Abstract
Background.
Shift-work is suggested to affect fetal development negatively. In particular, maternal hormonal disturbance arising from sleep deprivation or circadian rhythm changes may disturb fetal growth or lead to complications during pregnancy. Exposure to constant light is an environmental stressor that can affect the circadian system and has been shown to induce neurochemical and behavioral changes when used during the prenatal and/or postnatal period in experimental animals. However, studies investigating long-term effects of constant light in the offspring are sparse.
Methods.
An accidental power outage resulted in pregnant females being housed under constant light (LL) conditions for seven days of the offspring perinatal development (embryonic day 20 to postnatal day 4). The long-term effects of constant light on the behavior in the adult offspring were assessed by means of open field, object recognition, and water maze tests.
Results.
In adulthood, LL-animals displayed an intact recognition memory and no deficits in spatial learning or memory. In the open field test, LL-animals exhibited higher anxiety-like behavior, observed as significantly more thigmotaxis and less ambulation. These results were confirmed in the other behavioral tests as the LL-animals spent less time exploring the objects in the object recognition test, and showed thigmotactic behavior also in the water maze test.
Conclusion.
The results confirm that early life experience can cause changes in brain development that shape brain function and add to the sparse literature on long-term effects of constant light conditions during perinatal development on specific behaviors in adulthood.
Keywords: Anxiety, biological rhythms, circadian rhythm, cognitive function, development, open field, shift-work, stress, water maze
Introduction
Early life experiences can shape brain function and behavior in adulthood. Developmental processes throughout the perinatal period and the final postnatal maturation and reorganization include a variety of adaptation processes that affect brain function. During these developmental time windows the brain is highly sensitive to toxic or physical agents and environmental input (1-4). The belief that a mother's emotional or psychological state during pregnancy may influence the development of her fetus has existed since ancient times. For example, shift-work during pregnancy is a suspected risk factor for fetal development. In particular, maternal hormonal disturbance arising from sleep deprivation or circadian rhythm changes may disturb fetal growth or lead to complications of pregnancy (5).
Constant light conditions is an environmental stressor that can induce neurochemical changes during pre- and/or postnatal development as well as modify the rat circadian system, resulting in physiological and behavioral alterations (6,7). However, knowledge about the long-term consequences of exposure to constant light during the perinatal period is sparse. In the present study an accidental power outage resulted in pregnant females being housed under constant light (LL) conditions for 7 days of the offspring perinatal development (embryonic day 20 to postnatal day 4). The aim of the present study was therefore to examine the long-term behavioral effects of constant light for a total of 7 days during fetal and postnatal development in the adult offspring.
Material and methods
Time-mated, outbred Wistar rats were obtained from Taconic (Ejby, Denmark) and arrived at gestational day 15. Each dam was singly housed in polysulfone cages (59 × 38 × 20 cm) containing wood-chip bedding and nesting material. On postnatal day (PND) 1, all litters were arranged to contain an equal distribution of males and females. The animals were maintained on standard lab chow and water ad libitum, and were housed in temperature-controlled (21 ± 1°C) and humidity-controlled (50% ± 10%) cabinets in an animal room with a 12-h light/dark cycle (lights on at 6 a.m.). As a consequence of a power outage that interfered with the computer system that automatically controls the light/dark cycle in the animal facility 12 dams were exposed to 7 days of constant light (LL) from embryonic day (E) 20 and onwards after the birth of pups: E 20 until PND 4. Four of these dams were kept for investigation of effects of LL in the adult offspring. Litters from 12 dams housed under normal 12-h light/dark cycle served as controls. Pups from each housing condition were given one daily subcutaneous injection (20 µL/g) of Hanks' balanced salt solution on PNDs 9 and 10. The injections were given to all pups since the control group served as controls in a parallel study in which the LL-animals also were meant to be included (8). After weaning on PND 22 and onwards, the offspring were housed three per cage in polysulfone cages (59 × 38 × 20 cm). On PND 31 and onwards, the rats were housed under a reversed 12-h light/dark cycle (lights off at 7 a.m.) to allow for behavioral testing during the active period. The animals were allowed to adapt to the reversed light/dark cycle for over 2 months before the behavioral tests started at 13 weeks of age. The experimental procedures followed a protocol approved by the University veterinarian and the local Uppsala Animal Ethical Committee and were in accordance with the guidelines of the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Communities Council Directive (86/609/EEC).
The object recognition test
The object recognition (OR) test is based on the natural behavior of animals to spend more time exploring a novel, rather than a familiar, object. The test consists of three different phases: habituation, sample, and discrimination (9) and was conducted in an open field (OF) arena. The arena used in this study (circular, 90 cm in diameter) was as described elsewhere (10,11). The OF was divided into zones: that is, a central circle 30 cm in diameter (the center) surrounded by a middle circle (width 15 cm), which was surrounded by an outer circle (width 15 cm). The test was conducted at 13 weeks of age. The habituation phase consisted of two consecutive days with 10-min trials under dimmed light. Exploration of a novel OF arena, as in the habituation phase, is a commonly used test for studies of general exploration, ambulation, and emotional reactivity, and the 2-day trial protocol enables studies of habituation. For the two habituation trials, the time in seconds until the first visit to the defined zones (latency; LAT), number of visits (frequency; FRQ), duration (DUR) of visits in seconds, number of animals visiting the defined zones or performing the scored behavior (occurrence; OCC), total number of visits to the defined zones (TOTACT), the distance moved (TOTDISTANCE; centimeters), and rearing were registered.
In the sample phase, conducted 24 h after the last habituation trial, the samples (S) consisting of two identical objects (S1: cube 1 and cube 2; or S2: glass 1 and glass 2) were presented to the animals. The samples were placed in fixed positions in the middle circle for 5 min of exploration. The animals were then returned to their home cages for 1 h before the discrimination phase started. During the discrimination phase, one of the familiar objects was replaced by a novel object (N) for 5 min of exploration. The use of the two objects as sample or novel object was shifted equally between the animals, and the object locations were counterbalanced (8). Exploration of an object was defined as directing the nose at the object at a distance of less than 2 cm and/or touching it with the nose (9). The total exploration time (S1 + S2) or (S + N) and the discrimination ratio, N/(N + S) were calculated. Between each animal the OF arena was wiped clean with 10% ethanol and allowed to dry.
The water maze test
The water maze (WM) test is commonly used in studies of spatial learning and memory. The arena (160 cm in diameter) and the experimental conditions used in this study were as described elsewhere (8). The tank was divided into four equal quadrant zones, and the escape platform was placed in the south-west (SW) quadrant (target quadrant) during all acquisition trials. The acquisition trials were conducted for five consecutive days with four trials per day. The rats were started facing the wall using randomized starting positions, and allowed to search for the platform for 90 seconds. The animals were tested at 15–16 weeks of age. Parameters such as escape latency, latency to target quadrant, and swim distance were scored. Swimming distance to the wall was measured for evaluation of thigmotactic behavior. The retention test was conducted 72 h after the last acquisition session, as a 90-second single trial without the platform (probe trial). The animals were started in the north-east (NE) quadrant. In addition to the parameters analyzed during the acquisition trials, quadrant analyses were performed in the probe trial. Latency in the first crossing of the former platform location (target zone), number of crossings of the target zone, and number and duration of visits to the different quadrants were scored. Activity, defined as percentage of total time swimming faster than 2 cm/s, was scored for each quadrant.
Behavioral recordings
The animals were monitored on a TV-video set-up in all tests. Behavioral parameters in the OR tests were scored manually using Score 3.3 software (Pär Nyström; Soldis, Uppsala, Sweden) by an observer blinded as to the treatment. A visit to one of the defined zones was scored when both hind legs had crossed over into that section. Distance moved during the habituation phase of the OR test was automatically registered using the computerized tracking systems Ethovision version 2.3 (Noldus Information Technology, Wageningen, the Netherlands). Performance in the WM test was registered automatically using Viewer2 (Biobserve GmbH, Bonn, Germany).
Statistical analysis
Twelve animals per group were studied in the behavioral tests. One male from 12 different dams was tested in the control group (n = 12), and three males per litter from four different dams were tested in the LL-group (n = 12). The statistical analysis was based on number of litters (control group n = 12; LL-group n = 4). The non-parametric Mann–Whitney U test was used for intergroup comparisons of behavioral parameters. Measurements over time in the behavioral tests were analyzed using the non-parametric Friedman two-way ANOVA test and the Wilcoxon matched pairs test. Differences were considered statistically significant at P < 0.05. Values are expressed as mean ± SEM.
Results
Short-term findings
The exposure of the dams to constant light did not induce preterm birth or malformation in the offspring. No differences between the groups were detected in body weight gain during the postnatal period or in body weight in adulthood (data not shown).
Adult behavioral tests
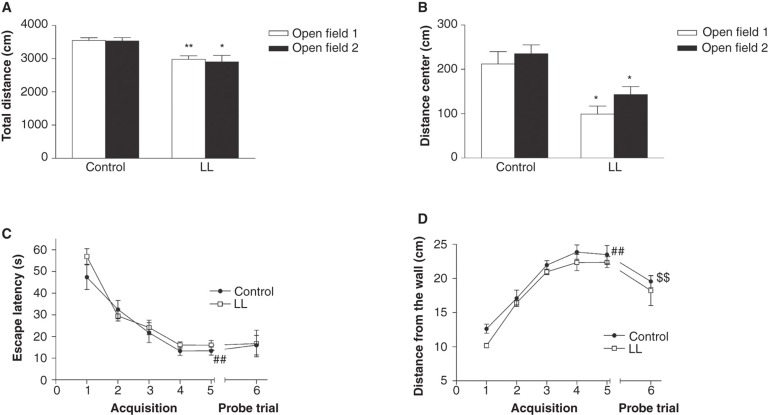
The OR test consisted of three different phases, habituation, sample, and discrimination, and was conducted at 13 weeks of age, in a circular OF arena. The two consecutive days of habituation were used for assessment of explorative strategies in a novel environment. The LL-animals had a significantly reduced locomotor activity (i.e. total activity and total distance moved) compared to the control group (Figure 1A and Table IA). The LL-animals moved significantly less in the center compared to controls in both trials, indicating higher levels of thigmotaxis (Figure 1B). The data analysis of trial 1 (Table IA) revealed that the LL-animals spent significantly more time in the outer circle and had fewer visits to the middle circle and center compared to controls. The time per visit in the outer circle was also significantly longer in the LL-group compared with the controls. A similar pattern was seen in the second habituation trial (Table IB).
Figure 1.
A-B: Spontaneous activity of rats exposed to constant light (LL) during the perinatal development or a 12-h light/dark cycle (control) examined in the open field (OF) test. A: The LL-animals had a significantly reduced locomotor activity compared to the control group. Neither of the groups had significantly reduced total distance moved in the arena between the two days. B: The LL-animals traveled a significantly shorter distance in the center compared to controls in both trials, interpreted as higher anxiety-like behavior. C-D: The performance in the water maze (WM) test. C: The escape latency (s), i.e. climbing up on the platform during the acquisition trials, and latency in first visiting the target zone during the probe trial. D: The average wall distance (cm) of the animals, used for interpretation of thigmotactic behavior. Values represent mean ± SEM. * P < 0.05, ** P < 0.01, compared to controls (Mann–Whitney U test); ## P < 0.01 compared to the first acquisition trial; $$ P<0.01 compared to the last acquisition trial (Wilcoxon matched pairs test). Twelve animals per group were tested, and the statistical analysis was based on number of litters (control group n = 12; LL-group n = 4).
Table I.
Behavioral parameters recorded during 10 min in the first (A) and second (B) open field (OF) tests in rats exposed to a 12-h light/dark cycle (control) or constant light (LL) during the perinatal development. Values represent mean ± SEM.
| Parameters | Control | LL |
|---|---|---|
| A: Open field test 1 | ||
| Total activity | 56 ± 4 | 36 ± 3** |
| Rearing | 24 ± 1 | 28 ± 2 |
| Outer circle | ||
| FRQ | 20 ± 1 | 14 ± 1** |
| DUR | 482 ± 9 | 522 ± 12* |
| DUR/FRQ | 25 ± 2 | 39 ± 2** |
| Middle circle | ||
| LAT | 9 ± 2 | 32 ± 5** |
| FRQ | 28 ± 2 | 17 ± 1** |
| DUR | 97 ± 7 | 67 ± 11* |
| DUR/FRQ | 3.6 ± 0.2 | 3.8 ± 0.6 |
| Center | ||
| LAT | 72 ± 20 | 135 ± 24 |
| FRQ | 8 ± 1 | 4 ± 1* |
| DUR | 23 ± 3 | 12 ± 1 |
| DUR/FRQ | 2.8 ± 0.3 | 3.2 ± 0.2 |
| B: Open field test 2 | ||
| Total activity | 52 ± 4 | 35 ± 3* |
| Rearing | 25 ± 1 | 27 ± 2 |
| Outer circle | ||
| FRQ | 18 ± 1 | 13 ± 1* |
| DUR | 489 ± 8 | 525 ± 7* |
| DUR/FRQ | 29 ± 3 | 46 ± 6* |
| Middle circle | ||
| LAT | 40 ± 16 | 70 ± 22 |
| FRQ | 26 ± 2 | 17 ± 2* |
| DUR | 84 ± 8 | 57 ± 5* |
| DUR/FRQ | 3.3 ± 0.2 | 3.4 ± 0.3 |
| Center | ||
| LAT | 95 ± 21 | 151 ± 13 |
| FRQ | 8 ± 1 | 5 ± 1* |
| DUR | 29 ± 3 | 19 ± 3 |
| DUR/FRQ | 3.7 ± 0.5 | 3.7 ± 0.2 |
*P < 0.05,
** P < 0.01 compared to controls (Mann–Whitney U test). Twelve animals per group were tested, and the statistical analysis was based on number of litters (control group n = 12; LL-group n = 4).
DUR = duration (s), total time spent in zone; DUR/FRQ = duration per visit (s); FRQ = frequency, number of visits; LAT = latency (s), time to first visiting a zone; Rearing = total number of rearings; Total activity = total number of visits to the defined zones.
In the OR sample phase, the LL-animals spent less time exploring the objects compared to controls, 33 ± 4 s and 44 ± 3 s, respectively (U = 8, P < 0.06). No difference was observed between LL-animals and controls in the cognitive part of the test. The LL-group had significantly (U = 6, P < 0.05) longer time to first exploration of the novel object compared to controls, 19 ± 5 s and 6 ± 1 s, respectively. However, both groups explored the novel object more than the familiar sample in the discrimination phase. The control group had a discrimination ratio of 0.70 and the LL-group had a ratio of 0.65, which both were significantly different from the probability discrimination ratio 0.5 (t test of single means).
The WM test was conducted at 15–16 weeks of age. There was no significant difference between LL-animals and controls in escape latency, and all groups learned the task in a similar manner, as indicated by reduced escape latencies over the 5 days of acquisition (Figure 1C). On the first acquisition day, the LL-animals swam closer to the wall when compared to the controls, 10 ± 1 cm and 13 ± 1 cm, respectively, indicating increased thigmotactic behavior, but the difference did not reach statistical significance (U = 9, P < 0.08). Both LL-animals and controls had reduced thigmotactic behavior over time (Figure 1D). In the probe trial, without the platform present, the LL-group swam longer and was more active (higher percentage of time with swim speed > 2 cm/s) compared to the controls, but the differences did not reach statistical significance (U = 12, P < 0.2 and U = 9, P < 0.08, respectively) (Table II).
Table II.
Behavioral parameters recorded during the 90-second probe trial of the water maze (WM) test. Values represent mean ± SEM.
| Parameters | Control | LL |
|---|---|---|
| Target zone crossings | 5.1 ± 0.6 | 4.5 ± 0.5 |
| Swim speed | 25 ± 0.8 | 27 ± 0.7 |
| Distance | 2228 ± 72 | 2434 ± 59 |
| Activity | 78 ± 2 | 86 ± 1 |
| NW quadrant | ||
| LAT | 9 ± 3 | 6 ± 1 |
| FRQ | 7 ± 0.5 | 7 ± 0.4 |
| DUR | 22 ± 2 | 19 ± 0.3 |
| Distance | 481 ± 42 | 490 ± 23 |
| Activity | 75 ± 3 | 87 ± 3* |
| Target (SW) quadrant | ||
| LAT | 3 ± 0.4 | 5 ± 0.6 |
| FRQ | 9 ± 1 | 9 ± 0.6 |
| DUR | 36 ± 2 | 36 ± 1 |
| Distance | 742 ± 45 | 834 ± 37 |
| Activity | 78 ± 3 | 85 ± 1 |
| NE quadrant | ||
| FRQ | 6 ± 1 | 7 ± 0.3 |
| DUR | 14 ± 1 | 16 ± 1 |
| Distance | 367 ± 44 | 425 ± 20 |
| Activity | 79 ± 3 | 84 ± 1 |
| SE quadrant | ||
| LAT | 7 ± 2 | 5 ± 1 |
| FRQ | 7 ± 1 | 8 ± 0.4 |
| DUR | 18 ± 1 | 19 ± 1 |
| Distance | 421 ± 38 | 516 ± 27 |
| Activity | 82 ± 2 | 89 ± 3 |
* P < 0.05 compared to controls (Mann–Whitney U test). Twelve animals per group were tested, and the statistical analysis was based on number of litters (control group n = 12; LL-group n = 4).
Activity = percentage of time in the whole pool or a defined quadrant with swim speed of more than 2 cm/s; Distance = swim distance (cm); DUR = duration (s), total time spent in zone; FRQ = frequency, number of visits; LAT = latency (s), time to first visiting a zone; Swim speed = average swim speed (cm/s) in the pool; Target zone crossing = target zone (former location of the platform) crossings.
Discussion
Shift-work is suggested to affect fetal development negatively. In particular, maternal hormonal disturbance arising from sleep deprivation or circadian rhythm changes may disturb fetal growth or lead to complications during pregnancy (5). In the present study, rats were exposed to constant light conditions for 7 days during pre- and postnatal development (E 20–PND 4), which corresponds to the last trimester of pregnancy in humans (12). To our knowledge this is the first study examining cognitive behaviors in adult rats after perinatal exposure to constant light. The LL-animals had increased anxiety-like behavior but no impairments in recognition memory or spatial learning and memory. These findings suggest that the brain systems important for these cognitive functions are not the main target for developmental changes due to constant light during this period of perinatal development. The reason for this is unknown, but granule cells of the dentate gyrus are one of the cell types in the brain with the last developmental trajectory, and cognitive behavior that is dependent on hippocampal integrity develops ontogenetically at about 1 month of age in rats (12,13). It is therefore possible that this environmental stressor only affects more mature neuronal systems directly or that the on-going development of hippocampus may reverse potential negative effects induced by constant light conditions on this brain region.
In the OF test, LL-rats displayed significantly more thigmotaxis and less ambulation compared to controls, commonly interpreted as anxiety-like behavior. The results were further strengthened by the findings in the OR test and the first WM trial. Interestingly, the increased anxiety-like behavior in the LL-animals after mainly postnatal light exposure is in accordance with a previous study, which showed that constant light during prenatal development (E 10–E 21) resulted in offspring with anxiety-like behavior in adulthood (7). The hypoactivity in the OF and the reduced exploration of the objects in the OR test, respectively, are most likely due to the anxiety-like characteristics in the LL-animals, but may also result from a direct effect on neuronal systems that control locomotion and exploration (14). On the other hand, the LL-animals swam longer and were more active compared to the controls in the probe trial of the WM test, which indicates that the effects on activity and exploration are somewhat context-dependent.
In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus contains a biological clock that controls the rhythmic expression of a number of physiological processes and behaviors including locomotor activity, sleep, and wakefulness. Disturbance in circadian rhythm has been associated with, and proposed to be a contributing factor to, mood disorders (15,16). The effects of constant light and constant darkness, respectively, during adulthood on physiological and behavioral processes have previously been assessed (14). For example, the circadian rhythm of the hypothalamus–pituitary–adrenal (HPA) axis, which is central for control of emotional processes (17), is synchronized via the SCN and has been shown to be dysregulated by constant light (18). Here, the animals were exposed to constant light during pre- and postnatal development, both of which may play a crucial role in the final organization of the circadian system and could be of importance for the observed behavioral effects (6,19,20).
In conclusion, the exposure of the dams to constant light from E 20 to PND 4 did not induce preterm birth or any observed short-term effects in the offspring. However, the 7 days of perinatal exposure to constant light induced an increased anxiety-like behavior in the offspring in adulthood. These findings add to the sparse knowledge of long-term consequences of constant light during early development and confirm that early life experiences can cause changes in brain development that shape brain function and affect specific behaviors in adulthood.
Acknowledgements
Ms Marita Berg, Professor Eva Brittebo, and Dr Samuel Rowley Uppsala University are gratefully acknowledged for technical assistance, for valuable comments during the preparation of the manuscript, and for language editing, respectively.
Declaration of interest: Funding from the Swedish Society for Medical Research (E.R.), the Facias Foundation (E.R. and O.K.), the Helge Ax:son Johnson Foundation (O.K.), and NN Jubileumsfond B (O.K.) supported this study. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Roman E, Nylander I. The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats . Stress. 2005;8:157–74. doi: 10.1080/10253890500188666. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson P. Developmental neurotoxicity of environmental agents in the neonate . Neurotoxicology. 1997;18:719–26. [PubMed] [Google Scholar]
- 3.Karlsson O, Berg AL, Lindstrom AK, Hanrieder J, Arnerup G, Roman E, et al. Neonatal exposure to the cyanobacterial toxin BMAA induces changes in protein expression and neurodegeneration in adult hippocampus . Toxicol Sci. 2012;130:391–404. doi: 10.1093/toxsci/kfs241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson O, Kultima K, Wadensten H, Nilsson A, Roman E, Andren PE, et al. Neurotoxin-induced neuropeptide perturbations in striatum of neonatal rats . J Proteome Res. 2013;12:1678–90. doi: 10.1021/pr3010265. [DOI] [PubMed] [Google Scholar]
- 5.Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies . BJOG. 2011;118:1429–37. doi: 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canal-Corretger MM, Vilaplana J, Cambras T, Diez-Noguera A. Functioning of the rat circadian system is modified by light applied in critical postnatal days . Am J Physiol Regul Integr Comp Physiol. 2001;280:R1023–30. doi: 10.1152/ajpregu.2001.280.4.R1023. [DOI] [PubMed] [Google Scholar]
- 7.Cisternas CD, Compagnucci MV, Conti NR, Ponce RH, Vermouth NT. Protective effect of maternal prenatal melatonin administration on rat pups born to mothers submitted to constant light during gestation . Braz J Med Biol Res. 2010;43:874–82. doi: 10.1590/s0100-879x2010007500083. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson O, Roman E, Berg AL, Brittebo EB. Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA (beta-N-methylamino-L-alanine) during the neonatal period . Behav Brain Res. 2011;219:310–20. doi: 10.1016/j.bbr.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data . Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson O, Roman E, Brittebo EB. Long-term cognitive impairments in adult rats treated neonatally with beta-N-methylamino-L-alanine . Toxicol Sci. 2009;112:185–95. doi: 10.1093/toxsci/kfp196. [DOI] [PubMed] [Google Scholar]
- 11.Roman E, Meyerson BJ, Hyytia P, Nylander I. The multivariate concentric square field test reveals different behavioural profiles in male AA and ANA rats with regard to risk taking and environmental reactivity . Behav Brain Res. 2007;183:195–205. doi: 10.1016/j.bbr.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat . Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 13.Douglas RJ, Peterson JJ, Douglas DP. The ontogeny of a hippocampus-dependent response in two rodent species . Behav Biol. 1973;8:27–37. doi: 10.1016/s0091-6773(73)80003-3. [DOI] [PubMed] [Google Scholar]
- 14.Benstaali C, Mailloux A, Bogdan A, Auzeby A, Touitou Y. Circadian rhythms of body temperature and motor activity in rodents their relationships with the light-dark cycle . Life Sci. 2001;68:2645–56. doi: 10.1016/s0024-3205(01)01081-5. [DOI] [PubMed] [Google Scholar]
- 15.Boyce P, Barriball E. Circadian rhythms and depression . Aust Fam Physician. 2010;39:307–10. [PubMed] [Google Scholar]
- 16.Giglio LM, Magalhaes PV, Kapczinski NS, Walz JC, Kapczinski F. Functional impact of biological rhythm disturbance in bipolar disorder . J Psychiatr Res. 2010;44:220–3. doi: 10.1016/j.jpsychires.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 17.de Kloet ER, de Jong IE, Oitzl MS. Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine . Eur J Pharmacol. 2008;585:473–82. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Walker JJ, Johnson NW, Zhao Z, Lightman SL, Spiga F. Constant light disrupts the circadian rhythm of steroidogenic proteins in the rat adrenal gland . Mol Cell Endocrinol. 2013;371:114–23. doi: 10.1016/j.mce.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Reppert SM, Schwartz WJ. Maternal suprachiasmatic nuclei are necessary for maternal coordination of the developing circadian system . J Neurosci. 1986;6:2724–9. doi: 10.1523/JNEUROSCI.06-09-02724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki K, Ino H, Chiba T, Adachi-Usami E. Light-induced apoptosis in the neonatal mouse retina and superior colliculus . Invest Ophthalmol Vis Sci. 1999;40:3079–83. [PubMed] [Google Scholar]