are important factors for the repair of peripheral nerve defects. Numerous studies have suggested that the design concept of the nerve conduits is very important[37,38,39,40,41,42]. The following are a list of properties that are believed to be important for developing an optimal conduit: biocompatibility, good flexibility, mechanical support, appropriate degradation velocity, neutral degradation products from polymer conduits, thickness, porosity, pore size, and wall micro-structure. Ideally the morphological characteristics of the conduit should be similar to the extracellular matrix. Nerve conduits alone are difficult for the repair of long segmental nerve defects. Neurotrophic factors and stem cells can be introduced into the conduit lumen to form a composite nerve conduit. These conduits not only serve to bridge the nerve gap, but also create a good microenvironment for nerve regeneration, with neurotrophic factors inducing chemotactic effects[43,44,45,46,47,48,49,50,51]. We look forward to the day when a nerve conduit is created that meets the physiological and biological requirements for promoting effective nerve repair.
Footnotes
Conflicts of interest: None declared.
(Edited by Mu WJ/Song LP)
REFERENCES
- [1].Sedaghati T, Yang SY, Mosahebi A, et al. Nerve regeneration with aid of nanotechnology and cellular engineering. Biotechnol Appl Biochem. 2011;58(5):288–300. doi: 10.1002/bab.51. [DOI] [PubMed] [Google Scholar]
- [2].Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010;15(2):93–103. doi: 10.1111/j.1529-8027.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- [3].Gu X, Ding F, Yang Y, et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93(2):204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [4].Pereira Lopes FR, Frattini F, Marques SA, et al. Transplantation of bone-marrow-derived cells into a nerve guide resulted in transdifferentiation into Schwann cells and effective regeneration of transected mouse sciatic nerve. Micron. 2010;41(7):783–790. doi: 10.1016/j.micron.2010.05.010. [DOI] [PubMed] [Google Scholar]
- [5].Carlstedt T. An overture to basic science aspects of nerve injuries. J Hand Surg Eur Vol. 2011;36(9):726–729. doi: 10.1177/1753193411422329. [DOI] [PubMed] [Google Scholar]
- [6].Cunha C, Panseri S, Antonini S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomedicine. 2011;7(1):50–59. doi: 10.1016/j.nano.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [7].Ishikawa N, Suzuki Y, Dezawa M, et al. Peripheral nerve regeneration by transplantation of BMSC-derived Schwann cells as chitosan gel sponge scaffolds. J Biomed Mater Res A. 2009;89(4):1118–1124. doi: 10.1002/jbm.a.32389. [DOI] [PubMed] [Google Scholar]
- [8].de Ruiter GC, Spinner RJ, Yaszemski MJ, et al. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 2009;20(1):91–105. doi: 10.1016/j.nec.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [9].Lavdas AA, Papastefanaki F, Thomaidou D, et al. Cell adhesion molecules in gene and cell therapy approaches for nervous system repair. Curr Gene Ther. 2011;11(2):90–100. doi: 10.2174/156652311794940755. [DOI] [PubMed] [Google Scholar]
- [10].Biazar E, Khorasani MT, Montazeri N, et al. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int J Nanomedicine. 2010;5:839–852. doi: 10.2147/IJN.S11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Toll EC, Seifalian AM, Birchall MA. The role of immunophilin ligands in nerve regeneration. Regen Med. 2011;6(5):635–652. doi: 10.2217/rme.11.43. [DOI] [PubMed] [Google Scholar]
- [12].Schnell E, Klinkhammer K, Balzer S, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [13].Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5(9):861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- [14].Lee AC, Yu VM, Lowe JB, 3rd, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- [15].Evans GR, Brandt K, Katz S, et al. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23(3):841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- [16].Spassky N, de Castro F, Le Bras B, et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22(14):5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sundback CA, Shyu JY, Wang Y, et al. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26(27):5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [18].Koh HS, Yong T, Chan CK, et al. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29(26):3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- [19].Chew SY, Mi R, Hoke A, et al. Aligned protein-polymer composite fibers enhance nerve regeneration: a potential tissue-engineering platform. Adv Funct Mater. 2007;17(8):1288–1296. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: Review of the literature. J Reconstr Microsurg. 2002;18(2):97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- [21].Battiston B, Geuna S, Ferrero M, et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25(4):258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- [22].Wang X, Gu X, Yuan C, et al. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68(3):411–422. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- [23].Yuan Y, Zhang P, Yang Y, et al. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25(18):4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]
- [24].Yang Y, Chen X, Ding F, et al. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials. 2007;28(9):1643–1652. doi: 10.1016/j.biomaterials.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [25].Wang X, Hu W, Cao Y, et al. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128(Pt 8):1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Ding F, Wu J, et al. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials. 2007;28(36):5526–5535. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [27].Hu J, Zhu QT, Liu XL, et al. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204(2):658–666. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- [28].Li Q, Ping P, Jiang H, et al. Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery. 2006;26(2):116–121. doi: 10.1002/micr.20192. [DOI] [PubMed] [Google Scholar]
- [29].Hou SY, Zhang HY, Quan DP, et al. Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience. 2006;140(1):101–110. doi: 10.1016/j.neuroscience.2006.01.066. [DOI] [PubMed] [Google Scholar]
- [30].Lu GY, Kong LJ, Sheng BY, et al. Degradation of covalently cross-linked carboxymethyl chitosan and its potential application for peripheral nerve regeneration. Eur Polym J. 2007;43(9):3807–3818. [Google Scholar]
- [31].Li W, Guo Y, Wang H, et al. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J Mater Sci Mater Med. 2008;19(2):847–854. doi: 10.1007/s10856-007-3087-5. [DOI] [PubMed] [Google Scholar]
- [32].Pabari A, Yang SY, Mosahebi A, et al. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156(1):2–10. doi: 10.1016/j.jconrel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [33].Jiang X, Lim SH, Mao HQ, et al. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223(1):86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- [34].Chhabra A, Williams EH, Wang KC, et al. MR neurography of neuromas related to nerve injury and entrapment with surgical correlation. AJNR Am J Neuroradiol. 2010;31(8):1363–1368. doi: 10.3174/ajnr.A2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92(3):245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- [36].Pabari A, Yang SY, Seifalian AM, et al. Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg. 2010;63(12):1941–1948. doi: 10.1016/j.bjps.2009.12.010. [DOI] [PubMed] [Google Scholar]
- [37].Siemionow M, Bozkurt M, Zor F, et al. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30(7):574–588. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- [38].Olakowska E, Woszczycka-Korczyńska I, Jędrzejowska- Szypułka H, et al. Application of nanotubes and nanofibres in nerve repair. A review . Folia Neuropathol. 2010;48(4):231–237. [PubMed] [Google Scholar]
- [39].Siemionow M, Brzezicki G, et al. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. doi: 10.1016/S0074-7742(09)87008-6. [DOI] [PubMed] [Google Scholar]
- [40].Coert JH. Pathophysiology of nerve regeneration and nerve reconstruction in burned patients. Burns. 2010;36(5):593–598. doi: 10.1016/j.burns.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [41].Rodrigues F, Schmidt I, Klämbt C. Comparing peripheral glial cell differentiation in Drosophila and vertebrates. Cell Mol Life Sci. 2011;68(1):55–69. doi: 10.1007/s00018-010-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yan H, Zhang F, Chen MB, et al. Chapter 10: Conduit luminal additives for peripheral nerve repair. Int Rev Neurobiol. 2009;87:199–225. doi: 10.1016/S0074-7742(09)87010-4. [DOI] [PubMed] [Google Scholar]
- [43].Pfister LA, Papalozos M, Merkle HP, et al. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12(2):65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- [44].Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ichihara S, Inada Y, Nakamura T. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 2008;39(Suppl 4):29–39. doi: 10.1016/j.injury.2008.08.029. [DOI] [PubMed] [Google Scholar]
- [46].Kemp SW, Walsh SK, Midha R. Growth factor and stem cell enhanced conduits in peripheral nerve regeneration and repair. Neurol Res. 2008;30(10):1030–1038. doi: 10.1179/174313208X362505. [DOI] [PubMed] [Google Scholar]
- [47].Yannas IV, Zhang M, Spilker MH. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J Biomater Sci Polym Ed. 2007;18(8):943–966. doi: 10.1163/156856207781494386. [DOI] [PubMed] [Google Scholar]
- [48].Dornseifer U, Matiasek K, Fichter MA, et al. Surgical therapy of peripheral nerve lesions: current status and new perspectives. Zentralbl Neurochir. 2007;68(3):101–110. doi: 10.1055/s-2007-984453. [DOI] [PubMed] [Google Scholar]
- [49].Grosheva M, Guntinas-Lichius O, Arnhold S, et al. Bone marrow-derived mesenchymal stem cell transplantation does not improve quality of muscle reinnervation or recovery of motor function after facial nerve transection in rats. Biol Chem. 2008;389(7):873–888. doi: 10.1515/BC.2008.100. [DOI] [PubMed] [Google Scholar]
- [50].Pitcher GM, Henry JL. Governing role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathy. Exp Neurol. 2008;214(2):219–228. doi: 10.1016/j.expneurol.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li ZY, Zhao Q, Bi R, et al. Construction of a three- dimensional bionic nerve conduit containing two neurotrophic factors with separate delivery systems for the repair of sciatic nerve defects. Neural Regen Res. 2011;6(13):988–994. [Google Scholar]


