Abstract
Background.
From spring of 2012, human papillomavirus (HPV) vaccine against cervical cancer is offered free of charge to all girls aged 10–12 years through a school-based vaccination programme in Sweden. The aim of this study was to explore how parents reason when they accept HPV vaccination for their young daughter and also their views on HPV-related information.
Methods.
Individual interviews with parents (n = 27) of 11–12-year-old girls. The interviews were recorded, transcribed verbatim, and analysed using thematic content analysis.
Results.
Three themes emerged through the analysis: Trust versus concern, Responsibility to protect against severe disease, and Information about HPV and HPV vaccination is important. The parents expressed trust in recommendations from authorities and thought it was convenient with school-based vaccination. They believed that cervical cancer was a severe disease and felt a responsibility to protect their daughter from it. Some had certain concerns regarding side effects and vaccine safety, and wished for a dialogue with the school nurse to bridge the information gaps.
Conclusions.
Trust in the recommendations from authorities and a wish to protect their daughter from a severe disease outweighed concerns about side effects. A school-based vaccination programme is convenient for parents, and the school nurse has an important role in bridging information gaps. The findings from this qualitative study cannot be generalized; however, it can provide a better understanding of how parents might reason when they accept the HPV vaccination for their daughter.
Keywords: Decision-making, HPV vaccination, parents, school-based vaccination, school nurses
Introduction
Several countries have included human papillomavirus (HPV) vaccine against cervical cancer as part of the general child vaccination programmes for girls between 10 and 14 years old (1,2). The rationale for publicly funded vaccination programmes is to achieve a high coverage, in order to attain herd immunity. School-based vaccination programmes generally have a higher uptake than other programmes (3).
In Sweden, from spring of 2012, the quadrivalent HPV vaccine is offered free of charge to all girls aged 10–12 years through a school-based vaccination programme. Information about HPV vaccination and informed consent is distributed to the parents by the school nurses. This information is standardized by the authorities, and its distribution is mandatory (4). Complementary information about HPV and HPV vaccine is optional and can be distributed verbally or in writing. Catch-up vaccination is offered to all girls born 1993–1998. HPV vaccines have been available since 2006 at a market cost of about 330€ for three doses, and since May 2007 reduced by the government subsidy to 200€. This opportunistic vaccination reached an uptake of about 25% of the age groups 13–17, strongly associated with parents education: higher chance of vaccination with higher parental education (5,6).
Several aspects affect parents' attitudes towards the HPV vaccination for their daughters (7–11). Recommendations from physicians (8–11), belief in the effectiveness of HPV vaccine or vaccines in general (8–10), belief in protection against cancer (10-12), care for daughters' health (9), and whether the parents had previously accepted childhood vaccinations (9,10) are examples of such factors. A survey of Swedish parents (n = 13,946) of 12–15-year-olds in 2007 found that parents' main concern about the HPV vaccine was whether the vaccine had any adverse effects and that information about HPV vaccine safety and efficacy was important to parents (13).
Barriers to vaccinating daughters against HPV are fear of side effects (8,9,12,14), the long-term safety of the vaccine (11), daughter's young age and a wish to wait until daughter is older (9–11). Some studies report worries of increased sexual risk-taking as a barrier (9,13), and some not (15,16).
Vaccination against HPV is a new addition to the Swedish school-based vaccination programme. It is the first time a vaccine is offered against a sexually transmitted infection (STI). The vaccine uptake can be affected by parents' attitudes, which may be different before the introduction of a programme compared to the day when they are to fill in a consent form for the vaccination. Knowledge of what factors actually affected parents' decision regarding HPV vaccination for their young daughter in a publicly funded school-based programme is limited (9). Therefore, the aim of this study was to explore how parents reason when they accept HPV vaccination for their young daughter and also their views on HPV-related information.
Material and methods
Design
An explorative qualitative study design was adopted using individual interviews with parents who had accepted HPV vaccination for their 11–12-year-old daughters.
Informants
Inclusion criteria were parents who had accepted HPV vaccination for their 11–12-year-old daughters. The parents were recruited from three strategically chosen municipalities in mid-Sweden where the vaccination programme had already been initiated in the schools. In several other municipalities the vaccination programme started one term later. School nurses (n = 100) from the three municipalities distributed an invitation letter about the study to all parents of 11–12-year-old girls (n = 1,888) in their schools. A total of 29 parents who had agreed to vaccinate their daughter volunteered to participate. Two parents were not interviewed due to practical issues. Characteristics of the participants (23 women, 4 men) are presented in Table I.
Table I.
Characteristics of the participants.
| Age | Mean 44 (range 36–52) |
| Sex | 23 women/4 men |
| Civil status | |
| Single | 2 |
| Living with partner | 6 |
| Married | 19 |
| Highest education | |
| University/College | 22 |
| High school | 4 |
| Vocational training/education | 1 |
| Country of birth | |
| Sweden | 24 |
| Other European country | 2 |
| Non-European country | 1 |
| More than one child | 25 (93%) |
Procedure
School nurses who agreed to assist with the recruitment of participants distributed an invitation letter to all parents of 11–12-year-old girls in their schools. Parents interested in participating in an interview were asked to contact the researchers for more information and to make an appointment for the interview. The parents determined the time and place of the interview, and all interviews took place 1–4 weeks after the parents had decided to vaccinate their daughter. Some girls had recently received their first injection, and some were to get it within a few days of the interview. Interviews took place at the parents' or researchers' work-place, at parents' home, at a café, or at a library. Every interview started with verbal information about the study and acknowledging that participation was voluntary. The parents were asked to sign a consent form and to fill in a short questionnaire with demographic questions. All interviews were audio-recorded and lasted an average of 20 minutes. The parents were offered a movie ticket in return for their participation. Data collection continued until little new information emerged from the interviews. The interviews were conducted by M.Go., M.Gr., C.S., and T.T. between March and April of 2012 and were then transcribed verbatim. All interviewers are experienced in qualitative interviewing and are registered nurses and midwives with experience in clinical work, for example in paediatric, adolescent, and maternal health.
Instrument
A semi-structured interview guide with two main open-ended questions was used for the study: ‘How did you (and your partner) reason before making a decision about the HPV vaccination for your daughter?' and ‘What did you think about the information you received from school?'
When necessary, the interviewer asked for clarifications or follow-up questions such as: ‘Could you tell me more about this?' Pilot interviews were conducted in February 2012 with three mothers of girls aged 12–16. These interviews did not indicate any need for changes in the interview guide.
Data analysis
The interviews were analysed with thematic content analysis as described by Burnard et al. (17). The interviews were read several times, and notes summarizing what was said were made in the margin. These notes were the initial codes, which were then collected from all the interviews and reviewed, removing duplicates. The number of codes was then reduced further into categories and themes, by grouping together overlapping or similar codes. Thereafter, the transcripts were read again and data that fit under a certain category were labelled accordingly. The initial analysis was conducted by M.Go. and M.Gr. To check for validity, four researchers (A.T.H., T.T., M.L., and C.S.) read three to six of the transcripts each, to identify categories. These category systems were similar to the initial category system. The categories and themes were then discussed with all authors until a consensus was reached. Examples of the analytical process are presented in Table II.
Table II.
Examples of the analytical process.
| Interview transcript | Initial coding framework | Category |
|---|---|---|
| We will probably know more about the side effects in 10 years. That we don't know today. And that you might be able to discern in 5–10 years while the pandemic side effect narcolepsy you could have discerned earlier. (Interview 1) | Takes time to see all the side effects. Compares it with the swine flu. | Unknown side effects worry |
To obtain trustworthiness in the study, the quality criteria for qualitative studies as outlined by Guba and Lincoln were considered; credibility, dependability, conformability, and transferability (18). Since little is known about Swedish parents' decision-making about HPV vaccine for their daughter the inductive approach was used and data were analysed without any predetermined theory.
Ethical requirements
The study was approved by the Regional Ethical Review Board in Uppsala, Sweden (D.nr. 2012/48).
Results
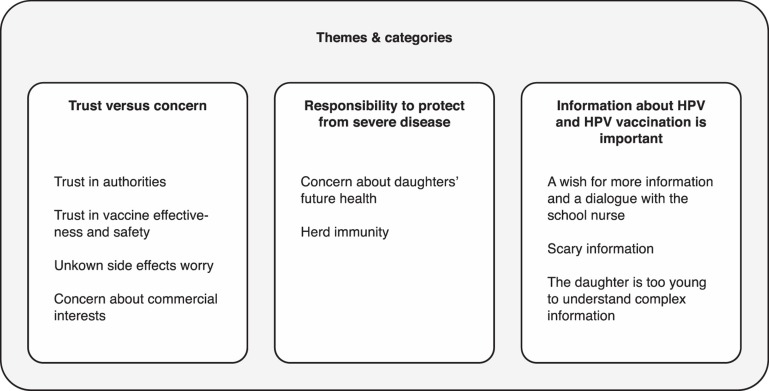
The analysis resulted in three main themes describing factors that had influenced parents' decision to accept HPV vaccination for their daughter: Trust versus concern, Responsibility to protect from severe disease, and Information about HPV and HPV vaccination is important. Each theme consists of two to four categories (Figure 1), which are presented below and illustrated by quotes.
Figure 1.
Themes and categories that emerged through the analysis.
Trust versus concern
Trust in authorities
Parents expressed a trust in vaccine recommendations from authorities and experts and said that the HPV vaccination was an offer they had decided to accept. Many had accepted the other vaccines offered in the child vaccination programme and were positive to vaccines in general. They believed the authorities make decisions that are good for the people; therefore, a vaccine included in the school-based vaccination programme is likely to be reliable.
It has been discussed and investigated and they have finally decided that this is what people must do, so I feel that we must, in any case, trust that the recommendations are right. (Mother, age 51, Interview #1).
It was also expressed that school-based vaccinations are very convenient and accessible for the parents. They believed that the vaccine coverage would increase through this system since it makes it easier for parents to accept the vaccine merely by filling in ‘yes' on a piece of paper. Several parents opined that the children trust the school nurse who administers the vaccination and feel comfortable with their classmates around them, further simplifying matters for parents. Parents could, however, also see a risk that peer pressure could be an issue for those who want to say no to the vaccination. Parents also viewed school-based vaccination as efficient from a socio-economic perspective, since the vaccination took place during school hours and parents did not have to be present.
It becomes more accessible, it rolls along by itself, automatically without having to make an appointment, driving yourself there … it is really good for us parents with limited time and so on … it's great … smoother. (Father, age 45, Interview #12).
Trust in vaccine effectiveness and safety
Even though many parents felt they had limited knowledge about the vaccine, they expressed trust in the vaccine's effectiveness and safety. Parents believed that the vaccine was well-tested in other parts of the world and that a large number of girls had already been vaccinated without severe side effects.
I was not particularly well informed but I think side effects are important, it seems pretty clear since there are so many people who have been vaccinated that the side effects should have been evident … so for me, it was obvious to vaccinate. (Mother, age 43, Interview #14)
However, all parents were not convinced about the vaccine's effectiveness even though they trusted its safety and thus had accepted the vaccination.
Unknown side effects worry
A worry about unknown side effects was expressed, and parents compared it to the mass swine flu vaccination in 2009–2010, which caused narcolepsy in several Swedish children. Many had not been worried about unknown side effects in vaccines before the swine flu vaccination. However, they estimated the risk for serious side effects of the HPV vaccine as being lower than the potential positive effects of the vaccine.
Vaccinations are good and bad, think about the swine flu from recent memory. The hysteria and how it was after, so one can feel that it has become difficult with vaccinations … but now I have become more observant about what I am going to put in her. (Mother, age 48, Interview #11)
Concern about commercial interests
Some parents were concerned about the underlying purpose of profit-making by the pharmaceutical industry. They discussed whether one could trust the vaccine trials or if the vaccine company could have influenced it.
The industry maybe has other purposes than to help people; they earn money too … they earn money in the first place. (Father, age 43, Interview #2).
Responsibility to protect from severe disease
Concern about daughters' future health
Parents had accepted HPV vaccination for their daughter to preserve her future health and to protect her from cancer. They felt that vaccination against cancer was an offer they had to accept.
I mean, a flu if you are normal, that you can overcome, but cervical cancer, that maybe you can't overcome. It is such a serious disease … if I say no to the vaccine and she gets sick, I would never be able to forgive myself. (Mother, age 46, Interview #20).
A common reason for accepting the HPV vaccination for their daughter was that they themselves or someone close to them had a history of an abnormal pap-smear, cervical cancer, or other cancer diagnosis. They had experienced the negative consequences of cancer and, therefore, felt that it was important to provide the best possible protection for their daughter.
I have myself had cervical cancer, so I think there is even more reason that my daughter will be vaccinated. There was no doubt, just a YES. (Mother, age 44, Interview #19).
Herd immunity
Most parents had their daughter vaccinated against HPV for her future health, but some also felt a responsibility to vaccinate her out of concern for others. They stated that in Sweden many childhood infections have been eliminated through the general vaccination programme which gives protection even for unvaccinated children. Therefore, some saw it as egoistic and irresponsible not to agree to vaccinations.
I think that it's a social responsibility since many of the diseases that we are vaccinated against under the general vaccination programme can cause a great havoc in our population and to not participate in the vaccination programme, I think, is irresponsible towards others. (Mother, age 46, Interview #10).
Information about HPV and HPV vaccination is important
A wish for more information and a dialogue with the school nurse
The information from the school was satisfactory according to many of the parents, but some requested further information about the virus, including the seriousness of cervical cancer, and the risks and benefits of the vaccination. Due to their limited knowledge about the virus and the vaccine, they requested a dialogue with the school nurse in addition to the written information they had received from the school. One parent requested more neutral information that addressed uncertainties with the vaccine. Some parents also requested better information for their daughters, who they believed had not received enough information regarding the vaccine at school.
I thought it was a pretty hard decision. I thought that I got quite insufficient information in the papers that came home from school … and the worst part, I think, is that when you have a school nurse who is going to vaccinate hundreds of children, and who is not well informed … because if you put a name and telephone number on a paper, then you should be able to answer parents' questions. (Mother, age 48, Interview #11).
I think that it would have been great if someone from the health care field could have come to a parent meeting … so that as a parent, one had the opportunity to ask questions … one of these papers can easily become lost in the backpack. (Mother, age 41, Interview #18).
Scary information
All parents had agreed to vaccinate their daughters, but two of the young girls refused vaccination because of fear after having read scaremongering leaflets. Several other girls had also heard scaremonger rumours and were worried about serious side effects of the vaccination. Their parents therefore felt unsure of the decision they had made and were uncertain of which sources to trust.
My daughter and her friend came home and were a little sad and wondered if there was rat poison in the vaccine. (Mother, age 38, Interview #17).
Another barrier the parents mentioned was the daughters' fear of injections. However, this was not a reason to reject the vaccination. Rather, they felt a need to discuss with their daughter more deeply the value of the vaccination and explain the vaccination procedure in order to make her more comfortable with the injection.
It is just this fear of needles, if the injection hurts; so it can be good to prepare your daughter that it will hurt. Can you use the numbing cream or routines around the vaccination itself? (Father, age 43, Interview #2).
The daughter is too young to understand complex information
Many parents wanted to talk to their daughter about other preventive methods for cervical cancer, such as condom use and pap-smear exam, but few thought the time around vaccination was a good time to do so. They were of the opinion that their daughter was too young to understand such information. They also felt it was difficult to talk about and did not know when was a good time to talk about the sexual transferability of the virus and protection against sexually transmitted infections (STI). However, some parents believed their daughter might understand more than they were aware of, and one parent believed that the age of 11–12 was a good age to talk about such issues. Many parents had informed their daughter briefly about the vaccination but most did not involve her in the decision-making.
I think that it might be too early in the middle school, but definitely in secondary school … seventh grade. I think so and then maybe continuously, it is a very important question … it is not everyone that gets this information at home for various reasons. (Mother, age 44, Interview #27)
Discussion
Discussion of the findings
The parents expressed trust in recommendations from authorities and believed it was important to protect their daughter against a severe disease. They thought it was convenient with school-based vaccination and appreciated that the vaccination was administered by the school nurse. However, they had concerns about the vaccine safety and side effects and, thus, requested additional and more adequate information about HPV and HPV vaccine. To our knowledge, this is the first study to explore how parents reason when they accept HPV vaccination for their young daughter, as part of the Swedish school-based vaccination programme.
For the parents, the fact that the vaccine was included in the school-based vaccination programme was a sign that the vaccine was safe and well-tested. Other studies have also found the importance of a vaccine being recommended by authorities and medical specialists (9,11). A school-based vaccination programme helped convince the parents of the safety of the vaccine, and also made the vaccination convenient and accessible for parents. School-based vaccination programmes are a way to facilitate vaccinations for all children regardless of socio-economic background (19).
Another finding was that, although the parents had trust in authorities, most of them brought up the swine flu vaccination in 2009–2010, when the vaccine was recommended for everybody in Sweden. Subsequently, this vaccination caused unforeseen cases of narcolepsy in children (20). This incident seemed to have affected most of the interviewed parents and made them more critical of new vaccines and concerned about unknown side effects. They considered the risks versus benefits carefully before making a decision about vaccinating their children. Despite this, the HPV vaccine coverage during the first year of the implementation of the school-based vaccination programme in Sweden was relatively high, specifically 79% (21).
In line with previous studies, the parents considered cervical cancer to be a serious disease and out of concern for their daughter's health, wished to protect her from it (9,11). Several of the parents in this study had experience of cancer either directly, or through family or friends. A previous study had a similar finding among parents accepting HPV vaccine for their daughter (11). Parents felt a responsibility to protect their daughter, but also a responsibility to protect others through containing the spread of the disease.
Scaremongering did not appear to have had a major impact on the decision-making for the parents in our study, but it had affected the decision in some families where the daughter became frightened and did not want to be vaccinated. Scare campaigns could be met with adequate and transparent information to enable parents to make an informed choice about the HPV vaccine (22).
Few of the parents in this study had discussed the virus and other future preventive methods, e.g. condom use, with their daughter. They felt that the girls were too young to discuss this subject. Challenges with information regarding HPV have previously been pointed out (23). Our study found that parents believe their children trust the school nurse. This underlines the importance of school nurses as key persons in spreading information about HPV and HPV prevention to school children. It is, therefore, of great importance that the school nurse is well educated in the area to be able to respond to questions from parents and to inform them as well as their children about HPV and HPV prevention. The school nurse is the hub for the vaccination in a school-based programme and can contribute to a well-functioning vaccination programme with a high coverage and satisfied parents.
Discussion of methods
The intention was to recruit a broad and varied sample through the choice of municipalities and schools in different socio-economic regions. To simplify participation, the parents were allowed to decide the time and place of the interview. The reason for choosing only those parents who accepted vaccination was to get a genuine understanding of their reasoning before the decision. The sample consisted mainly of female parents with a post-secondary education. This over-representation of well-educated parents could be a selection bias, and it is possible that the results would have been different with more participants having a lower education level. Further studies among parents with low education level and also among those who declined vaccinations for their daughters are needed.
As with all qualitative studies, the results cannot be generalized but can provide a better understanding of which factors influence parents' decision to accept HPV vaccination for their daughter and how they view the information they received.
The analysis process was systematic and rigorous, all data transcripts were thoroughly analysed, and the main findings as well as contrary findings have been presented in the results section supported with illuminating quotes to ensure credibility. Even though the results cannot be generalized, the authors believe that they can be transferred to other groups of well-educated parents.
To check for validity and to avoid lone researcher bias (17), several researchers individually read the transcripts to identify categories. These category systems were then compared to the initial analysis. All researchers took part in discussing the categories and themes until a consensus was reached.
Conclusion
Parents accepted HPV vaccination since they trusted the recommendations from authorities and wanted to protect their daughter from a severe disease. This outweighed the concerns they had about side effects and commercial interests. The parents thought it was convenient with a school-based vaccination programme and identified the school nurse as an important source of information about HPV and HPV vaccination. The findings from this qualitative study cannot be generalized; however, they can provide a better understanding of how parents might reason when they accept the HPV vaccination for their daughter.
Acknowledgements
This study was funded by the Swedish Cancer Society, the Uppsala-Örebro Regional Research Council, the Medical Faculty at Uppsala University, the Solstickan Foundation, and the Swedish Society of Nursing. We are grateful to the parents who took part in the interviews and to the school nurses who helped with the recruitment.
Declaration of interest: In 2010, M.Go. and T.T. lectured about their own research on events for school nurses organized by Sanofi Pasteur (MSD). The authors have no other potential competing interests to declare. The authors alone are responsible for the content and writing of the paper.
References
- 1.Mosina L, Martin R, Eckert L. HPV vaccination in Europe: Experience from National Immunization Programmes WHO . http://www.who.int/immunization_delivery/systems_policy/HPV_vaccination_in_Europe.pdf. 2010 Available from. accessed 9 January 2012.
- 2.The National Board of Health and Welfare [Socialstyrelsen] Background to a vaccination programme for the human papilloma virus in Sweden 2007; 2008 . http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/8868/2008-132-2_20081322.pdf. Available from. accessed 14 August 2012.
- 3.Limia A, Pachon I. Coverage of human papillomavirus vaccination during the first year of its introduction in Spain . http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19873. Euro Surveill. 2011;16(pii):19873. Available from. [PubMed] [Google Scholar]
- 4.Swedish Association of Local Authorities and Regions [Sveriges Kommuner och Landsting, SKL] Consentform HPV vaccination Stockholm, Sweden . http://www.skl.se/vi_arbetar_med/halsaochvard/lakemedel/vacciner/samtyckesblankett. 2013 Available from. accessed 28 March 2013.
- 5.Leval A, Herweijer E, Arnheim-Dahlstrom L, Walum H, Frans E, Sparen P, et al. Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability . J Infect Dis. 2012;206:860–6. doi: 10.1093/infdis/jis405. [DOI] [PubMed] [Google Scholar]
- 6.Leval A, Herweijer E, Ploner A, Eloranta S, Fridman Simard J, Dillner J, et al. Quadrivalent HPV-vaccine effectiveness: a Swedish national cohort study . J Natl Cancer Inst. 2013;105:469–74. doi: 10.1093/jnci/djt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widgren K, Simonsen J, Valentiner-Branth P, Molbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark . Vaccine. 2011;29:9663–7. doi: 10.1016/j.vaccine.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents' health beliefs and HPV vaccination of their adolescent daughters . Soc Sci Med. 2009;69:475–80. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie G, Anderson M, Marra F, McNeil S, Pielak K, Dawar M, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: parental factors associated with HPV vaccine receipt . PLoS Med. 2010;7:e1000270. doi: 10.1371/journal.pmed.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trim K, Nagji N, Elit L, Roy K. Parental knowledge, attitudes, and behaviours towards human papillomavirus vaccination for their children: a systematic review from 2001 to 2011 . Obstet Gynecol Int. 2012;2012:921236. doi: 10.1155/2012/921236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus . Ann Epidemiol. 2009;19:531–8. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins RB, Pierre-Joseph N, Marquez C, Iloka S, Clark JA. Why do low-income minority parents choose human papillomavirus vaccination for their daughters? . J Pediatr. 2010;157:617–22. doi: 10.1016/j.jpeds.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlstrom LA, Tran TN, Lundholm C, Young C, Sundstrom K, Sparen P. Attitudes to HPV vaccination among parents of children aged 12–15 years—a population-based survey in Sweden . Int J Cancer. 2010;126:500–7. doi: 10.1002/ijc.24712. [DOI] [PubMed] [Google Scholar]
- 14.Marlow LA. HPV vaccination among ethnic minorities in the UK: knowledge, acceptability and attitudes . Br J Cancer. 2011;105:486–92. doi: 10.1038/bjc.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area . Sex Transm Dis. 2011;38:197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall H, Ryan P, Roberton D, Baghurst P. A cross-sectional survey to assess community attitudes to introduction of human papillomavirus vaccine . Aust N Z J Public Health. 2007;31:235–42. doi: 10.1111/j.1467-842x.2007.00054.x. [DOI] [PubMed] [Google Scholar]
- 17.Burnard P, Gill P, Stewart K, Treasure E, Chadwick B. Analysing and presenting qualitative data . Br Dent J. 2008;204:429–32. doi: 10.1038/sj.bdj.2008.292. [DOI] [PubMed] [Google Scholar]
- 18.Guba E, Lincoln Y. Fourth generation evaluation. Newbury Park, CA: Sage publications; 1989. [Google Scholar]
- 19.Ward KF, Menzies RI, Quinn HE, Campbell-Lloyd S. School-based vaccination in NSW . N S W Public Health Bull. 2010;21:237–42. doi: 10.1071/NB10046. [DOI] [PubMed] [Google Scholar]
- 20.Medical Products Agency [Läkemedelsverket] The Medical Products Agency coordinates research around vaccine safety and narcolepsy . http://www.lakemedelsverket.se/Alla-nyheter/NYHETER-2012/Lakemedelsverket-samordnar-forskning-kring-vaccinsakerhet-och-narkolepsi-/ 2012 Available from. cited 18 June 2012.
- 21.Swedish Institute for Communicable Disease Control [Smittskyddsinstitutet] Statistics for HPV vaccinations . http://www.smittskyddsinstitutet.se/nyhetsarkiv/2013/stor-andel-flickor-vaccinerade-sig-mot-hpv/ 2013 Available from. cited 8 February 2013.
- 22.Abdelmutti N, Hoffman-Goetz L. Risk messages about HPV, cervical cancer, and the HPV vaccine Gardasil: a content analysis of Canadian and U.S. national newspaper articles . Women Health. 2009;49:422–40. doi: 10.1080/03630240903238776. [DOI] [PubMed] [Google Scholar]
- 23.Cooper Robbins SC, Bernard D, McCaffery K, Brotherton J, Garland S, Skinner SR. "Is cancer contagious?": Australian adolescent girls and their parents: making the most of limited information about HPV and HPV vaccination . Vaccine. 2010;28:3398–408. doi: 10.1016/j.vaccine.2010.02.078. [DOI] [PubMed] [Google Scholar]