Abstract
Purpose.
Cardiovascular diseases are the leading cause of death in patients with non-alcoholic steatohepatitis (NASH). We aimed to investigate the presence of endothelial dysfunction and whether serum concentrations of liver enzymes may reflect the severity of such an endothelial dysfunction in patients with NASH.
Methods.
Fifty patients with NASH diagnosed by liver biopsies and 30 healthy controls were included. Blood samples after fasting were harvested for measurements of glucose, insulin, cholesterol, triglyceride, and liver enzymes. All patients underwent transthoracic echocardiography and brachial and carotid artery Doppler ultrasonography to evaluate flow-mediated dilatation (FMD) and carotid artery intima-media thickness (CIMT).
Results.
Patients with NASH had impaired FMD (4.9 ± 2.8% to 9.3 ± 4.4%, P < 0.001) and higher CIMT (0.79 ± 0.16 mm to 0.64 ± 0.11 mm, P < 0.001) when compared with healthy controls. Linear regression analyses revealed that serum concentrations of gamma glutamyl transferase (GGT) and alanine transaminase (ALT) were associated with FMD and CIMT.
Conclusions.
Patients with NASH have impaired FMD and increased CIMT when compared with healthy controls. In patients with NASH, serum concentrations of GGT and ALT might have a predictive value for FMD and CIMT.
Keywords: Alanine transaminase, endothelial dysfunction, gamma glutamyl transferase, non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of elevated liver enzymes and chronic liver disease in Western countries (1). Patients with elevated liver enzymes in the absence of alcohol consumption and secondary causes of liver disease are described as having NAFLD (2). Non-alcoholic steatohepatitis (NASH) is a more severe form of NAFLD. It is characterized by steatosis, lobular inflammation, ballooning, and fibrosis in liver biopsies, which is the gold standard for diagnosis of NASH (3). The prevalence of cardiovascular disease and death from cardiovascular causes is increased in patients with NAFLD (4). Carotid intima-media thickness and presence of coronary plaques were also increased in patients with NAFLD (5,6). Although the association between cardiovascular diseases and NAFLD is well known, data concerning the role of endothelial dysfunction in patients with NASH—a more severe subgroup of NAFLD—are limited.
Endothelial dysfunction is associated with an increased risk of myocardial infarction, coronary revascularization, and cardiovascular death (7). Measurements of flow-mediated dilatation of the brachial artery with two-dimensional and Doppler ultrasonography have been used for the diagnosis of endothelial dysfunction, and a high correlation with angiographic results has been demonstrated (8). In this study we aimed to investigate the presence of endothelial dysfunction and the role of liver enzymes in predicting endothelial function in patients with NASH.
Methods
Study population
Fifty patients previously diagnosed with NASH (27 male and 23 female, mean age 42 ± 9 years) by liver biopsies in the Gastroenterology Department of Kayseri Education and Research Hospital between May 2010 and April 2012 were included. The indication for liver biopsy in these patients had been elevated liver enzymes in blood tests over 6 months. All patients underwent transthoracic echocardiography and brachial and carotid artery Doppler studies after careful physical examination. Also, blood samples after fasting were harvested for biochemical analyses, including lipids, glucose, insulin, creatinine, aspartic acid transaminase (AST), alanine transaminase (ALT), and gamma glutamyl transferase (GGT) concentrations (Olympus AU-640 analyser, Mishima Olympus Co. Ltd, Shizuoka, Japan). All blood tests were repeated three times, and mean values were used for statistical analyses. To diminish any confounders that might influence endothelial function, patients with established coronary artery disease (CAD), peripheral arterial disease, hypertension, hyperlipidemia, diabetes, a history of smoking, and use of anti-hyperlipidemic drugs were excluded. Thirty healthy volunteers, age- and sex-matched (19 male and 11 female, mean age 41 ± 6 years), were selected as a control group.
Flow-mediated dilatation and carotid intima-media thickness
Endothelial dysfunction was evaluated using brachial artery flow-mediated dilatation (FMD). Brachial artery FMD was measured with a high-frequency (7.0–13.0 MHz) ultrasound scanning probe to obtain longitudinal images of the brachial artery at a point 5 to 10 cm proximal to the antecubital fossa (Siemens Medical Sol, Mountain View, CA, USA). All scans were performed by the same sonographer. Two-dimensional images were obtained at baseline with Doppler ultrasound scanning to assess arterial diameter. Angle correction software was used during Doppler imaging to approximate a 20-degree angle of incidence to blood flow. Increased shear stress was achieved by producing reactive hyperemia. A pressure cuff placed on the right forearm was inflated up to 50 mmHg higher than systolic blood pressure for 5 minutes in order to induce ischemia by occluding arterial flow. Arterial diameter measurements were repeated within 60 seconds of cuff deflation. Arterial diameter was measured from the right arm at the intima-media interface of the clearest echocardiography line. Images were acquired with electrocardiogram gating, with measurements made in end diastole corresponding to the onset of the R wave. Measurements were reported as % change in diameter.
Carotid artery intima-media thickness measurements were performed in the supine position with the neck extended and the chin turned away from the side being examined. The right and left common carotid arteries were imaged proximal to the bulb in multiple longitudinal planes for clearest resolution of the intima-media thickness (IMT) of the far wall. The mean IMT was obtained by manually tracing the intima-media in the far wall of the artery. Measurements were performed on three end diastolic images and averaged.
Statistical analysis
All analyses were carried out using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were given as mean ± standard deviation; categorical variables were defined as percentages. The variables were investigated using the Kolmogorov–Smirnov test to determine whether or not they were normally distributed. The t test was used to compare continuous variables between the two groups. Non-parametric values were compared with Mann–Whitney U test. Chi-square test was used to compare categorical data. A multiple linear regression model was used to identify the statistical significance of relationships between selected variables and FMD and carotid artery intima-media thickness (CIMT). The model fit was assessed using appropriate residual and goodness-of-fit statistics. A two-tailed P value < 0.05 was considered as statistically significant.
Results
Fifty patients with NASH and 30 healthy volunteers were included (Table I). There were no differences between patients and controls in terms of age and gender. Moreover, concentrations of serum glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, and creatinine were similar. However, serum concentrations of GGT, AST, ALT, and insulin were elevated in the NASH patients. Likewise, the homeostasis model assessment (HOMA) index and body mass index were higher in patients with NASH when compared with controls (Table I).
Table I.
Comparison of baseline demographics, laboratory features, and Doppler studies of patients with non-alcoholic steatohepatitis and controls.
| NASH (n = 50) | Controls (n = 30) | p value | |
|---|---|---|---|
| Age (years) | 42 ± 9 | 41 ± 6 | 0.686 |
| Gender (male/female) | 27/23 | 19/11 | 0.414 |
| BMI (kg/m2) | 30 ± 4 | 28 ± 3 | 0.004 |
| Glucose (mg/dL) | 90 ± 12 | 92 ± 8 | 0.662 |
| Total cholesterol (mg/dL) | 221 ± 36 | 205 ± 38 | 0.065 |
| LDL cholesterol (mg/dL) | 126 ± 27 | 119 ± 39 | 0.359 |
| HDL cholesterol (mg/dL) | 42 ± 6 | 43 ± 7 | 0.782 |
| Triglyceride (mg/dL) | 172 ± 57 | 174 ± 33 | 0.835 |
| Creatinine (mg/dL) | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.325 |
| GGT (U/L) | 55 ± 18 | 36 ± 20 | <0.001 |
| AST (U/L) | 48 ± 10 | 30 ± 6 | <0.001 |
| ALT (U/L) | 61 ± 22 | 45 ± 13 | 0.001 |
| Insulin (µU/mL) | 13 ± 3 | 5 ± 2 | <0.001 |
| HOMA-IR | 3.3 ± 1.5 | 1.5 ± 0.3 | <0.001 |
| Ejection fraction (%) | 65 ± 6 | 66 ± 5 | 0.183 |
| BA baseline diameter (mm) | 40 ± 6 | 39 ± 4 | 0.172 |
| FMD (%) | 4.9 ± 2.8 | 9.3 ± 4.4 | <0.001 |
| CIMT (mm) | 0.79 ± 0.16 | 0.64 ± 0.11 | <0.001 |
AST = aspartic acid transaminase; ALT = alanine transaminase; BA = brachial artery; BMI = body mass index; CIMT = carotid intima-media thickness; FMD = flow-mediated dilatation; GGT = gamma glutamyl transferase; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
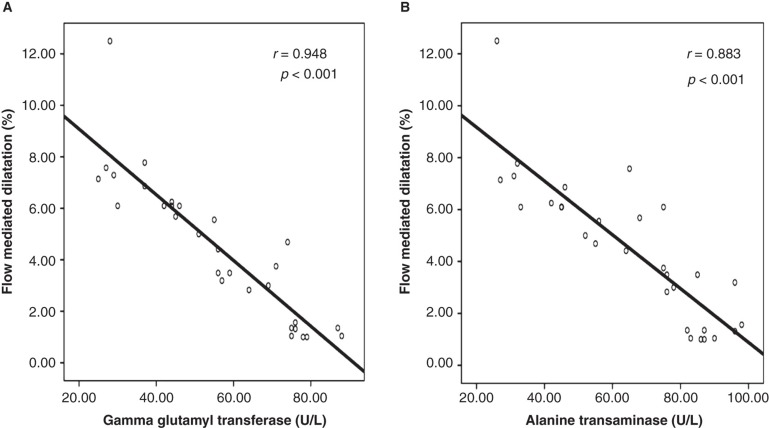
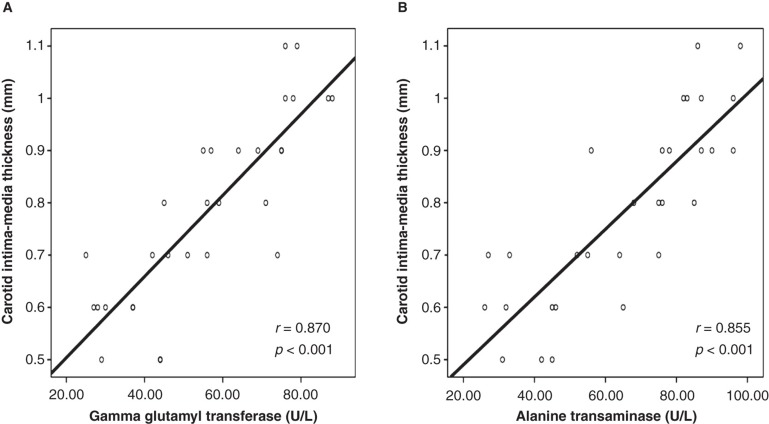
Doppler studies revealed that patients with NASH had an impaired flow-mediated dilatation when compared with the controls (Table I). Furthermore, carotid artery sonographic imaging showed that CIMT was higher in patients with NASH. Linear regression analysis showed that serum GGT and ALT concentrations were significantly associated with FMD (GGT: coefficient β: –0.36, P = 0.007; and ALT: coefficient β: –0.43, P = 0.002) and CIMT (GGT: coefficient β: 0.40, P = 0.021; and ALT: coefficient β: 0.38, P = 0.027) (Tables II and III). Also, correlation analyses showed that there was a statistically significant correlation between serum concentrations of GGT and ALT and FMD and CIMT, respectively (Figures 1 and 2).
Table II.
Linear regression analysis showing relationships between several variables and flow-mediated dilatation of brachial artery.
| Coefficient β | p value | |
|---|---|---|
| Age | –0.09 | 0.298 |
| BMI | 0.01 | 0.958 |
| AST | –0.09 | 0.335 |
| ALT | –0.43 | 0.002 |
| GGT | –0.36 | 0.007 |
| Insulin | –0.05 | 0.610 |
| HOMA index | –0.09 | 0.414 |
| Total cholesterol | 0.16 | 0.846 |
Table III.
Linear regression analysis showing relationships between several variables and carotid artery intima-media thickness.
| Coefficient β | p value | |
|---|---|---|
| Age | 0.09 | 0.415 |
| BMI | 0.09 | 0.401 |
| AST | 0.18 | 0.133 |
| ALT | 0.38 | 0.027 |
| GGT | 0.40 | 0.021 |
| Insulin | 0.03 | 0.821 |
| HOMA index | 0.09 | 0.536 |
| Total cholesterol | 0.01 | 0.902 |
Figure 1.
In patients with non-alcoholic steatohepatitis, serum gamma glutamyl transferase and alanine transaminase concentrations were negatively correlated with flow-mediated dilatation of the brachial artery. A: Graphics showing correlation between gamma glutamyl transferase concentration and flow-mediated dilatation. B: Graphics showing correlation between alanine transaminase concentration and flow-mediated dilatation.
Figure 2.
In patients with non-alcoholic steatohepatitis, serum gamma glutamyl transferase and alanine transaminase concentrations were highly correlated with the carotid intima-media thickness. A: Graphics showing correlation between serum gamma glutamyl transferase concentration and carotid intima-media thickness. B: Graphics showing correlation between serum alanine transaminase concentration and carotid intima-media thickness.
Discussion
Non-alcoholic steatohepatitis is a more severe subgroup of NAFLD which ranges from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis. NAFLD is defined histologically in liver biopsies by exhibiting macrovesicular steatosis, lobular inflammation, balloon degeneration of hepatocytes, and zone 3 pericellular fibrosis (9). Despite sampling variability and inter-observer discordance, a liver biopsy is the gold standard for diagnosis of NASH (10). Patients with NASH are more prone to develop CAD than patients with simple steatosis; however, it is still unclear whether liver enzymes could be used as specific markers of endothelial dysfunction in these patients.
Elevated serum GGT enzyme activity has been widely used as a marker of alcohol abuse and liver dysfunction including NAFLD. The concentration in serum of GGT is a highly sensitive laboratory measurement for the presence of NAFLD (11,12). Banderas et al. showed that serum GGT and triglycerides and obesity were predictors of NAFLD in patients with the metabolic syndrome (13). Alanine aminotransferase is a simple marker for NAFLD and has been shown to be associated with liver fat accumulation as measured by magnetic resonance (MR) proton spectroscopy (14,15). Dixon et al. found that ALT was an independent predictor for the presence of NASH and advanced pericellular fibrosis in liver biopsies of 105 obese patients undergoing obesity surgery (16).
Accumulating data suggest that GGT and ALT also might be markers of endothelial dysfunction and atherosclerosis besides their role in NAFLD. The serum GGT concentration was shown to be significantly associated with endothelial dysfunction, extensive atherosclerotic cardiovascular involvement, and adverse cardiac events and mortality during the course of acute coronary syndromes (17-19). Several studies investigating the role of GGT in stable CAD, acute coronary syndrome, and ST segment elevation myocardial infarction revealed that GGT is an independent predictor of short- and long-term mortality (19,20). Similarly, serum ALT concentrations were also found to be associated with endothelial dysfunction as measured by FMD and CIMT in patients with diabetes (13,21). However, in patients with NASH relations between ALT and endothelial dysfunction have previously not been studied.
Brachial artery FMD is a simple and readily available tool for assessing endothelial function (22). Impaired FMD is an early sign of subclinical atherosclerosis and may be useful in identifying patients carrying a high risk of complications of atherosclerotic vascular diseases (23). It is well known that, in patients with NAFLD, CAD is the leading cause of that, followed by hepatic malignancy and cirrhosis (24). In patients with NAFLD, impaired FMD of brachial artery and increased CIMT have been reported in several studies (25,26). However, CIMT and FMD have previously not been studied in patients with NASH.
In our study serum concentrations of both GGT and ALT were found to be predictors of endothelial function in patients with NASH. Several mechanisms have been employed in this association linking GGT and ALT with both NASH and endothelial dysfunction. First, increased serum concentrations of GGT and ALT reflect oxidative stress, which may play a role in the development of both NASH and endothelial dysfunction. GGT acts as a mediator in the transportation of extracellular glutathione into most types of cells. Production of free radicals leads to reduction in glutathione and induces GGT to protect glutathione levels (27). Serum GGT concentrations usually increase as a response to pre-existing oxidative stress. Results from the CARDIA study showed that a high serum GGT concentration is a result of pre-existing oxidative stress and GGT is expressed as a cellular defender against oxidative stress (28). High serum concentrations of GGT documented in patients with coronary artery disease are thought to be a result of oxidative stress active in the formation of atherosclerotic plaque (29-31). However, oxidative stress also plays a major role in the progression of NAFLD to NASH (32). Lipid peroxidation products and 8-hydroxy-deoxyguanosine were demonstrated in both plasma and liver biopsies of patients with NASH, indicating contribution of oxidative stress to progression of liver disease (33). Additionally, elevation in ALT concentrations represent fat deposition in liver and other organs with non-adipose tissues, and an increase of intracellular triglyceride in these tissues might be a mediator for production of reactive oxygen species which may further lead to organ dysfunction in both liver and vascular endothelium (34). In our study, increased serum concentrations of GGT and ALT in patients with NASH and impaired FMD suggest that pre-existing oxidative stress plays a role in the development and progression of both NASH and endothelial dysfunction. Moreover, high serum GGT and ALT concentrations might also reflect the inflammatory state which may contribute to the development of endothelial dysfunction and fibrosis in NASH (35,36). Results of a previous study conducted by Lee and colleagues showed that GGT is a component of subclinical inflammation, which is also involved in the pathogenesis of NASH and atherosclerosis (37,38). In a previous study which included patients with the metabolic syndrome, Kerner et al. showed that high serum ALT concentrations were correlated with high C-reactive protein levels. The authors concluded that hepatic inflammation as a result of hepatic steatosis might be a potential contributor to low-grade systemic inflammation seen in patients with NASH (35).
This study has some limitations. First, the number of patients included is relatively small to draw definite conclusions from. However, we included a homogeneous, biopsy-proven NASH population. Second, the absence of measurements of oxidative and inflammatory markers is also a limitation. Nevertheless, we conclude that patients with NASH have impaired FMD and increased CIMT when compared with healthy controls. In patients with NASH, serum concentrations of GGT and ALT might have a predictive value for FMD and CIMT, and these biomarkers might therefore be useful for the demonstration of endothelial dysfunction.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis . Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease . Diabet Med. 2005;22:1129–33. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- 3.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity . Gastroenterology. 1999;116:1413–19. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 4.Kostapanos MS, Athyros VG, Karagiannis A, Mikhailidis DP. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease . Dig Dis Sci. 2012;57:1109. doi: 10.1007/s10620-012-2066-y. [DOI] [PubMed] [Google Scholar]
- 5.Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study . Arterioscler Thromb Vasc Biol. 2005;25:1045–50. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 6.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease . Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 7.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease . Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 8.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease . Am J Cardiol. 1998;82:1535–9. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 9.Pascale A, Pais R, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions . J Gastrointestin Liver Dis. 2010;19:415–23. [PubMed] [Google Scholar]
- 10.Brunt EM. Pathology of nonalcoholic steatohepatitis . Hepatol Res. 2005;33:68–71. doi: 10.1016/j.hepres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Franzini M, Fornaciari I, Fierabracci V, Elawadi HA, Bolognesi V, Maltinti S, et al. Accuracy of b-GGT fraction for the diagnosis of non-alcoholic fatty liver disease . Liver Int. 2012;32:629–34. doi: 10.1111/j.1478-3231.2011.02673.x. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher LM, Kwoh-Gain I, Powell EE, Powell LW, Halliday JW. Markers of chronic alcohol ingestion in patients with nonalcoholic steatohepatitis: an aid to diagnosis . Hepatology. 1991;13:455–9. [PubMed] [Google Scholar]
- 13.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramírez JC, Castro MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome . Eur J Gastroenterol Hepatol. 2012;24:805–10. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 14.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study . Atherosclerosis. 2007;191:391–6. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Westerbacka J, Cornér A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen AM, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk . Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese . Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 17.Yagmur J, Ermis N, Acikgoz N, Cansel M, Atas H, Karakus Y, et al. Elevated serum gamma-glutamyl transferase activity in patients with cardiac syndrome X and its relationship with carotid intima media thickness . Acta Cardiol. 2010;65:515–19. doi: 10.1080/ac.65.5.2056237. [DOI] [PubMed] [Google Scholar]
- 18.Aksakal E, Tanboga IH, Kurt M, Kaygın MA, Kaya A, Isik T, et al. The relation of serum gamma-glutamyl transferase levels with coronary lesion complexity and long-term outcome in patients with stable coronary artery disease . Atherosclerosis. 2012;221:596–601. doi: 10.1016/j.atherosclerosis.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Dogan A, Icli A, Aksoy F, Varol E, Erdogan D, Ozaydin M, et al. Gamma-glutamyltransferase in acute coronary syndrome patients without ST elevation and its association with stenotic lesion and cardiac events . Coron Artery Dis. 2012;23:39–44. doi: 10.1097/MCA.0b013e32834e4ed0. [DOI] [PubMed] [Google Scholar]
- 20.Akpek M, Elcik D, Kalay N, Yarlioglues M, Dogdu O, Sahin O, et al. The prognostic value of serum gamma glutamyl transferase activity on admission in patients with STEMI undergoing primary PCI . Angiology. 2012;63:579–85. doi: 10.1177/0003319711431880. [DOI] [PubMed] [Google Scholar]
- 21.Schindhelm RK, Diamant M, Bakker SJ, van Dijk RA, Scheffer PG, Teerlink T, et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus . Eur J Clin Invest. 2005;35:369–74. doi: 10.1111/j.1365-2362.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoner L, Erickson ML, Young JM, Fryer S, Sabatier MJ, Faulkner J, et al. There's more to flow-mediated dilation than nitric oxide . J Atheroscler Thromb. 2012;19:589–600. doi: 10.5551/jat.11973. [DOI] [PubMed] [Google Scholar]
- 23.Charakida M, Masi S, Lüscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation . Eur Heart J. 2010;31:2854–61. doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 24.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up . Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 25.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease . Hepatology. 2005;42:473–80. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 26.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease . Am J Med. 2008;121:72–8. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Turgut O, Yilmaz A, Yalta K, Karadas F, Yilmaz MB. Gamma glutamyl transferase is a promising biomarker for cardiovascular risk . Med Hypotheses. 2006;67:1060–4. doi: 10.1016/j.mehy.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Lee DH, Gross MD, Jacobs DR. Jr; Cardiovascular Risk Development in Young Adults Study. The association of serum carotenoids and tocopherols with gamma glutamyltransferase the CARDIA study . Clin Chem. 2004;50:582–8. doi: 10.1373/clinchem.2003.028852. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Blomhoff R, Jacobs DR. Is serum gamma glutamyltransferase a marker of oxidative stress? . Free Radic Res. 2004;38:535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study . Arterioscler Thromb Vasc Biol. 2007;27:127–33. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 31.Demirkan B, Güray Y, Güray Ü, Turak O, Hajro E, Korkmaz S. The relationship between saphenous coronary bypass graft occlusion and serum gamma-glutamyltransferase activity . Turk Kardiyol Dern Ars. 2010;38:321–6. [PubMed] [Google Scholar]
- 32.Nseir W, Shalata A, Marmor A, Assy N. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease . Dig Dis Sci. 2011;56:3439–49. doi: 10.1007/s10620-011-1767-y. [DOI] [PubMed] [Google Scholar]
- 33.Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? . Gut. 2002;50:585–8. doi: 10.1136/gut.50.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger RH. Lipotoxic diseases . Annu Rev Med. 2002;53:319–36. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 35.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome . Arterioscler Thromb Vasc Biol. 2005;25:193–7. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
- 36.Turgut O, Tandogan I. Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward . J Atheroscler Thromb. 2011;18:177–81. doi: 10.5551/jat.6189. [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, Jacobs DR., Jr Association between serum gamma-glutamyltransferase and C-reactive protein . Atherosclerosis. 2005;178:327–30. doi: 10.1016/j.atherosclerosis.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Lee DH, Jacobs DR, Gross M, Kiefe CI, Roseman J, Lewis CE, et al. Gamma glutamyltransferase is a predictor of incident diabetes and hypertension: the CARDIA Study . Clin Chem. 2003;49:1358–66. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]