Abstract
OBJECTIVE:
To analyze the distribution characteristics of cardiac autonomic nerves and to explore the correlation between cardiac autonomic nerve distribution and arrhythmia.
DATA RETRIEVAL:
A computer-based retrieval was performed for papers examining the distribution of cardiac autonomic nerves, using heart, autonomic nerve, sympathetic nerve, vagus nerve, nerve distribution, rhythm and atrial fibrillation as the key words.
SELECTION CRITERIA:
A total of 165 studies examining the distribution of cardiac autonomic nerve were screened, and 46 of them were eventually included.
MAIN OUTCOME MEASURES:
The distribution and characteristics of cardiac autonomic nerves were observed, and immunohistochemical staining was applied to determine the levels of tyrosine hydroxylase and acetylcholine transferase (main markers of cardiac autonomic nerve distribution). In addition, the correlation between cardiac autonomic nerve distribution and cardiac arrhythmia was investigated.
RESULTS:
Cardiac autonomic nerves were reported to exhibit a disordered distribution in different sites, mainly at the surface of the cardiac atrium and pulmonary vein, forming a ganglia plexus. The distribution of the pulmonary vein autonomic nerve was prominent at the proximal end rather than the distal end, at the upper left rather than the lower right, at the epicardial membrane rather than the endocardial membrane, at the left atrium rather than the right atrium, and at the posterior wall rather than the anterior wall. The main markers used for cardiac autonomic nerves were tyrosine hydroxylase and acetylcholine transferase. Protein gene product 9.5 was used to label the immunoreactive nerve distribution, and the distribution density of autonomic nerves was determined using a computer-aided morphometric analysis system.
CONCLUSION:
The uneven distribution of the cardiac autonomic nerves is the leading cause of the occurrence of arrhythmia, and the cardiac autonomic nerves play an important role in the occurrence, maintenance, and symptoms of arrhythmia.
Keywords: cardiac autonomic nerve, sympathetic nerve, parasympathetic nerve, vagus nerve, arrhythmia, norepinephrine, catecholamine, adrenergic receptor, acetylcholine, muscarinic M receptor, tyrosine hydroxylase, acetylcholine transferase
Research Highlights
-
(1)
The distribution of cardiac sympathetic and parasympathetic nerves was examined. Sympathetic nerves function on cardiac adrenergic receptors through the release of norepinephrine and catecholamine. The adrenergic receptors include α and β isoforms, and the regulatory functions of sympathetic nerves are mainly regulated by a β-adrenergic effect.
-
(2)
The main markers of cardiac autonomic nerves are tyrosine hydroxylase and acetylcholine transferase. Immunohistochemical staining revealed that cardiac autonomic nerves are unevenly distributed in different sites, and are mainly located at the surface of the atrium and pulmonary vein, forming a ganglia plexus.
-
(3)
Cardiac autonomic nerves play an important role in the occurrence, maintenance and symptoms of arrhythmia.
INTRODUCTION
The autonomic nervous system is also termed the vegetative nervous system. Cardiac autonomic nerves are mainly distributed in cardiac vessels, glands and other sites. The central nerves are localized within the brain and spinal cord, while peripheral nerves include visceral efferent fibers and visceral sensory afferent fibers, forming the visceral motor nerve and visceral sensory nerve, respectively.
DISTRIBUTION AND CHARACTERISTIC OF CARDIAC AUTONOMIC NERVES
Cardiac autonomic nerves include sympathetic and parasympathetic systems. Sympathetic nerves can excite the heart, while parasympathetic nerves inhibit the heart. Atrial autonomic nerves are composed of parasympathetic and ventricular autonomic nerves, and are mainly sympathetic.
Distribution of cardiac sympathetic nerves
The sympathetic nerve originates from the hypothalamus and projects out of spinal cord T1-5 segments, where it exchanges into neurons in the cervicothoracic ganglion and the stellate ganglion, producing sympathetic postganglionic fibers, and then travels below the epicardium[1] to control cardiac function[2]. Release of norepinephrine and catecholamines activates adrenergic receptors in the myocardial cell membrane to accelerate rhythm and atrioventricular junction conduction, and strengthen the contraction of atrial and ventricular muscles.
Adrenergic receptors are divided into α and β subtypes, including α1, α2, β1, β2 and β3[3]. The functions of sympathetic nerves are mainly regulated by a β adrenergic effect, with the β1 receptor dominant in heart and β1 receptor content especially high in ventricular muscle; more than 75% of β1 receptors are visible in myocardial fibers. The distribution of β receptors within human cardiac tissue is markedly higher than that for α receptors. Cardiac tissue, similar to other organs, is innervated by sympathetic nerves, and possesses presynaptic and postsynaptic adrenergic receptors. Presynaptic α receptors are mainly the α2 subtype, which can inhibit nerve endings and release noradrenaline after excitation; this receptor reduces stimulation on postsynaptic α and β receptors through a feedback mechanism. Postsynaptic α receptors are mainly the α1 subtype. The density of α receptors within the left and right ventricles is very similar.
Distribution of cardiac parasympathetic nerve
Cardiac parasympathetic preganglionic fibers originate from the medulla, and project out of dorsal vagal nucleus or ambiguous nucleus to enter the thoracic cavity along the bilateral neck. The ganglion is located at the junction of the pulmonary vein, inferior vena cava and lower left atrium, as well as the atrioventricular groove. The ganglial plexus in these tissues is mainly distributed in the adipose tissue below the epicardial membrane, with a small part in the myocardium, and few in the endocardium. These adipose tissues form island-like fat pad structures on the epicardial membrane, where parasympathetic postganglionic fibers changed into neurons in the intracardiac ganglion to innervate the sinoatrial node, atrioventricular bundle and its branches and atrial muscle[4].
Parasympathetic nerves regulate cardiac function through the release of acetylcholine, which is an important neurotransmitter for central and peripheral nerves, as well as for sympathetic and parasympathetic nerve preganglionic neurons. In the central nervous system, cholinergic neurons are critical for autonomic nerve regulation. These neurons are located in the ventral medulla oblongata, dorsal nucleus of vagus nerve, salivatory nucleus and nucleus of the solitary tract. Parasympathetic nerves release acetylcholine, which subsequently acts on muscarinic cholinergic M receptors on the myocardial cell membrane, leading to a slowing of cardiac rhythm and atrioventricular conduction velocity, as well as attenuation of myocardial contractility. The M receptor is divided into M1, M2, M3, M4 and M5 subtypes, which are all G-protein coupled receptors and are expressed in the heart.
The autonomic nervous system is unevenly distributed within the heart. The vagus nerve is mainly distributed in the sinus node, atrioventricular node, atrial septum and atrial tissue, but is rarely seen in the ventricular muscle. By contrast, sympathetic fibers can be found in the sinus node, atrioventricular node, atrial tissue, and ventricular surface. The right vagus nerve mainly dominates the right atrium, especially the sinoatrial node, while the left vagus nerve mainly dominates the atrioventricular node. The right sympathetic nerve innervates the right side of the heart and the ventricular anterior wall, while the left sympathetic nerve is responsible for the left side of the heart and the ventricular posterior wall. The distribution and density of cardiac autonomic nerves are the main differences distinguishing humans from other experimental animal models[5,6,7]).
Functions of cardiac autonomic nerves
Regulation of periodic variation
The cardiac autonomic nerves mainly act on the sinus node, stimulating and inhibiting the nerve to delay or accelerate the heartbeat cycle.
Regulation of intensity change
The cardiac autonomic nerves directly act on the myocardium. Inhibiting the nerves can attenuate myocardial contraction intensity, while exciting the nerves can enhance myocardial contraction strength.
Regulation of conduction change
The cardiac autonomic nerves mainly acts on the stimulus-transmitting system, and plays a negative role on the contraction conduction velocity and inhibition of nerves, and a positive role in nerve excitation.
In addition, cardiac autonomic nerves possess antagonistic actions against the myocardial threshold value, contraction velocity, refractory period duration, chronaxie and metabolism. When the cardiac autonomic nerves are disturbed, arrhythmia often occurs, which is the abnormal origin, frequency, rhythm, conduction velocity and conduction sequence of cardiac excitation.
Arrhythmia type
Improvement of autorhythmicity
The alteration of sinoatrial node pacemaker function and the appearance of ectopic pacemaker activity can both cause arrhythmias, which are divided into a normal autorhythmicity mechanism change and an abnormal autorhythmicity mechanism formation[8].
Trigger of activity
Impulse formation results from the after-depolarization (depolarization of the second threshold after the first action potential)[8].
Reentry
Following one impulse transmission, the myocardial tissue can turn back along another circular pathway and excite again, which is an important mechanism leading to fast arrhythmia. The circular pathway is divided into an anatomical circular pathway and a functional circular pathway. The anatomical circular pathway is dependent on the presence of an anatomical loop, in which reciprocal conduction is blocked or the effective refractory period is not consistent in each site, and the turning-back impulse drops beyond the refractory period of the previously excited myocardium[8].
MARKERS ASSOCIATED WITH THE DISTRIBUTION OF CARDIAC AUTONOMIC NERVE
The main markers used for identification of cardiac autonomic nerves are tyrosine hydroxylase and acetylcholine transferase. Tyrosine hydroxylase is a rate-limiting enzyme for catalyzing the synthesis of catecholamine neurotransmitter, and is highly expressed in the sympathetic ganglia and sympathetic noradrenergic neurons; the expression level may represent the distribution of sympathetic nerves in the heart[9]. Acetylcholine transferase is the synthesis enzyme of acetylcholine and a specific marker of cholinergic neurons. Acetylcholine transferase expression can accurately display cholinergic nerve distribution and activity, and be used to determine the distribution and redistribution of the vagus nerve.
Measurement of nerve density
The nerve distribution density was determined with a computer aided morphometric analysis system, where the colored autonomic nerve was recognized by computer, the pixel area was calculated and the nerve density represented as the area of measured nerve/the total area (μm2/mm2). Each section was observed under 40 × magnification, and three visual fields with the maximal nerve density were selected to calculate the average nerve density of each section[10].
Protein gene product 9.5
Protein gene product 9.5 is a specific ubiquitin hydroxyl hydrolase in nerve fibers, and can serve as an axonal marker[11,12,13]. Protein gene product 9.5-positive nerve fibers can be labeled using immunofluorescence or immunohistochemistry. At present, protein gene product 9.5 is widely used in clinical studies of peripheral nerve injury, and can also label the cardiac autonomic nerve[14].
Growth associated protein
Growth associated protein is a fast transport membrane phosphorylated protein expressed in the growth hillock of sprouting axons, and is widely distributed in the autonomic nervous system where it plays a critical role in neural development, axon regeneration, synaptic reconstruction and neurotransmitter release. The presence of this protein can be used to evaluate the activity of autonomic nerve growth. Nerve growth factor is the most important neurotrophic factor for support of neuronal differentiation, maturation, survival, repair after injury and axonal regeneration, and the expression level of nerve growth factor is related to the density of local sympathetic nerves[15].
REGULATION OF CARDIAC AUTONOMIC NERVES ON CARDIAC RHYTHM
Basic experiments and clinical studies have confirmed that the mechanism of arrhythmia reentrant is predominantly related to cardiac anatomy, while cardiac autonomic nerves can directly or indirectly induce cardiac arrhythmia by altering its electrophysiological characteristics. Autonomic nerve abnormalities may lead to cardiac dysfunction and increase the incidence of arrhythmia[16]. The cardiac autonomic nerves play an important role in the occurrence of arrhythmias, and its maintenance and symptoms[17,18,19].
The cardiac α receptor is involved in intricate signaling mechanisms and influences ion exchange through transmembrane ion channels. Under normal conditions, the α1 adrenergic receptor-mediated electrophysiological responses occur as a result of decreased autorhythmicity and a prolonging refractory period, and can theoretically prevent arrhythmia. When myocardial ischemia occurs, the α1 adrenergic effect is enhanced and fatal arrhythmia can occur via numerous mechanisms including accumulation of local catecholamine storage, increased α1 receptor density, enhanced receptor signaling and an uneven excitatory α1 adrenergic receptor response. However, there is insufficient evidence to prove that α receptor excitation may induce or prevent arrhythmia in other pathological conditions.
The sympathetic nervous system is considered to be important in fatal arrhythmia. Following central or peripheral adrenergic stimulation, exogenous catecholamines and stress can activate the sympathetic nervous system, increase the vulnerability of the normal and ischemic heart and increase the incidence of arrhythmia. β receptor blockers can prevent this sympathetic nervous system activation, and accordingly prevent the occurrence of arrhythmia. The supraventricular tachycardia that occurs in some reentrant mechanisms is more likely to occur after activation of the sympathetic nervous system, and vagal activation can inhibit the reentrant tachycardia involving the atrioventricular node, and even terminate the attack. For atrial muscle with pathological damage, activation of the sympathetic nervous system can promote the occurrence of atrial fibrillation. In clinical treatment, sympathetic nerve excitation-related arrhythmia is frequently seen in patients with acute myocardial ischemia or myocardial infarction, as well as in long QT interval syndromes and in tension conditions.
β receptor blockers can prevent the occurrence of sudden cardiac death in patients with coronary heart disease and heart failure. The β receptor may directly influence impulse formation and conduction, increase cellular and Purkinje fiber automaticity at the atrioventricular junction, increase the early after- depolarization, prolong the QT interval, increase repolarization dispersion, reduce ventricular fibrillation threshold and increase the degree of T wave electrical alterations. β receptor blockers can also prevent myocardial ischemia-induced ventricular fibrillation.
Mechanisms of β receptor blockers preventing myocardial ischemia-induced ventricular fibrillation
These mechanisms include reducing myocardial oxygen consumption to prevent myocardial ischemia, suppression of central and local release of catecholamines and reversal of the adverse effects of catecholamines on myocardial electrophysiology, thus maintaining the electrical stability of ischemic myocardium and improving ventricular fibrillation threshold[20], reducing the circadian rhythm peak of catecholamine release, attenuating the damage of catecholamine to atherosclerotic plaque, preventing sudden death especially during sleep and in early morning, inhibiting platelet aggregation function, and improving myocardial diastolic function and local motion abnormality.
Influence of cardiac autonomic nerve on cardiac rhythm
Atrial fibrillation: Sympathetic nerve excitability can shorten the atrial refractory period, while vagus nerve excitation makes the atrial refractory period irregular, leading to paroxysmal atrial fibrillation. Sympathetic nerve- and vagus nerve-mediated atrial fibrillation has different clinical characteristics. Vagus nerve-mediated paroxysmal atrial fibrillation is mainly found in patients with non-organic heart disease. At night, at rest, and after meals, especially after dinner drinking, the vagal activity is enhanced, and the heart rate slows and shows a sinus bradycardia heartbeat prior to episodes of atrial fibrillation. Parasympathetic nerve-mediated atrial fibrillation is frequently seen in patients with organic heart disease, and occurs during the day, especially in the morning, when the subject is emotional or exercising. Prior to atrial fibrillation onset the heart rate accelerates.
Supraventricular tachycardia: Enhancement of vagal activity can terminate or reduce the incidence of supraventricular tachycardia, especially when the atrioventricular node is part of the reentrant ring. Increased sympathetic nerve activity is beneficial for the occurrence and maintenance of supraventricular tachycardia.
Ventricular tachycardia: Ventricular arrhythmia occurs when enhanced vagal activity causes sinus bradycardia or vagal activity weakening induces sinus tachycardia. The autonomic nerve has various effects on ventricular arrhythmia under different conditions. In general, vagal excitation slows down heart rate and prolongs the diastolic blood supply time and its negative inotropic effect, thus reducing myocardial oxygen consumption, directly influencing cardiac electrophysiological properties and ultimately suppressing ventricular arrhythmia. The inhibitory effect of vagus nerve impulses on ventricular arrhythmias is more apparent in acute myocardial ischemia than in non-acute myocardial ischemia. This may be due to the fact that the function of nerve fibers and receptors is destroyed in the infarction region, thus weakening the role of the vagus nerve[21,22].
Sympathetic and parasympathetic agonists can also cause arrhythmia, although the mechanism is very complicated and includes reciprocal inhibition and simultaneous activation. The interaction between sympathetic nerves and the vagus nerve involves inhibition of norepinephrine release from adrenergic nerve endings following muscarinic receptor activation, while activation of the preganglionic α1 receptor at parasympathetic nerve endings is blocked. Neuropeptide Y and norepinephrine at the sympathetic nerve endings can reduce the release of acetylcholine and norepinephrine[23]. At the same time, activation of the vagus nerve is beneficial for the sinus node, but negatively effects the atrioventricular node, the conduction tract and the ventricular muscle; these effects are markedly different from the distribution density of the vagus nerve[24,25]. In addition, central nervous system and peripheral nervous system stimulation can induce cardiac arrhythmia.
DATA SOURCES AND METHODOLOGY
Data retrieval
A computer-based retrieval was performed in domestic and foreign databases between January 1992 and December 2011 for papers examining the distribution of cardiac autonomic nerves[26,27] using the key words of “heart, autonomic nerve, vagus nerve, sympathetic nerve, nerve distribution, rhythm, and atrial fibrillation”. A total of 165 studies were selected on November 2012.
Inclusion criteria
Publications examining cardiac autonomic nerve distribution were selected, and articles published in the authoritative journals were preferred in the same field.
Exclusion criteria
Reproduced studies, conference abstracts, comments, essays or letters were excluded, and all remaining studies were carefully screened by reading the title and abstract. A total of 46 papers were selected for final analyses.
Flowchart of screening results (Figure 1)
Figure 1.
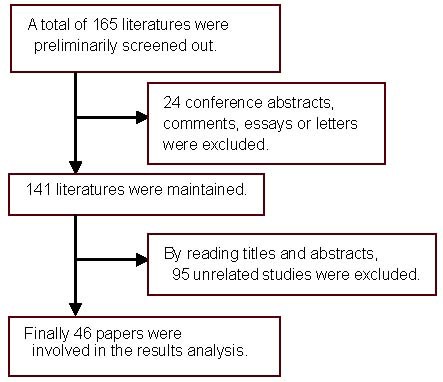
Flowchart of literature screening.
Literature on the distribution of cardiac autonomic nerve (Table 1)
Table 1.
Literature on the distribution of cardiac autonomic nerve in the database
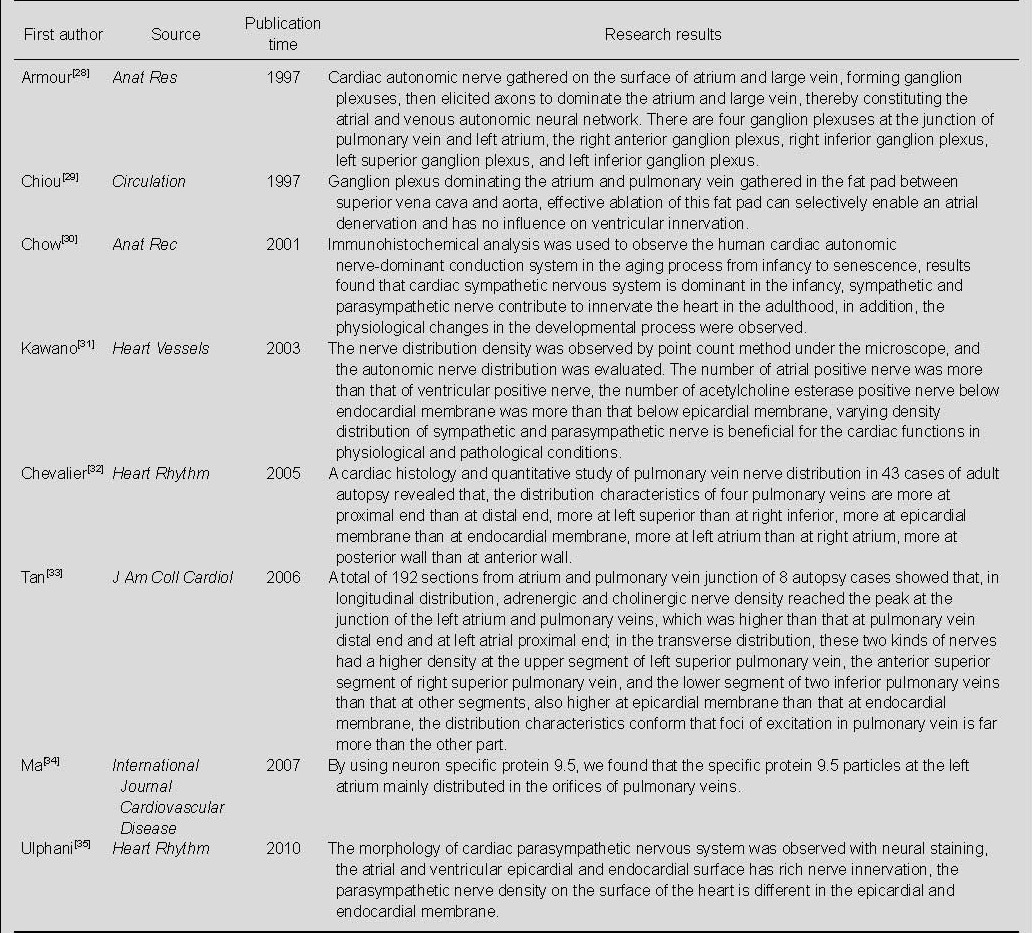
CONCLUSION
The regulatory effects of sympathetic and parasympathetic nerves on cardiac function are complex, and show different influences and electrophysiological properties at different cardiac sites. The parasympathetic nerves function in the atrium and conduction system, while sympathetic nerves function in the ventricle. Both systems can shorten the atrial refractory period, but have reverse effects on the electrophysiological properties of the atrioventricular node and the ventricles. Sinus rhythm frequency is maintained by vagus nerve activity. Thus, stimulation of sympathetic nerves may shorten the refractory periods in the right atrium, right ventricle and left ventricle, as well as maintain, extend or shorten the atrioventricular nodal conduction time. When the atrioventricular modal conduction time is maintained constantly, the atrial and ventricular refractory period is still shortened. The heart rate is the same between daytime waking state and nocturnal sleep state and the QT interval at night is prolonged, suggesting that the sinus heart rate and atrioventricular conduction time cannot completely reflect the degree of cardiac autonomic nerve activity in other parts of the heart. The cardiac autonomic nervous system plays an important role in the occurrence, maintenance and symptoms of arrhythmia. The majority of arrhythmia reentry mechanisms are related to cardiac anatomy, although the majority of fast arrhythmia episodes are usually paroxysmal, indicating that one or more factors plays a key or auxiliary function in the occurrence of arrhythmia. The autonomic nervous system can affect cardiac electrophysiological properties and accordingly induce cardiac arrhythmia. A large number of clinical trials using β receptor blockers have confirmed that intervention can prevent sudden cardiac death. Central and peripheral nerve stimulation also triggers arrhythmia, as can drugs that excite sympathetic and parasympathetic nerves and various kinds of pressure load tests.
As vagal preganglionic fibers are localized in the atrial fat pad, there are fewer nerve fibers across the fat pad that directly enters the atrial muscle. Therefore, radiofrequency ablation of the fat pad can remove atrial vagus nerve controland[36] provide partial treatment of atrial fibrillation[37]. Thoracoscopic detection for epicardial ablation can reduce vagus nerve-mediated atrial fibrillation, and clinical studies have shown that it is feasible to remove the atrial vagal nerve by direct ablation of the fat pad, which is a safe and effective procedure for some patients with atrial fibrillation[38].
Footnotes
Conflicts of interest: None declared.
(Edited by Yang Y/Song LP)
REFERENCES
- [1].Martins JB, Zipes DP. Epicardial phenol interrupts refractory period responses to sympathetic but not vagal stimulation in canine left ventricular epicardium and endocardium. Circ Res. 1980;47(1):33–40. doi: 10.1161/01.res.47.1.33. [DOI] [PubMed] [Google Scholar]
- [2].James TN. Combinatorial roles of the human intertruncal plexus in mediating both afferent and efferent autonomic neural traffic and in producing a cardiogenic hypertensive chemoreflex. Prog Cardiovasc Dis. 2004;46(6):539–572. doi: 10.1016/j.pcad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- [3].Niu YH, Xie ZX, Yin YH. Association of 13-adrenoceptor gene polymorphism with resting heart rate. Shengwu Yixue Gongchengxue Zazhi. 2007;24(2):399–403. [PubMed] [Google Scholar]
- [4].Yin XM, Yang YZ. The role of vagal tone in modulating atrial electrophysiological property. Zhonghua Xinxueguan Bing Zazhi. 2010;38(2):186–188. [PubMed] [Google Scholar]
- [5].Crick SJ, Sheppard MN, Anderson RH, et al. A quantitative study of nerve distribution in the conduction system of the guinea pig heart. J Anat. 1996;188(2):403–416. [PMC free article] [PubMed] [Google Scholar]
- [6].Crick SJ, Anderson RH, Ho SY, et al. Localisation and quantitation of autonomic innervation in the porcine heart II: endocardium, myocardium and epicardium. J Anat. 1999;195(3):359–373. doi: 10.1046/j.1469-7580.1999.19530359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crick SJ, Sheppard MN, Ho SY, et al. Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J Anat. 1999;195(3):341–357. doi: 10.1046/j.1469-7580.1999.19530341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Auerbach DS, Grzda KR, Furspan PB, et al. Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers. J Physiol. 2011;589(9):2363–2381. doi: 10.1113/jphysiol.2010.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nori SL, Gaudino M, Alessandrini F, et al. Immunohistochemical evidence for sympathetic denervation and reinnervation after necrotic injury in rat myocardium. Cell Mol Biol (Noisy-le-grand) 1995;41(6):799–807. [PubMed] [Google Scholar]
- [10].Gao CH, Wang F, Zhang J, et al. Neuromechanism of complex fractionated atrial electrogram in dog with atrial fibrillation. Jiefangjun Yixue Zazhi. 2010;35(11):1357–1360. [Google Scholar]
- [11].Hsieh ST, Lin WM, Chiang HY, et al. Skin innervation and its effects on the epidermis. J Biomed Sci. 1997;4(5):264–268. doi: 10.1007/BF02253428. [DOI] [PubMed] [Google Scholar]
- [12].Hasan W, Cowen T, Barnett PS, et al. The sweating apparatus in growth hormone deficiency, following treatment with r-hGH and in acromegaly. Auton Neurosci. 2001;89(1-2):100–109. doi: 10.1016/S1566-0702(01)00257-0. [DOI] [PubMed] [Google Scholar]
- [13].Loo LS, Ng YK, Zhu YZ, et al. Cortical expression of endothelin receptor subtypes A and B following middle cerebral artery occlusion in rats. Neuroscience. 2002;112(4):993–1000. doi: 10.1016/s0306-4522(02)00043-x. [DOI] [PubMed] [Google Scholar]
- [14].Chow LT, Chow SS, Anderson RH, et al. Autonomic innervation of the human cardiac conduction system: changes from infancy to senility--an immunohistochemical and histochemical analysis. Anat Rec. 2001;264(2):169–182. doi: 10.1002/ar.1158. [DOI] [PubMed] [Google Scholar]
- [15].Liu ZQ, Sun HS, Gao CJ, et al. Nerve terminal protection effects of nerve growth factor on chemical sympathectomy by 6-OHDA. Zhonghua Shiyan Waike Zazhi. 2005;22(2):194–195. [Google Scholar]
- [16].Zhang Y, Wang Z, Zhang Y, et al. Efficacy of cardiac autonomic denervation for atrial fibrillation: a meta- analysis. J Cardiovasc Electrophysiol. 2012;23(6):592–600. doi: 10.1111/j.1540-8167.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- [17].Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- [18].Jardine DL, Charles CJ, Frampton CM, et al. Cardiac sympathetic nerve activity and ventricular fibrillation during acute myocardial infarction in a conscious sheep model. Am J Physiol Heart Circ Physiol. 2007;293(1):433–439. doi: 10.1152/ajpheart.01262.2006. [DOI] [PubMed] [Google Scholar]
- [19].Passariello G, Peluso A, Moniello G, et al. Effect of autonomic nervous system dysfunction on sudden death in ischemic patients with anginal syndrome died during electrocardiographic monitoring in Intensive Care Unit. Minerva Anestesiol. 2007;73(4):207–212. [PubMed] [Google Scholar]
- [20].Rostock T, Steven D, Lutomsky B, et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 2008;51(22):2153–2160. doi: 10.1016/j.jacc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- [21].Mazgalev T, Dreifus LS, Michelson EL. A new mechanism for atrioventricular nodal gap-vagal modulation of conduction. Circulation. 1989;79(2):417–430. doi: 10.1161/01.cir.79.2.417. [DOI] [PubMed] [Google Scholar]
- [22].Meesmann M, Karagueuzian HS, Ino T, et al. The role of enhanced vagal activity on ischemic ventricular tachycardia: pharmacologic basis of inefficiency. Am Heart J. 1991;121(6):1703–1713. doi: 10.1016/0002-8703(91)90016-b. [DOI] [PubMed] [Google Scholar]
- [23].Deneke T, Chaar H, de Groot JR, et al. Shift in the pattern of autonomic atrial innervation in subjects with persistent atrial fibrillation. Heart Rhythm. 2011;8(9):1357–1363. doi: 10.1016/j.hrthm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- [24].Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47(6):1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- [25].Sharifov OF, Fedorov VV, Beloshapko GG, et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43(3):483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- [26].Thomson Scientific. 2012. Nov 03, http://ip-science.thomsonreuters.com/mjl .
- [27].China National Knowledge Infrastructure. 2012. Nov 03, http://www.cnki.net .
- [28].Armour JA, Murphy DA, Yuan BX, et al. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Res. 1997;247(2):289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [29].Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes The third fat pad. Circulation. 1997;95(11):2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- [30].Chow LT, Chow SS, Anderson RH, et al. Autonomic innervation of the human cardiac conduction system: changes from infancy to senility--an immunohistochemical and histochemical analysis. Anat Rec. 2001;264(2):169–182. doi: 10.1002/ar.1158. [DOI] [PubMed] [Google Scholar]
- [31].Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18(1):32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- [32].Chevalier P, Tabib A, Meyronnet D, et al. Quantitative study of nerves of the human left atrium. Heart Rhythm. 2005;2(5):518–522. doi: 10.1016/j.hrthm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- [33].Tan AY, Li H, Wachsmann-Hogiu S, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48(1):132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- [34].Ma L, Hou YM. Cardiac autonomic nervous and atrial fibrillation. Guoji Xinxueguan Bing Zazhi. 2007;34(2):94–95. [Google Scholar]
- [35].Ulphani JS, Cain JH, Inderyas F, et al. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm. 2010;7(8):1113–1119. doi: 10.1016/j.hrthm.2010.03.043. [DOI] [PubMed] [Google Scholar]
- [36].Lu Z, Scherlag BJ, Lin J, et al. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res. 2009;84(2):245–252. doi: 10.1093/cvr/cvp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koduri H, Ng J, Cokic I, et al. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012;5(4):640–649. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109(3):327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]


