Abstract
Patients with non-small-cell lung cancer (NSCLC) appear to gain particular benefit from treatment with epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitors (TKI) if their disease tests positive for EGFR activating mutations. Recently, several large, controlled, phase III studies have been published in NSCLC patients with EGFR mutation-positive tumours. Given the increased patient dataset now available, a comprehensive literature search for EGFR TKIs or chemotherapy in EGFR mutation-positive NSCLC was undertaken to update the results of a previously published pooled analysis. Pooling eligible progression-free survival (PFS) data from 27 erlotinib studies (n = 731), 54 gefitinib studies (n = 1802) and 20 chemotherapy studies (n = 984) provided median PFS values for each treatment. The pooled median PFS was: 12.4 months (95% accuracy intervals [AI] 11.6–13.4) for erlotinib-treated patients; 9.4 months (95% AI 9.0–9.8) for gefitinib-treated patients; and 5.6 months (95% AI 5.3–6.0) for chemotherapy. Both erlotinib and gefitinib resulted in significantly longer PFS than chemotherapy (permutation testing; P = 0.000 and P = 0.000, respectively). Data on more recent TKIs (afatinib, dacomitinib and icotinib) were insufficient at this time-point to carry out a pooled PFS analysis on these compounds. The results of this updated pooled analysis suggest a substantial clear PFS benefit of treating patients with EGFR mutation-positive NSCLC with erlotinib or gefitinib compared with chemotherapy.
Keywords: epidermal growth factor receptor (EGFR), tyrosine-kinase inhibitor, erlotinib, gefitinib, non-small-cell lung cancer (NSCLC), mutation, first line, afatinib, icotinib
Introduction
The identification of new molecular targets for the treatment of lung cancer has revolutionized treatment paradigms for this disease. Non-small-cell lung cancer (NSCLC) therapy has benefited from the discovery of the epidermal growth factor receptor (EGFR) as a key mediator of cell proliferation. Efforts to identify agents to target EGFR led to the development of the EGFR tyrosine-kinase inhibitors (TKIs), which target the tyrosine-kinase (TK) domain of the receptor. Two EGFR TKIs have been approved for use in NSCLC in Europe and North America, erlotinib and gefitinib. Erlotinib has shown efficacy for second- or third-line treatment of NSCLC [1], as maintenance therapy [2] and for the first-line treatment of EGFR mutation-positive disease [3,4]. Gefitinib has shown efficacy for the treatment of locally advanced or metastatic NSCLC with activating EGFR mutations [5–7].
Low mutation rate and low availability of tumour samples limited the sample size for most of the efficacy analyses of erlotinib or gefitinib in patients with EGFR mutation-positive tumours. This prompted a retrospective pooled analysis by Paz-Ares et al. in 2010 [8], which reinforced the evidence that the preferential use of EGFR TKIs in patients with EGFR mutation-positive tumours may be warranted. Several additional large, phase III studies have since reported data in EGFR mutation-positive populations, including the WJTOG/802 [9], NEJ002 [10], OPTIMAL [3] and EURTAC [4] studies. Other anti-EGFR agents are also under investigation. Afatinib (an irreversible HER-family blocker), dacomitinib (an irreversible TKI of EGFR, HER2 and HER4) and icotinib (an EGFR TKI) have shown activity in EGFR mutation-positive NSCLC [11–16]. To date, the datasets for these compounds remain relatively limited although the results of one phase III trial have been recently reported [11].
The molecular biology of the EGFR mutation was reviewed extensively in the previous pooled analysis publication [8]. Briefly, EGFR plays a role in the mediation of cell signalling by regulating proliferation, angiogenesis and apoptosis [17,18]. Ninety per cent of NSCLC EGFR mutations comprise a leucine to arginine substitution at position 858 in exon 21 (L858R) or various deletion mutations in exon 19 [19–23]. Epidermal growth factor receptor mutations alter the TK pocket of the receptor, enhancing its sensitivity to EGFR TKIs.
Epidermal growth factor receptor mutations are found in around 10% of NSCLC in Caucasians and 30% of NSCLC in East-Asians [22]. Correlations between EGFR mutation-positive status and clinical characteristics have been reported, however, with the mutations being more common in the tumours of never-smokers and females, and in adenocarcinomas [22,24,25]. This correlation is not exclusive and patients cannot be assumed to have EGFR mutation-positive disease based on clinical profile alone. Therefore, EGFR mutation testing is essential; currently the European Society for Medical Oncology guidelines indicate that EGFR mutation testing is recommended as standard in non-squamous NSCLC [26]. Tumour specimens from curative surgery or bronchial biopsy are the gold standard for testing, but less than one-third of patients are suitable for surgery [27] and bronchial biopsy is impractical for poorly accessible tumours. Cytological samples, such as fine-needle aspirates, bronchial brushings, serum, plasma, circulating tumour cells and pleural effusion samples have all been used for EGFR mutation testing, but are considered less reliable because of heterogeneity of tissue samples and sparse cellularity [28].
Genotyping of EGFR mutations can be accomplished by several techniques. Direct DNA sequencing e.g. pyrosequencing and dideoxy ‘Sanger’ sequencing, will reveal any mutation. Detection by PCR (e.g. PCR-fragment length analysis) or real-time quantitative PCR (qPCR) is routinely employed to detect known, pre-specified EGFR mutations [29]. Locked nucleic acid genotyping is also used. In the clinical setting, rapid diagnostic testing may be employed with real-time PCR kits, which detect a specific number of mutations. Sequencing is still required to detect the rarer mutations. This pooled analysis focuses primarily on studies that included patients with exon 19 or exon 21 mutated NSCLC; multiple techniques have proven efficacious at detecting these classical mutations with high specificity and variable levels of detection. Cases identified by Sanger sequencing or highly sensitive methods appear to respond similarly to EGFR TKI [3,4].
The increased number of studies that have examined the efficacy of the EGFR TKIs in patients with exon 19 or exon 21 mutated NSCLC provides an expanded dataset for analysis. This paper describes an updated literature search for clinical studies of erlotinib, gefitinib and other EGFR TKIs in patients with EGFR mutation-positive NSCLC, and reports the results of a pooled analysis of erlotinib, gefitinib and chemotherapy, with the aim of providing updated median pooled progression-free survival (PFS) values. This study should help to provide robust recommendations for the clinical management of patients in this important patient subset.
Materials and methods
Selection criteria
All prospective and retrospective studies that examined erlotinib, gefitinib, icotinib, afatinib or dacomitinib as single-agent therapy or chemotherapy as single- or multiple-agent treatment for patients with EGFR mutation-positive NSCLC were eligible for inclusion. The studies were not critically assessed for methodology of EGFR mutation status determination.
Literature search strategy
The literature was reviewed to identify studies for inclusion in the pooled analysis. The Datastar Web search engine was used to search Medline, BIOSIS Previews and Embase. The search date was 14 November 2011 and the search string used was (‘epidermal growth factor’ or ‘EGFR’) AND (‘lung’ OR ‘NSCLC’) AND (‘mutation’ OR ‘mutations’). Congress abstracts were searched, also on 14 November 2011, by using the same search string. The congresses searched were: the American Society of Clinical Oncology (ASCO) 2009–2011; the World Congress on Lung Cancer (WCLC) 2009–2011; and the European Cancer Organisation-European Society for Medical Oncology (ECCO-ESMO) 2009–2011. The references used in the original pooled analysis [8] were checked for updates (e.g. updated analyses or full papers). Only English language papers from 2004 or later were included. The papers were initially filtered by manually scanning the titles. Abstracts of the remaining papers were reviewed and filtered further. The remaining papers were then reviewed in full. The search excluded studies where: the results were only given graphically; two TKIs were given in sequence; PFS data for EGFR TKIs were presented as a class and not split by drug; patients were treated in the maintenance or adjuvant setting; EGFR TKI or chemotherapy was used in combination with any other therapy (including surgery); the PFS/time-to-progression (TTP) was not given for EGFR mutation-positive NSCLC; information was ambiguous; only updates were reported; EGFR mutation status analysis was performed with blood samples; results were presented for patients only with ‘other’ EGFR mutations (i.e. non-exon 19 deletion or L858R mutation); or patients received a non-standard dose. The search also excluded studies where patients were selected for EGFR mutation status plus: another biomarker; specific types or patterns of metastases; having very poor performance status (3+); having benefited from an EGFR TKI previously (including long-term responders). Individual case studies were excluded.
Data extraction
The chosen end-point was PFS. For the purposes of the pooled analysis, TTP was considered equivalent to PFS. Where both PFS and TTP were reported, PFS was used in preference to TTP. The PFS/TTP data were extracted from the report by one individual and entered onto a database. Entries were validated by at least one other analyst. All references were checked for duplication; only the most recent publication was used for data (with verification against prior publications).
Statistical analysis
The methodology was as described in Paz-Ares et al. [8], and is reported only briefly here. Individual data points were not available for PFS/TTP, therefore calculation of a pooled median PFS was made from the study medians. In some studies, a median PFS was not available, and simplifying assumptions (exponential distribution) were made to approximate the study median. In one study, a mean PFS value instead of median PFS was reported; based on the simplifying assumption of an underlying exponential distribution the median is simply the mean multiplied by ln(2). In some studies PFS was reported as ‘after T(time) months a fraction of the patients were without progression….’. The median PFS was estimated based on the assumptions of an exponential distribution by T/{ln(PFS) ln(x)}. Finally, in some cases multiple reports of PFS by time and percentage were reported. Here, the average of the multiple medians was taken to approximate the overall median in this study. The pooled median PFS (MPFSall) was then obtained by a weighted average of the single study medians, which was calculated by multiplying the study median (MPFS(i)) by the size of the study and summing over all studies. The result was divided by the total number of patients in all the studies (Nall):
Valid confidence intervals (CIs) to assess inherent variation can only be calculated when individual data are given. These were not available for all studies so a surrogate ‘accuracy interval’ was calculated to reflect the comparative accuracy of the median estimates. The reported medians were treated as maximum likelihood estimates of the parameters of the exponential distribution to determine the pertaining confidence bands as ‘accuracy intervals’; the 90% and 95% confidence bands were used as surrogate ‘accuracy intervals’ (single studies and pooled median estimates, respectively).
Random permutations [30] across studies were generated for 20,000 runs to test the null hypothesis that there is no difference between treatments. This comparative test is statistically valid, but only refers to the given study pool (conditional test) and cannot be readily extrapolated to the total patient population.
The potential for publication bias was assessed by using funnel plots with the pertaining accuracy intervals.
Sensitivity analysis
Sensitivity analyses were performed to assess the adequacy of the calculated accuracy intervals by using a resampling technique (‘bootstrap’) (e.g. Hesterberg et al. [30]) with 5000 runs.
Results
Breakdown of eligible studies
The number of studies identified and excluded is shown in Figure 1. In total, 92 papers or abstracts contained PFS values that were eligible for this pooled analysis. There were 26 studies that evaluated erlotinib, 54 studies that evaluated gefitinib, 20 that evaluated chemotherapy, two that evaluated icotinib and one that evaluated afatinib (Table 1). Of the 27 erlotinib studies, 10 were first line, 17 of 54 gefitinib studies were first line and 17 of 20 chemotherapy studies were first line. There were two erlotinib phase III trials, seven gefitinib phase III trials, nine chemotherapy phase III trials and one icotinib phase III trial. There were 10 retrospective erlotinib trials (n = 127), 26 retrospective gefitinib trials (n = 861), and 11 retrospective chemotherapy trials (n = 439). The total number of patients included in the pooled analysis was 3521 (731 were treated with erlotinib, 1802 were treated with gefitinib and 984 were treated with chemotherapy). Afatinib and icotinib were not included in the pooled analysis calculations, but studies were identified that included 129 afatinib-treated patients (US and Taiwanese) and 29 icotinib-treated patients (all Chinese). In studies where mutation types were reported individually the most common EGFR mutations were exon 19 deletions (53%) and L858R mutations (38%).
Fig. 1.
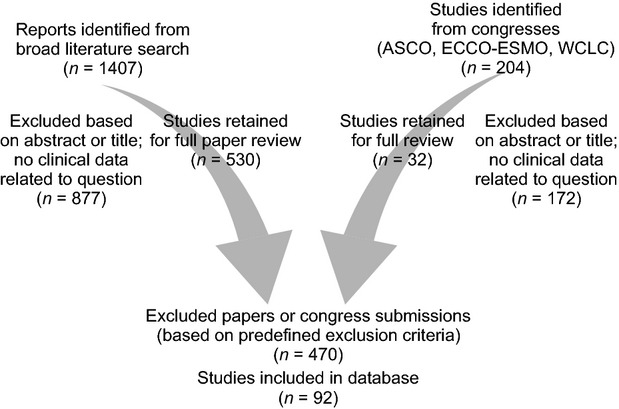
Breakdown of citations retrieved from literature searches and number of trials included in the analysis. ASCO: American Society of Clinical Oncology; ECCO-ESMO: European Cancer Organisation-European Society for Medical Oncology; WCLC: World Congress on Lung Cancer.
Table 1.
Characteristics of the studies included in the pooled analysis to evaluate the effects of single-agent erlotinib, single-agent gefitinib or chemotherapy in patients with EGFR mutation-positive NSCLC (studies not included in the original analysis are highlighted)
| Study | Design | Patients | Treatment | PFS/TTP |
|---|---|---|---|---|
| Erlotinib | ||||
| Ahn et al. [31] | Prospective | n = 24: Korean; ≥1 prior treatment; exon 19 deletion (n = 17); L858R (n = 5); exon 20 mutation (n = 1); exon 18 mutation and exon 19 deletion (n = 1) | Erlotinib 150 mg/day | TTP: 8.6 months |
| Jackman et al. [32] | Ph II, single-arm | n = 9: primarily white; chemo-naїve; ≥70 years; exon 19 deletion (n = 3); L858R (n = 5); L861Q and exon 19 deletion (n = 1) | Erlotinib 150 mg/day | TTP: 13 months |
| Jackman et al. [33] | Ph II, single-arm | n = 33: female; chemo-naїve; adenocarcinoma; EGFR mutations | Erlotinib 150 mg/day | TTP: 12.6 months |
| Miller et al. [34] | Ph II, single-arm | n = 18: BAC and adenocarcinoma, BAC subtype; 0–1 prior treatments; exon 19 or 21 EGFR mutation | Erlotinib 150 mg/day | PFS: 13 months |
| Pirker et al. [35] | Prospective (TRUST study) | n = 11: primarily white; chemo-naïve or previously treated; exon 19 deletion (n = 7); L858R (n = 5) | Erlotinib 150 mg/day | PFS: 405 days |
| Riely et al. [36] | Retrospective | n = 12: primarily white; chemo-naїve or previously treated; exon 19 deletion (n = 8); L858R (n = 4) | Erlotinib 150 mg/day | PFS: 12 months |
| Rosell et al. [37] | Prospective, Ph II | n = 12: Spanish; non-squamous cell carcinoma; exon 19 or 21 EGFR mutation | Erlotinib 150 mg/day | PFS: 13 months |
| Zhou et al. [38] | Retrospective | n = 6: Chinese; ≥1 previous treatment; EGFR mutations | Erlotinib 150 mg/day | TTP: 15.8 months |
| Amann et al. [39] | Retrospective; Ph II single-arm | n = 3: primarily white; mainly chemo-naïve; L858R (n = 3) | Erlotinib 150 mg/day | TTP: 13.1 months |
| Ciuleanu et al. [40] | Ph III; randomized comparison versus chemotherapy (TITAN) | n = 7: predominantly white; previously treated; EGFR mutation | Erlotinib 150 mg/day | PFS: 8.4 months |
| Choi et al. [41] | Retrospective; Ph II; single-arm | n = 21: Korean; chemo-naïve; exon 19 or exon 21 mutation | Erlotinib 150 mg/day | PFS: 11.5 months |
| De Greve et al. [42] | Prospective; Ph II; single-arm (FIELT) | n = 46: Belgian; chemo-naïve; exon 18 (n = 2), exon 19 (n = 27), exon 20 (n = 3), exon 21 (n = 15) | Erlotinib 150 mg/day | PFS rate at 3 months: 83% PFS rate at 6 months: 74% Median TTP not reached: 44+ weeks |
| Fiala et al. [43] | Retrospective | n = 9: Czech; EGFR mutation | Erlotinib 150 mg/day | TTP: 8.4 months |
| Rosell et al. [44] | Ph III; randomized comparison versus chemotherapy (EURTAC) | n = 86: primarily white; chemo-naïve; EGFR mutation | Erlotinib 150 mg/day | PFS: 9.7 months |
| Janne et al. [45] | Ph II; randomized comparison versus erlotinib plus chemotherapy (CALGB30406) | n = 33: primarily white; chemo-naïve; EGFR mutation | Erlotinib 150 mg/day | PFS: 14.1 months |
| Lynch et al. [46] | Ph II; randomized comparison versus erlotinib + bortezomib | n = 4: primarily white; mainly chemo-naïve; EGFR mutations | Erlotinib 150 mg/day | PFS: 4.3 months |
| Okano et al. [47] | Prospective; Ph II; single-arm | n = 10: Japanese; previously treated; EGFR mutation | Erlotinib 150 mg/day | PFS: 418 days (13.75 months) |
| Pallis et al. [48] | Prospective; Ph II; single-arm | n = 9: Greek; chemo-naïve; exon 19 deletion (n = 4); L858R (n = 5) | Erlotinib 150 mg/day | PFS: 12.4 months |
| Puente et al. [49] | Retrospective; single-arm | n = 23: chemo-naïve; EGFR mutation | Erlotinib 150 mg/day | PFS: 11 months |
| Rosell et al. [50] | Prospective; single-arm | n = 217: primarily white; chemo-naïve or previously treated; exon 19 deletion (n = 135); L858R (n = 82) | Erlotinib 150 mg/day | PFS: 14 months |
| Rotella et al. [51] | Retrospective; single-arm | n = 8: previously treated; exon 19 (n = 6); exon 21 (n = 2) | Erlotinib 150 mg/day | PFS: 18 months |
| Spigel et al. [52] | Prospective, ph II | n = 3: primarily white; 1–2 prior treatments; EGFR mutations | Erlotinib 150 mg/day | PFS: 9.23 months |
| Sun et al. [53] | Retrospective; single-arm | n = 35: Korean; mainly previously treated; exon 19 deletion or L858R | Erlotinib 150 mg/day | PFS: 8.0 months |
| Takahashi et al. [54] | Retrospective; Ph II; single-arm | n = 2; Japanese; chemo-naïve or previously treated; exon 19 deletion | Erlotinib 150 mg/day | TTP: Patient 1: 308 days (10.1 months) Patient 2: >973 days (>32 months) |
| Zhou et al. [3] | Ph III; randomized comparison versus carboplatin/gemcitabine (OPTIMAL) | n = 82: Chinese; chemo-naïve; exon 19 deletion (n = 43); L858R (n = 39) | Erlotinib 150 mg/day | PFS: 13.1 months |
| Zhu et al. [55] | Retrospective; single-arm | n = 8: Chinese; chemo-naïve or previously treated; exon 19 deletion (n = 5); L858R (n = 3) | Erlotinib 150 mg/day | PFS: 15.2 months |
| Gefitinib | ||||
| Asahina et al. [56] | Ph II, single-arm | n = 16: Japanese; chemo-naive; exon 19 deletion (n = 13); L858R (n = 3) | Gefitinib 250 mg/day | PFS: 8.9 months |
| Bell et al. [57] | Retrospective (Ph II IDEAL studies) | n = 14: ≥1 previous treatment; exon 19 deletion (n = 11); L858R (n = 2); InsG771 (n = 1) | Gefitinib 250 or 500 mg/day | TTP: 3.8 months |
| Buckingham et al. [58] | Retrospective | n = 17: ≥1 previous treatment; EGFR mutation | Gefitinib 250 mg/day | PFS: 13.6 months |
| Chou et al. [59] | Retrospective | n = 33: Taiwanese; prior platinum therapy; exon 18 substitution (n = 4); exon 19 deletion (n = 11); exon 20 substitution or deletion (n = 4); exon 21 substitution (n = 12); ≥1 mutation (n = 2) | Gefitinib 250 mg/day | PFS: 7.6 months |
| Cortes-Funes et al. [60] | Retrospective | n = 10: Spanish; ≥1 previous treatment; exon 19 deletion (n = 8); L858R (n = 2) | Gefitinib 250 mg/day | TTP: 12.3 months |
| D'Addario et al. [61] | Ph II, single-arm | n = 4: Swiss; chemo-naїve; exon 19 deletion (n = 2); L858R (n = 2) | Gefitinib 250 mg/day | TTP: 7.5 months |
| Dongiovanni et al. [62] | Retrospective | n = 9: Italian; chemo-naїve or previously treated; exon 19 deletion (n = 8); L858R (n = 1) | Gefitinib 250 mg/day | TTP: 14.9 months |
| Fukuoka et al. [63] | Ph III IPASS; randomized comparison with carboplatin/paclitaxel | n = 132: East-Asian; adenocarcinoma; never-smokers; chemo-naїve; EGFR mutation | Gefitinib 250 mg/day | PFS: 9.5 months |
| Han et al. [64] | Retrospective | n = 21: Korean; previously treated; exon 19 deletion (n = 12); L858R (n = 6); G719A (n = 3) | Gefitinib 250 mg/day | TTP: 13.8 months |
| Hirsch et al. [65] | Pooled analysis | n = 43: Italian or US; chemo-naїve or previously treated; exon 21 mutations (n = 31); exon 19 deletions (n = 11); mutations in exons 19 and 21 (n = 1) | Gefitinib 250 or 500 mg/day | PFS: 3 months |
| Ichihara et al. [66] | Retrospective | n = 30: Japanese; chemo-naїve and previously treated; exon 19 deletion (n = 16); L858R (n = 14) | Gefitinib 250 mg/day | PFS: 11.3 months |
| Inoue et al. [67] | Ph II, single-arm | n = 29: Japanese; chemo-naїve; poor performance status; exon 19 deletion (n = 18); L858R (n = 10), L861Q (n = 1) | Gefitinib 250 mg/day | PFS: 6.5 months |
| Inoue et al. [68] | Ph II, non-randomized comparison with standard chemotherapy | n = 16: Japanese; chemo-naїve; exon 19 deletion (n = 9); L858R (n = 7) | Gefitinib 250 mg/day | PFS: 9.7 months |
| Kim et al. [69] | Retrospective | n = 8: Korean; ≥1 previous treatment; exon 19 deletion (n = 5); L858R (n = 1) | Gefitinib 250 mg/day | TTP: 12.6 months |
| Kimura et al. [70] | Prospective, single-arm | n = 9: Japanese; chemo-naїve and previously treated; exon 19 deletion (n = 4); L858R (n = 4); V689L (n = 1) | Gefitinib 250 mg/day | PFS: 6.4 months |
| Kobayashi et al. [71] | Ph III, randomized comparison with carboplatin/paclitaxel | n = 98: chemo-naïve, EGFR mutation | Gefitinib 250 mg/day | PFS: 10.4 months |
| Koyama et al. [72] | Retrospective | n = 18: Japanese; chemo-naїve or previous treatment; G719C (n = 2); G719C and W731R (n = 1); P733S (n = 1); exon 19 deletion (n = 6); V738–I744 ins (n = 2); S768C (n = 1); T790M (n = 1); Q812R (1); V843I (n = 1); L858R (n = 2) | Gefitinib 250 mg/day | Mean TTP: 13.7 months |
| Massarelli et al. [73] | Retrospective | n = 7: Asian or Caucasian; chemo-naїve or previous treatment; exon 19 deletion (n = 6); G719A (n = 1) | Gefitinib 250 mg/day | TTP: 9.3 months |
| Oshita et al. [74] | Retrospective | n = 11: Japanese; ≥1 previous treatment; EGFR mutation | Gefitinib 250 mg/day | PFS: 16 months |
| Pallis et al. [75] | Retrospective | n = 11: Greek; ≥1 previous treatment; exon 19 deletion (n = 6); L858R (n = 3); G719D (n = 1); E746V (n = 1) | Gefitinib 250 mg/day | TTP: 14.7 months |
| Riely et al. [36] | Retrospective | n = 22: primarily white; chemo-naїve or previously treated; exon 19 deletion (n = 15); L858R (n = 7) | Gefitinib 250 mg/day | PFS: 12 months |
| Sequist et al. [76] | Ph II, single-arm | n = 31: primarily non-Asian; chemo-naїve; exon 19 deletion (n = 17); L858R (n = 8); atypical mutation (n = 6) | Gefitinib 250 mg/day | PFS: 9.2 months |
| Shao et al. [77] | Ph II, single-arm | n = 51: Taiwanese; chemo-naїve; EGFR mutation | Gefitinib 250 mg/day | PFS: 8.8 months |
| Shoji et al. [78] | Retrospective | n = 20; Japanese; chemo-naїve and previously treated; exon 19 deletion (n = 10); L858R (n = 8); E709A and G719S (n = 1); L858R and Y725Y (n = 1) | Gefitinib 250 mg/day | PFS: 14 months |
| Sugio et al. [79] | Ph II, single-arm | n = 19: Japanese; exon 19 deletion (n = 7); L858R (n = 10); exon 19 deletion and L858R (n = 1); exon 19 deletion and G796A (n = 1) | Gefitinib 250 mg/day | PFS: 7.1 months |
| Sunaga et al. [80] | Ph II, single-arm | n = 21: Japanese; chemo-naїve or previously treated; exon 19 deletion (n = 17); L858R (n = 4) | Gefitinib 250 mg/day | PFS: 12.9 months |
| Sutani et al. [81] | Ph II, single-arm | n = 27: Japanese; 0–1 previous treatments; exon 19 deletion; L858R, L861Q | Gefitinib 250 mg/day | TTP: 9.4 months |
| Takano et al. [82] | Retrospective | n = 85: Japanese; chemo-naїve or previously treated; exon 19 deletion (n = 49); L858R (n = 36) | Gefitinib 250 mg/day | PFS: 9.2 months |
| Tamura et al. [83] | Ph II, single-arm | n = 28: Japanese; 0–2 previous treatments; exon 19 deletion (n = 14); L858R (n = 14) | Gefitinib 250 mg/day | PFS: 11.5 months |
| Varella-Garcia et al. [84] | Retrospective | n = 27: Japanese; chemo-naїve or previously treated; EGFR mutations | Gefitinib 250 mg/day | TTP: 10.2 months |
| Xu et al. [85] | Retrospective | n = 32: Chinese; chemo-naїve and previous treatment; exon 19 deletion (n = 11); exon 19 – not deletion (n = 6); L858R (n = 6); exon 18 mutation (n = 6); exon 20 mutation (n = 2); exon 23 mutation (n = 1) | Gefitinib 250 mg/day | TTP: 15 months |
| Zhang et al. [86] | Retrospective | n = 12: Chinese; ≥1 previous treatment; exon 19 deletion (n = 4); L858 (n = 8) | Gefitinib 250 mg/day | PFS: 10 months |
| Asami et al. [87] | Prospective; Ph II; single-arm | n = 17: Japanese; exon 19 deletion (n = 7); L858R (n = 10) | Gefitinib 250 mg/day | PFS: 12.9 months |
| Azuma et al. [88] | Retrospective | n = 47: Japanese; chemo-naїve or previously treated; exon 19 deletion (n = 27); L858R (n = 20) | Gefitinib 250 mg/day | PFS: 6.7 months |
| Chen et al. [89] | Prospective, single-arm | n = 26: Chinese; chemo-naїve; EGFR mutation | Gefitinib 250 mg/day | PFS: 9.0 months |
| Chen et al. [90] | Prospective, Ph II; randomized comparison of gefitinib ± tegafur/uracil | n = 16: Taiwanese or Chinese; previously treated; exon 19 deletion (n = 12); L858R (n = 4) | Gefitinib 250 mg/day | PFS: 7.6 months |
| Douillard et al. [7] | Ph III INTEREST study; randomized, comparison with docetaxel | n = 19: primarily white; prior platinum chemotherapy; EGFR mutation | Gefitinib 250 mg/day | PFS: 7.0 months |
| Giovannetti et al. [91] | Retrospective, single-arm | n = 9: Italian; chemo-naїve or previously treated; exon 19 deletion (n = 7); L858R (n = 2) | Gefitinib 250 mg/day | TTP: 9.0 months |
| Inoue et al. [92] | Prospective; Ph II; single-arm (NEJ003) | n = 31: Japanese; chemo-naїve; EGFR mutation | Gefitinib 250 mg/day | PFS: 13.6 months |
| Lee et al. [93] | Ph III; randomized comparison with cisplatin/gemcitabine (First-SIGNAL) | n = 27: Korean; chemo-naïve; EGFR mutation | Gefitinib 250 mg/day | PFS: 7.9 months |
| Kim et al. [94] | Prospective; Ph II; single-arm | n = 45: Korean; chemo-naïve; exon 19 deletion (n = 29); L858R (n = 15); L861Q (n = 1) | Gefitinib 250 mg/day | PFS: 398 days (13.1 months) |
| Maemondo et al. [10] | Ph III; randomized comparison with carboplatin/paclitaxel (NEJSG002) | n = 114: Japanese; chemo-naïve; exon 19 deletion (n = 58); L858R (n = 49); Other (n = 7) | Gefitinib 250 mg/day | PFS: 10.8 months |
| Masago et al. [95] | Retrospective; single-arm | n = 47; Japanese; chemo-naїve or previously treated; EGFR mutation | Gefitinib 250 mg/day | PFS: 342 days (11.3 months) |
| Mitsudomi et al. [9] | Ph III; randomized comparison with cisplatin/docetaxel (WJTOG3405) | n = 86: Japanese; chemo-naïve; exon 19 deletion (n = 50); L858R (n = 36) | Gefitinib 250 mg/day | PFS: 9.2 months (8.4 months in stage IIIB/IV) |
| Moiseyenko et al. [96] | Prospective; single-arm | n = 25: Russian; chemo-naïve; exon 19 deletion (n = 17); L858R (n = 8) | Gefitinib 250 mg/day | PFS: 8.0 months |
| Park et al. [97] | Prospective, Ph II, single-arm | n = 3: Korean; previously treated; exon 19 deletion (n = 2); L858R (n = 1) | Gefitinib 250 mg/day | PFS: 5.8 months |
| Sun et al. [53] | Retrospective; single-arm | n = 42: Korean; mainly previously treated; exon 19 deletion or L858R | Gefitinib 250 mg/day | PFS: 11.9 months |
| Sun et al. [15] | Ph III; randomized comparison with icotinib (ICOGEN) | n = 39: Chinese; previously treated; EGFR mutation | Gefitinib 250 mg/day | PFS: 158 days |
| Uruga et al. [98] | Retrospective; single-arm | n = 9: Japanese; chemo-naїve or previously treated; exon 19 deletion (n = 6); L858R (n = 3) | Gefitinib 250 mg/day | PFS: 396 days |
| Wu et al. [99] | Retrospective; single-arm | n = 272: Taiwanese; chemo-naїve or previously treated; exon 19 deletion (n = 106); L858R (n = 114); other (n = 52) | Gefitinib 250 mg/day | PFS: 7.8 months |
| Wu et al. [100] | Retrospective; single-arm | n = 32: Chinese; previously treated; exon 19 deletion or L858R | Gefitinib 250 mg/day | PFS: 8.0 months |
| Yamaguchi et al. [101] | Retrospective; single-arm | n = 16: Japanese; chemo-naїve or previously treated; exon 19 deletion (n = 4); L858R (n = 8); exon 19 deletion + L858R (n = 4) | Gefitinib 250 mg/day | PFS: 246 days(8.1 months) |
| Yoshida et al. [102] | Prospective | Japanese; chemo-naïve (n = 23); exon 19 deletion or L858R Japanese; previously treated (n = 15); exon 19 deletion or L858R | Gefitinib 250 mg/day | PFS: 7.8 months PFS: 6.5 months |
| Chemotherapy | ||||
| Bell et al. [57] | Retrospective (Ph III INTACT studies; randomized comparison with gefitinib) | n = 9: primarily white; chemo-naїve; exon 19 deletion; L858R; other mutations | Paclitaxel/carboplatin or gemcitabine/cisplatin | PFS: 6.7 months |
| Eberhard et al. [103] | Retrospective (Ph III TRIBUTE study; randomized comparison with erlotinib plus carboplatin/paclitaxel) | n = 14: primarily white; chemo-naїve; exon 19 deletion; L858R; other mutations | Carboplatin/paclitaxel | TTP: 6.6 months |
| Fukuoka et al. [63] | Ph III; randomized comparison with gefitinib (IPASS) | n = 129: East-Asian; adenocarcinoma; never-smokers; chemo-naїve; EGFR mutation | Carboplatin/paclitaxel | PFS: 6.3 months |
| Inoue et al. [68] | Ph II; non-randomized comparison with gefitinib | n = 9: Japanese; chemo-naїve; exon 19 deletions (n = 8); L858R (n = 1) | Standard chemotherapy | PFS: 7.6 months |
| Lee et al. [104] | Retrospective | n = 17: Korean; chemo-naїve; patients receiving platinum-based chemotherapy; EGFR mutation | Platinum-based chemotherapy | TTP: 8 months paclitaxel, 9.7 months; gemcitabine, 7.4 months |
| Tambo et al. [105] | Retrospective | n = 26: Japanese; chemo-naïve; EGFR mutations | Chemotherapy | PFS: 8.4 months |
| Ciuleanu et al. [40] | Ph III; randomized comparison versus erlotinib (TITAN) | n = 4: predominantly white; previously treated; EGFR mutation | Docetaxel or pemetrexed (single-agent) | PFS: 9.9 months |
| Douillard et al. [7] | Ph III; randomized comparison with gefitinib (INTEREST) | n = 19; primarily white; prior platinum chemotherapy; EGFR mutation | Docetaxel | PFS: 4.1 months |
| Rosell et al. [44] | Ph III; randomized comparison versus erlotinib (EURTAC) | n = 87: primarily white; chemo-naïve; EGFR mutation | Platinum-based chemotherapy | PFS: 5.2 months |
| Kalikaki et al. [106] | Retrospective; single-arm | n = 9; Greek; chemo-naïve; EGFR mutation | Chemotherapy | TTP: 6.1 months |
| Kim et al. [107] | Retrospective | n = 67: Korean; chemo-naïve; EGFR mutation | Platinum-based chemotherapy | PFS: 7.1 months |
| Lin et al. [108] | Retrospective; single-arm | n = 56: East-Asian; chemo-naïve; primarily exon 19 deletion or L858R | Predominantly platinum-based chemotherapy | PFS: 6.1 months |
| Maemondo et al. [10] | Ph III; randomized comparison with gefitinib (NEJSG002) | n = 114: Japanese; chemo-naïve; exon 19 deletion (n = 59); L858R (n = 48); other (n = 7) | Carboplatin/paclitaxel | PFS: 5.4 months |
| Matsumoto et al. [109] | Retrospective; single-arm | n = 26: Japanese; chemo-naïve; EGFR mutation | Chemotherapy (50% platinum doublet, 50% non-platinum) | PFS: 6.9 months |
| Mitsudomi et al. [9] | Ph III; randomized comparison with gefitinib (WJTOG3405) | n = 86: Japanese; chemo-naïve; exon 19 deletion (n = 37); L858R (n = 49) | Cisplatin/docetaxel | PFS: 6.3 months (5.3 months in stage IIIB/IV) |
| Sun et al. [53] | Retrospective; single-arm | n = 67: Korean; chemo-naïve; exon 19 deletion or L858R | Chemotherapy | PFS: 5.1 months |
| Wu et al. [99] | Retrospective; single-arm | n = 93: Taiwanese; previously treated; exon 19 deletion (n = 43); L858R (n = 37); other (n = 13) | Pemetrexed monotherapy | PFS: 3.9 months |
| Wu et al. [100] | Retrospective; single-arm | n = 55: Chinese; chemo-naïve; exon 19 deletion (n = 32); L858R (n = 21); exon 19 deletion and L858R (n = 2) | Predominantly platinum-based chemotherapy | PFS: 4 months |
| Yoshida et al. [102] | Prospective | Japanese; chemo-naïve (n = 25) or previously treated (n = 20); exon 19 deletion or L858R | Cytotoxic chemotherapy | PFS: chemo-naïve: 5.1 months; previously treated: 4.0 months |
| Zhou et al. [3] | Ph III; randomized comparison versus erlotinib (OPTIMAL) | n = 72: Chinese; chemo-naïve; exon 19 deletion (n = 39); L858R (n = 33) | Carboplatin/gemcitabine | PFS: 4.6 months |
| Afatinib | ||||
| Yang et al. [110] | Ph II; randomized, single-arm | n = 129; Taiwanese and US; chemo-naïve, or one previous line of chemotherapy; exon 19 deletion (n = 52); L858R (n = 54); other (n = 23) | Afatinib 40 mg or 50 mg/day | PFS: 14 months |
| Icotinib | ||||
| Ren et al. [16] | Ph I; single-arm | n = 7: Chinese; previously treated; exon 19 deletion (n = 3); L858R (n = 4) | Icotinib (varied dose and schedule) | PFS: 141 days (4.6 months) |
| Sun et al. [15] | Ph III; randomized comparison with gefitinib (ICOGEN) | n = 27: Chinese; previously treated; EGFR mutation | Icotinib 125 mg three times/day | PFS: 198 days (6.5 months) |
Ph: phase, BAC: bronchioloalveolar carcinoma.
There was a mixture of ethnicities included in the pooled analysis. Since only patients with EGFR activating mutations were included, no effect of ethnicity on efficacy was expected. Subgroup analyses revealed no striking differences between Asian and Caucasian patients with EGFR activating mutations and therefore, no adjustments were made in the analysis for ethnicity.
When the median PFS data are examined individually, there is a trend for erlotinib and gefitinib to report longer PFS times than chemotherapy (Fig. 2). This trend is confirmed when the pooled median PFS values are considered (Fig. 3). When analysed for significance by permutation testing (Table 2) there was a statistically significant increase in PFS for erlotinib compared with chemotherapy in the first line (P = 0.000), in lines other than first (P = 0.0022) and in all lines (P = 0.000). There was also a statistically significant increase in PFS for gefitinib compared with chemotherapy in the first line (P = 0.000), in lines other than first (P = 0.0039) and in all lines (P = 0.000). There were only three chemotherapy studies (n = 116) in treatment lines other than first; which limits the interpretation of this result, despite the significant P-value. However, the pooled median PFS value of 4.1 months for these three studies was not unexpected when the 5.8-month pooled median PFS for first-line chemotherapy-treated patients was considered.
Fig. 2.
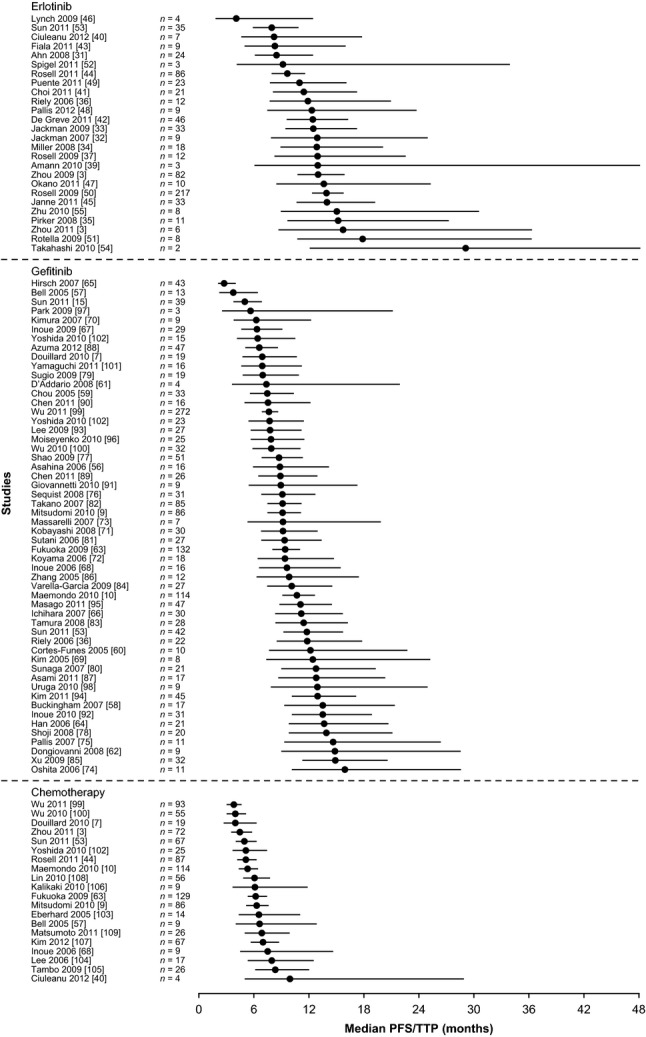
Forest plot showing analysis of median pooled PFS or TTP and 90% accuracy intervals during treatment with single-agent erlotinib, single-agent gefitinib or chemotherapy in all lines of treatment in patients with EGFR mutation-positive NSCLC. PFS: progression-free survival; TTP: time to progression; EGFR: epidermal growth factor receptor; NSCLC: non-small-cell lung cancer.
Fig. 3.
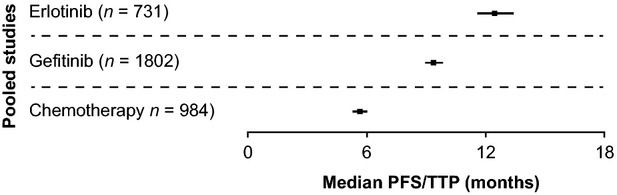
Forest plot showing pooled median PFS/TTP and 95% accuracy intervals with single-agent erlotinib, single-agent gefitinib or chemotherapy in patients with EGFR mutation-positive NSCLC in all lines of therapy. PFS: progression-free survival; TTP: time to progression; EGFR: epidermal growth factor receptor; NSCLC: non-small-cell lung cancer.
Table 2.
Pooled median PFS with 95% accuracy intervals for patients with EGFR mutation-positive tumours
| Treatment | Number of studies | Pooled number of patients | Pooled median PFS | Accuracy interval | Bootstrap estimated 95% confidence limits |
|---|---|---|---|---|---|
| Any line of therapy | |||||
| Single-agent erlotinib | 26 | 731 | 12.4 | 11.6–13.4 | 10.9–13.4 |
| Single-agent gefitinib | 54 | 1802 | 9.4 | 9.0–9.8 | 8.7–10.2 |
| Chemotherapy | 20 | 984 | 5.6 | 5.3–6.0 | 5.1–6.2 |
| Predominantly first line* | |||||
| Single-agent erlotinib | 10 | 354 | 12.0 | 10.8–13.3 | 10.8–13.1 |
| Single-agent gefitinib | 16 | 703 | 9.8 | 9.1–10.5 | 9.0–10.6 |
| Chemotherapy | 17 | 868 | 5.8 | 5.5–6.2 | 5.4–6.4 |
| Lines of therapy other than first | |||||
| Single-agent erlotinib | 17 | 377 | 12.9 | 11.6–14.3 | 10.0–13.9 |
| Single-agent gefitinib | 37 | 1099 | 9.2 | 8.6–9.7 | 8.3–10.5 |
| Chemotherapy | 3 | 116 | 4.1 | 3.5–5.0 | n/a† |
Predominantly first line is ≥90% of patients treated in the first-line setting.
Because of the low number of studies in this pool the bootstrap estimate is not trustworthy.
Comparing lines of treatment (Table 3), the pooled median PFS for chemotherapy was different when given as predominantly first-line treatment versus other lines of treatment (5.8 versus 4.1 months, respectively, P = 0.012). The analysis did not discriminate between single-agent chemotherapy and doublet chemotherapy. The pooled median PFS values for erlotinib and gefitinib were not statistically different between lines of treatment (erlotinib: 12.0 versus 12.9 months, for first and other lines, respectively, P = 0.678; gefitinib: 9.7 versus 9.1 months, for first and other lines, respectively, P = 0.283).
Table 3.
Statistical analysis based on permutation testing
| Study pool A | Study pool B | Differential median pooled PFS (A-B) | Estimated P-value (based on 20,000 random permutations) |
|---|---|---|---|
| Any line of therapy | |||
| Chemotherapy | EGFR TKI | −4.7 | 0.000 |
| Chemotherapy | Gefitinib | −3.8 | 0.000 |
| Chemotherapy | Erlotinib | −6.8 | 0.000 |
| Predominantly first line* | |||
| Chemotherapy | EGFR TKI | −4.7 | 0.000 |
| Chemotherapy | Gefitinib | −4.0 | 0.000 |
| Chemotherapy | Erlotinib | −6.2 | 0.000 |
| Lines of therapy other than first | |||
| Chemotherapy | EGFR TKI | −6.1 | 0.0028 |
| Chemotherapy | Gefitinib | −5.1 | 0.0039 |
| Chemotherapy | Erlotinib | −8.8 | 0.0022 |
| Chemotherapy | |||
| Predominantly first line* | Lines of therapy other than first | 1.7 | 0.012 |
| Erlotinib | |||
| Predominantly first line* | Lines of therapy other than first | −0.9 | 0.678 |
| Gefitinib | |||
| Predominantly first line* | Lines of therapy other than first | 0.6 | 0.283 |
Predominantly first line is ≥90% of patients treated in the first-line setting. This comparative test is statistically valid, but only refers to the given study pool (conditional test) and cannot be readily extrapolated to the total patient population.
Statistical analysis of a pooled median PFS could not be established for afatinib and icotinib because of lack of data. In one study for afatinib (n = 129), the PFS was 14 months [110], and in two studies (n = 27) and (n = 7) the PFS with icotinib was 6.5 months and 4.6 months, respectively [15,16] (Table 1). No eligible dacomitinib studies were identified at the time of analysis.
Publication bias was assessed by using funnel plots with PFS/TTP as the outcome. These were symmetrical for each of the treatment groups (Fig. 4A–C).
Fig. 4.
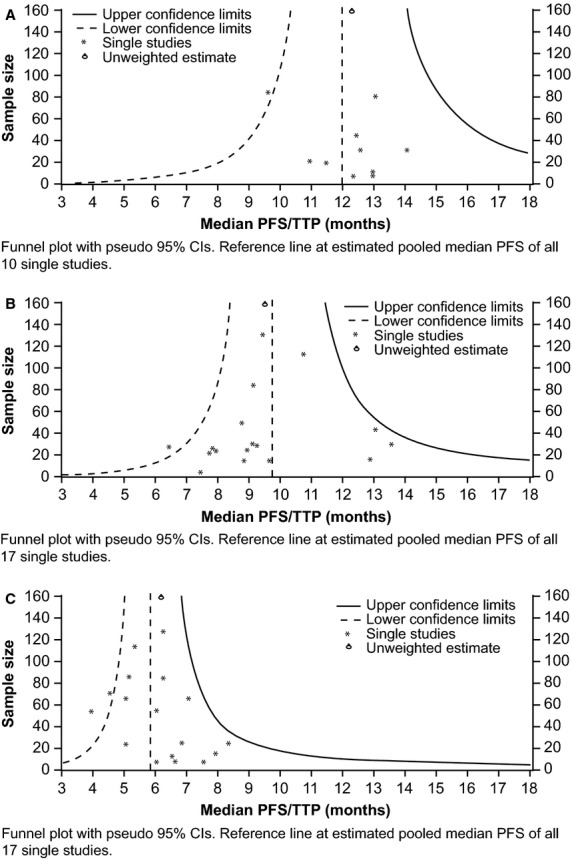
Funnel plots by using PFS/TTP as an outcome for (A) single-agent erlotinib (B) single-agent gefitinib and (C) chemotherapy in the first-line setting. PFS: progression-free survival; TTP: time to progression; CI: confidence interval.
Discussion
The dataset analyzed here was almost double the size of that previously assessed [8]. The patient number was updated from 365 to 731 in the erlotinib arm, from 1069 to 1802 in the gefitinib arm and from 375 to 984 in the chemotherapy studies. Progression-free survival was again chosen as the end-point to assess. Because of high levels of crossover in post-study therapy, the use of overall survival as an end-point was not considered to be able to discern differences between treatments. This analysis indicates that PFS is longer in patients with EGFR mutation-positive NSCLC when treated with erlotinib (12.4 months) or gefitinib (9.4 months), compared with conventional chemotherapy (5.6 months). Permutation testing indicated that the difference in PFS was statistically significant, but this should be interpreted carefully, given that this significance applies to the current study pool and it cannot be readily extrapolated to the total patient population since a controlled randomized trial has not been carried out to confirm this. The bootstrap runs underlined the adequacy of the used accuracy intervals and thus provided confidence in the validity of the main analysis. The results are similar to those reported previously [8], in which statistically different PFS values for erlotinib (13.2 months) and gefitinib (9.8 months) were shown, as compared with chemotherapy (5.9 months).
As for the previous pooled analysis [8], there are limitations to this analysis. Statistical comparisons were made in this pooled retrospective analysis between erlotinib, gefitinib and chemotherapy based on PFS. Only high level information like median PFS was obtained from the publications, which could be used to calculate the pooled median PFS. In order to determine accuracy intervals, the simplifying assumption that PFS followed an exponential distribution was necessary but this was not verifiable. However, a bootstrap run confirmed the approximate validity of the accuracy intervals. Also, the schedule of visits for the progression of disease may have differed, according to the trial protocol. Furthermore, as the composition of the different patient groups (with respect to relevant risk factors) cannot be assessed, the results should be interpreted with due caution. However, there is no indication that the study pool and its treatment subgroups were not representative of the total patient population. Because of the comprehensive nature of the study pool, the differences reported have resulted from data collated from almost the entire body of evidence published up to November 2011. Furthermore, the line of treatment represents an important clinical risk factor that was considered as part of this study pool; the differences between treatments were confirmed through the treatment lines investigated, lending weight to the analyses. These points suggest that, despite the inherent limitations, the differences seen in the study pool deserve attention when the total patient population is considered. Because of the comprehensive approach to pool all available evidence, retrospective studies were included and no quality analysis of the data or mutation testing was possible. Additionally, this pooled analysis compares only median PFS values, and does not include other measures of response or safety/toxicity. Finally, PFS is not always assessed in the same way across all studies, a further source of variability.
The present analysis included large, phase III studies that prospectively evaluated the treatments in patients with EGFR mutation-positive NSCLC, which augment the dataset with robust data. The dataset reported here seems to be in agreement with the primary results of the additional phase III trials, which all reported significantly longer PFS with EGFR TKI therapy compared with chemotherapy [3,4,10,111]. The gefitinib data reported here are also in agreement with results of a recently reported phase IV study of gefitinib in Caucasian patients with EGFR mutation-positive NSCLC (n = 106), which observed a median PFS of 9.7 months [112].
There is a need for randomized trials among the EGFR TKIs to directly compare efficacy and toxicity. Trials are ongoing or recently completed, which should provide further data. For example, a randomized, open-label trial recently reported a longer PFS, but slightly more adverse events, with dacomitinib compared with erlotinib in patients with previously treated advanced NSCLC (n = 188) [113]. The LUX-Lung 7 study is a comparative study of afatinib versus gefitinib (NCT01466660) for EGFR mutation-positive NSCLC and is currently recruiting patients. Finally, CTONG 0901 (NCT01024413) was a randomized, phase II trial comparing first-line erlotinib with first-line gefitinib in patients with advanced NSCLC with exon 21 mutations; results have not yet been reported.
Afatinib and icotinib were included in the literature search and pooled analysis. However, there are currently very limited data on these two EGFR TKIs, and statistical analysis of a pooled median PFS could not be accomplished. Further clinical trials are required to establish the role of these agents in the treatment of patients with EGFR mutation-positive NSCLC. Recently, a phase III clinical trial of afatinib was completed and showed that patients receiving afatinib (n = 230) had a median PFS of 11.1 months (compared with 6.9 months with chemotherapy; hazard ratio [HR] = 0.58, 95% CI 0.43–0.78, P = 0.0004) [11]. This study was reported after the pooled analysis was complete, and is therefore not included in the analysis. In a subset of patients with exon 19 and L858R mutations, the median PFS was 13.6 months for afatinib, compared with 6.9 months for chemotherapy (HR = 0.47, 95% CI 0.34–0.65, P < 0.0001). Tolerability of anti-EGFR agents is also important; afatinib had relatively high levels of treatment-related adverse events (diarrhoea: 95%, leading to discontinuation in 1% of patients; rash: 62% and paronychia: 57%).
This pooled analysis utilizes data from a variety of ethnicities, ages and smoking histories, and includes both male and female patients. There are also a variety of EGFR mutations included, although the majority are exon 19 deletions and L858R mutations. The clear efficacy benefits across this range of clinical characteristics confirm the necessity of EGFR mutation testing, rather than reliance on clinical characteristics. Studies in which patients were ‘unselected’ or selected by clinical characteristics, demonstrate this. The First-SIGNAL study comparing the efficacy of single-agent gefitinib with gemcitabine plus cisplatin as first-line therapy for Korean ‘never-smokers’ with stage IIIB or IV lung adenocarcinoma not selected by EGFR mutation status [114] was unable to demonstrate superiority for gefitinib over chemotherapy. The IPASS study (phase III, n = 609, gefitinib or carboplatin plus paclitaxel in first line) showed non-inferiority of gefitinib to chemotherapy in non-smokers (or former light smokers) with adenocarcinoma. Only 60% of this preselected population had EGFR mutation-positive tumours. However, a significant PFS benefit of gefitinib compared with chemotherapy was reported in patients with established EGFR mutated-disease (9.5 months versus 6.3 months, respectively, HR = 0.48, 95% CI 0.36–0.64 P < 0.0001) [5]. Obviously, clinical characteristics are not an appropriate surrogate for EGFR mutation testing.
Furthermore, although common mutations, such as exon 19 deletions and L858R mutations in exon 21 have been associated with response to EGFR TKIs, many other mutations are detected only occasionally, and correlations with response are not defined. A recent study screened 681 cases and found 18 rare mutations; responses to EGFR TKIs were reported on a case by case basis and varied by mutation [115]. For example, exon 20 and 21 mutations were more likely to confer resistance to erlotinib or gefitinib, while exon 18 and 19 mutations were more often associated with improved efficacy outcome. An analysis of ‘other’ mutations from the SATURN, TRUST and TITAN trials suggested that some mutations (e.g. in exon 18) conferred a clinical benefit from erlotinib and others (e.g. in exon 20) had a prognostic influence on OS [116]. However, further data are needed.
Erlotinib and gefitinib also have differing responses in the common mutations, and new EGFR TKIs would be expected to also have differences. Exon 19 deletions and L858R mutations have shown similar in vitro sensitivity to gefitinib [22]; however, erlotinib and gefitinib have shown different clinical efficacy depending on whether exon 19 deletions and L858R mutations are present [117,118]. Despite these differences, both drugs have efficacy in patients with both of these mutations and these differences would not influence treatment selection.
As the number of clinical trials evaluating EGFR TKIs continues to increase, the number of patients eligible for pooled analyses such as this one will increase. Updating the dataset will enable more information to be gathered on the effect of TKIs on patients with NSCLC with rare mutations, as well as efficacy outcome of the newer agents. Assessing the benefit of different treatment regimens will also be important, for example, sequential intercalated chemotherapy and erlotinib is being investigated as a promising approach [119].
Conclusions
A comprehensive review of PFS with EGFR TKI therapy or chemotherapy in the treatment of EGFR mutation-positive NSCLC was carried out, and included more than 3500 patients in a pooled analysis. The results demonstrate a clear PFS benefit of treating patients with EGFR mutation-positive NSCLC with an EGFR TKI compared with chemotherapy, with median pooled PFS values of 12.4 months (erlotinib), 9.4 months (gefitinib) and 5.6 months (chemotherapy) reported. This confirms that all patients should be tested for EGFR mutation status immediately on diagnosis of NSCLC, or as soon as feasible. This pooled dataset is in agreement with several large, prospective phase III studies that examined EGFR TKIs as first-line therapy, and strengthens the recommendation that EGFR mutation-positive NSCLC should be treated with erlotinib or gefitinib in the first line. If first-line therapy with EGFR TKIs is not achievable, then consideration should be given to treating with an anti-EGFR agent in any line of therapy, as PFS benefits over chemotherapy are obvious. Further trials should provide more insight into the role of second-generation EGFR TKIs for EGFR mutation-positive NSCLC.
Acknowledgments
Support for third-party writing assistance for this manuscript was provided by F. Hoffmann–La Roche Ltd.
Author contribution
Luis Paz-Ares contributed to the design of the study, data interpretation and writing the manuscript. Denis Soulières contributed to the design of the study, data interpretation and writing the manuscript. Joachim Moecks contributed to the design of the study, statistical approaches, data interpretation and writing the manuscript and analyzing the data. Ilze Bara contributed to the design of the study, data interpretation and writing the manuscript. Tony Mok contributed to the design of the study, data interpretation and writing the manuscript. Barbara Klughammer contributed to the design of the study, data interpretation and writing the manuscript and analyzing the data.
Conflicts of interest
Dr Luis Paz-Ares has received honoraria from F. Hoffman-La Roche Ltd, AstraZeneca, Pfizer and Boehringer Ingelheim. Dr Barbara Klughammer is an employee of F. Hoffman-La Roche Ltd and holds stock options in F. Hoffman-La Roche Ltd. Dr Ilze Bara is an employee of F. Hoffman-La Roche Ltd. Dr Joachim Moecks is a past-employee of F. Hoffman-La Roche Ltd. Dr Soulières has served on advisory boards for F. Hoffman-La Roche and has received payment for speaking engagements from F. Hoffman-La Roche. Prof Tony Mok has received honoraria from, and acted as a consultant for, AstraZeneca, Roche, Eli Lilly, Merck Serono, Eisai, BMS, Beigene, AVEO, Pfizer, Taiho, BI, and GSK Biologicals, and has received research funding from AstraZeneca.
References
- 1.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 2.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Mok T, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–42. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Soulieres D, Melezinek I, et al. Clinical outcomes in non-small cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med. 2010;14:51–69. doi: 10.1111/j.1582-4934.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 10.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC-H, Schuler MH, Yamamoto N, et al. LUX-Lung 3: a randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol. 2012;30:LBA7500. [Google Scholar]
- 12.Mok T, Spigel DR, Park K, et al. Efficacy and safety of PF299804 as first-line treatment (tx) of patients (pts) with advanced (adv) NSCLC selected for activating mutation (mu) of epidermal growth factor receptor (EGFR) Ann Oncol. 2010;21:LBA18. [Google Scholar]
- 13.Boyer MJ, Blackhall FH, Park K, et al. Efficacy and safety of PF299804 versus erlotinib (E): a global, randomized phase II trial in patients (pts) with advanced non-small cell lung cancer (NSCLC) after failure of chemotherapy (CT) J Clin Oncol. 2010;28:LBA7523. [Google Scholar]
- 14.Ou S-H. Second generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol. 2012;83:407–21. doi: 10.1016/j.critrevonc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Shi Y, Zhang L, et al. A randomized, double-blind phase III study of icotinib versus gefitinib in patients with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN) J Clin Oncol. 2011;29:Abstract 7522. [Google Scholar]
- 16.Ren GJ, Zhao YY, Zhu YJ, et al. Tumor gene mutations and messenger RNA expression: correlation with clinical response to icotinib hydrochloride in non-small cell lung cancer. Chin Med J. 2011;124:19–25. [PubMed] [Google Scholar]
- 17.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nature Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 18.Gaughan EM, Costa DB. Genotype-driven therapies for non-small cell lung cancer: focus on EGFR, KRAS and ALK gene abnormalities. Ther Adv Med Oncol. 2011;3:113–25. doi: 10.1177/1758834010397569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 20.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 21.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumours to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Nat Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 23.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 24.Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 25.Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–41. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 26.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 27.Hotta K, Matsuo K, Ueoka H, et al. Role of adjuvant chemotherapy in patients with resected non–small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol. 2004;22:3860–7. doi: 10.1200/JCO.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 28.Smouse JH, Cibas ES, Janne PA, et al. EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer Cytopathol. 2009;117:67–72. doi: 10.1002/cncy.20011. [DOI] [PubMed] [Google Scholar]
- 29.Gately K, O'Flaherty J, Cappuzzo F, et al. The role of molecular footprint of EGFR in tailoring treatment decisions in NSCLC. J Clin Pathol. 2012;65:1–7. doi: 10.1136/jclinpath-2011-200275. [DOI] [PubMed] [Google Scholar]
- 30.Hesterberg T, Monaghan S, Moore DS. Bootstrap methods and permutation tests. Companion chapter 18. In: Moore D, McCabe G, Duckworth WM, Alwan LC, et al., editors. The practice of business statistics. 2nd ed. New York: W.H. Freeman and Co; 2009. pp. 18-3–74. [Google Scholar]
- 31.Ahn MJ, Park BB, Ahn JS, et al. Are there any ethnic differences in molecular predictors of erlotinib efficacy in advanced nonsmall cell lung cancer? Clin Cancer Res. 2008;14:3860–6. doi: 10.1158/1078-0432.CCR-07-4608. [DOI] [PubMed] [Google Scholar]
- 32.Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 33.Jackman DM, Cioffredi L, Lindeman NI, et al. Phase II trial of erlotinib in chemotherapy-naive women with advanced pulmonary adenocarcinoma. J Clin Oncol. 2009;27:423s. [Google Scholar]
- 34.Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26:1472–8. doi: 10.1200/JCO.2007.13.0062. [DOI] [PubMed] [Google Scholar]
- 35.Pirker R, Minar W, Filipits M. Integrating epidermal growth factor receptor-targeted therapies into platinum-based chemotherapy regimens for newly diagnosed non-small-cell lung cancer. Clin Lung Cancer. 2008;9:S109–15. doi: 10.3816/CLC.2008.s.016. [DOI] [PubMed] [Google Scholar]
- 36.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–44. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 37.Rosell R, Perez-Roca L, Sanchez JJ, et al. Customized treatment in non-smallcell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PLoS ONE. 2009;4:e5133. doi: 10.1371/journal.pone.0005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S, Ren S, Yan L, et al. Clinical efficacy of erlotinib in patients previously treated for advanced non-small cell lung cancer. Respirology. 2009;14:709–15. doi: 10.1111/j.1440-1843.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 39.Amann JM, Lee JW, Roder H, et al. Genetic and proteomic features associated with survival after treatment with erlotinib in first-line therapy of non-small cell lung cancer in Eastern Cooperative Oncology Group 3503. J Thorac Oncol. 2010;5:169–78. doi: 10.1097/JTO.0b013e3181c8cbd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciuleanu T, Stelmakh S, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–8. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 41.Choi DR, Lee DH, Choi CM, et al. Erlotinib in first-line therapy for non-small cell lung cancer: a prospective phase II study. Anticancer Res. 2011;31:3457–62. [PubMed] [Google Scholar]
- 42.De Greve J, Van Meerbeeck JP, Vansteenkiste J, et al. First-line erlotinib in patients (pts) with advanced non-small cell lung cancer (NSCLC) carrying an activating EGFR mutation: a multicentre academic phase II study in Belgium. J Thorac Oncol. 2011;6:S1270. doi: 10.1371/journal.pone.0147599. (P3.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiala O, Pesek M, Krejci J, et al. Chemotherapy versus erlotinib in the second line treatment of advanced nonsmall cell lung cancer. J Thorac Oncol. 2011;6:S1259. [Google Scholar]
- 44.Rosell R, Gervais R, Vergnenegre A, et al. Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: interim results of the European Erlotinib versus Chemotherapy (EURTAC) phase III randomized trial. J Thorac Oncol. 2011;6:S314. [Google Scholar]
- 45.Janne PA, Wang X, Socinski MA, et al. Outcome of advanced NSCLC patients with EGFR exon 19 and 21 mutations treated with erlotinib (E) alone or in combination with carboplatin/paclitaxel (CP) in CALGB 30406. J Thorac Oncol. 2011;6:S447. [Google Scholar]
- 46.Lynch TJ, Fenton D, Hirsh V, et al. A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:1002–9. doi: 10.1097/JTO.0b013e3181aba89f. [DOI] [PubMed] [Google Scholar]
- 47.Okano Y, Shinohara T, Machida H, et al. Phase II trial of erlotinib in previously treated patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:S1175. [Google Scholar]
- 48.Pallis AG, Voutsina A, Kentepozidis N, et al. A phase II trial of erlotinib as front-line treatment in clinically selected patients with non-small-cell lung cancer. Clin Lung Cancer. 2012;13:129–35. doi: 10.1016/j.cllc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Puente J, Gonzalez Larriba JL, Gajate P, et al. Clinical impact of the feasibility of epidermal growth factor receptor (EGFR) mutations detection in cytological samples of patients (pt) with non-small cell lung cancer (NSCLC) treated with erlotinib (E) at first line. J Clin Oncol. 2011;29:Abstract e18051. [Google Scholar]
- 50.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 51.Rotella V, Mazzoni F, Pratesi N, et al. Erlotinib as a second-line therapy in advanced non small cell lung cancer: correlation between clinical characteristics and biomarkers. Eur J Cancer Suppl. 2009;7:545. [Google Scholar]
- 52.Spigel DR, Burris HA, 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2582–9. doi: 10.1200/JCO.2010.30.7678. [DOI] [PubMed] [Google Scholar]
- 53.Sun J-M, Won Y-W, Kim ST, et al. The different efficacy of gefitinib or erlotinib according to epidermal growth factor receptor exon 19 and exon 21 mutations in Korean non-small cell lung cancer patients. J Cancer Res Clin Oncol. 2011;134:687–94. doi: 10.1007/s00432-010-0928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi T, Yamamoto N, Nukiwa T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010;30:557–63. [PubMed] [Google Scholar]
- 55.Zhu YJ, Xia Y, Ren GJ, et al. Efficacy and clinical/molecular predictors of erlotinib monotherapy for Chinese advanced non-small cell lung cancer. Chin Med J. 2010;123:3200–5. [PubMed] [Google Scholar]
- 56.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23:8081–92. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 58.Buckingham LE, Coon JS, Morrison LE, et al. The prognostic value of chromosome 7 polysomy in non-small cell lung cancer patients treated with gefitinib. J Thorac Oncol. 2007;2:414–22. doi: 10.1097/01.JTO.0000268675.02744.b0. [DOI] [PubMed] [Google Scholar]
- 59.Chou TY, Chiu CH, Li LH, et al. Mutation in the tyrosine-kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:3750–7. doi: 10.1158/1078-0432.CCR-04-1981. [DOI] [PubMed] [Google Scholar]
- 60.Cortes-Funes H, Gomez C, Rosell R, et al. Epidermal growth factor receptor activating mutations in Spanish gefitinib treated non-small-cell lung cancer patients. Ann Oncol. 2005;16:1081–6. doi: 10.1093/annonc/mdi221. [DOI] [PubMed] [Google Scholar]
- 61.D'Addario G, Rauch D, Stupp R, et al. Multicenter phase II trial of gefitinib firstline therapy followed by chemotherapy in advanced non-small-cell lung cancer (NSCLC): SAKK protocol 19/03. Ann Oncol. 2008;19:739–45. doi: 10.1093/annonc/mdm564. [DOI] [PubMed] [Google Scholar]
- 62.Dongiovanni D, Daniele L, Barone C, et al. Gefitinib (ZD1839): therapy in selected patients with non-small cell lung cancer (NSCLC)? Lung Cancer. 2008;61:73–81. doi: 10.1016/j.lungcan.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Fukuoka M, Wu Y, Thongprasert S, et al. Biomarker analyses from a phase III, randomized, open-label, first-line study of gefitinib (G) versus carboplatin/paclitaxel (C/P) in clinically selected patients (pts) with advanced non-small cell lung cancer (NSCLC) in Asia (IPASS) J Clin Oncol. 2009;27:408s. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 64.Han SW, Kim TY, Lee KH, et al. Clinical predictors versus epidermal growth factor receptor mutation in gefitinib-treated nonsmall-cell lung cancer patients. Lung Cancer. 2006;54:201–7. doi: 10.1016/j.lungcan.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Hirsch FR, Varella-Garcia M, Cappuzzo F, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2007;18:752–60. doi: 10.1093/annonc/mdm003. [DOI] [PubMed] [Google Scholar]
- 66.Ichihara S, Toyooka S, Fujiwara Y, et al. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer. 2007;120:1239–47. doi: 10.1002/ijc.22513. [DOI] [PubMed] [Google Scholar]
- 67.Inoue A, Kobayashi K, Usui K, et al. Firstline gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 68.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24:3340–6. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 69.Kim KS, Jeong JY, Kim YC, et al. Predictors of the response to gefitinib in refractory non-small cell lung cancer. Clin Cancer Res. 2005;11:2244–51. doi: 10.1158/1078-0432.CCR-04-2081. [DOI] [PubMed] [Google Scholar]
- 70.Kimura H, Suminoe M, Kasahara K, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA) Br J Cancer. 2007;97:778–84. doi: 10.1038/sj.bjc.6603949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi K, Inoue A, Usui K, et al. First-line gefitinib for poor PS patients with EGFR mutations. J Clin Oncol. 2008;26:8070s. [Google Scholar]
- 72.Koyama N, Jinn Y, Takabe K, et al. The characterization of gefitinib sensitivity and adverse events in patients with non-small-cell lung cancer. Anticancer Res. 2006;26:4519–25. [PubMed] [Google Scholar]
- 73.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine-kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 74.Oshita F, Matsukuma S, Yoshihara M, et al. Novel heteroduplex method using small cytology specimens with a remarkably high success rate for analysing EGFR gene mutations with a significant correlation to gefitinib efficacy in non-small-cell lung cancer. Br J Cancer. 2006;95:1070–5. doi: 10.1038/sj.bjc.6603396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pallis AG, Voutsina A, Kalikaki A, et al. ‘Classical’ but not ‘other’ mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer. 2007;97:1560–6. doi: 10.1038/sj.bjc.6604068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harbouring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 77.Shao Y, Lin Z, Hu F, et al. Quality of life in advanced non-small cell lung cancer patients receiving first-line gefitinib monotherapy. J Clin Oncol. 2009;27:511s. [Google Scholar]
- 78.Shoji F, Yano T, Yoshino I, et al. The characteristics and failure pattern of gefitinib responders with postoperative recurrence of pulmonary adenocarcinoma. Eur J Surg Oncol. 2008;34:89–93. doi: 10.1016/j.ejso.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Sugio K, Uramoto H, Onitsuka T, et al. Prospective phase II study of gefitinib in non-small cell lung cancer with epidermal growth factor receptor gene mutations. Lung Cancer. 2009;64:314–8. doi: 10.1016/j.lungcan.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Sunaga N, Tomizawa Y, Yanagitani N, et al. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer. 2007;56:383–9. doi: 10.1016/j.lungcan.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 81.Sutani A, Nagai Y, Udagawa K, et al. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br J Cancer. 2006;95:1483–9. doi: 10.1038/sj.bjc.6603466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takano T, Ohe Y, Tsuta K, et al. Epidermal growth factor receptor mutation detection using high-resolution melting analysis predicts outcomes in patients with advanced non small cell lung cancer treated with gefitinib. Clin Cancer Res. 2007;13:5385–90. doi: 10.1158/1078-0432.CCR-07-0627. [DOI] [PubMed] [Google Scholar]
- 83.Tamura K, Okamoto I, Kashii T, et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Thoracic Oncology Group trial (WJTOG0403) Br J Cancer. 2008;98:907–14. doi: 10.1038/sj.bjc.6604249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varella-Garcia M, Mitsudomi T, Yatabe Y, et al. EGFR and HER2 genomic gain in recurrent non-small cell lung cancer after surgery: impact on outcome to treatment with gefitinib and association with EGFR and KRAS mutations in a Japanese cohort. J Thorac Oncol. 2009;4:318–25. doi: 10.1097/JTO.0b013e31819667a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu JM, Han Y, Duan HQ, et al. EGFR mutations and HER2/3 protein expression and clinical outcome in Chinese advanced non-small cell lung cancer patients treated with gefitinib. J Cancer Res Clin Oncol. 2009;135:771–82. doi: 10.1007/s00432-008-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang XT, Li LY, Mu XL, et al. The EGFR mutation and its correlation with response of gefitinib in previously treated Chinese patients with advanced non-small-cell lung cancer. Ann Oncol. 2005;16:1334–42. doi: 10.1093/annonc/mdi340. [DOI] [PubMed] [Google Scholar]
- 87.Asami K, Koizumi T, Hirai K, et al. Gefitinib as first-line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: results of a Nagano Lung Cancer Research Group study. Clin Lung Cancer. 2011;12:387–92. doi: 10.1016/j.cllc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Azuma K, Okamoto I, Kawahara A, et al. Association of the expression of mutant epidermal growth factor receptor protein as determined with mutation-specific antibodies in non-small cell lung cancer with progression-free survival after gefitinib treatment. J Thorac Oncol. 2012;7:122–7. doi: 10.1097/JTO.0b013e31822eeba2. [DOI] [PubMed] [Google Scholar]
- 89.Chen X, Li W, Hu X, et al. Effect of gefitinib challenge to initial treatment with non-small cell lung cancer. Biomed Pharmacother. 2011;65:542–6. doi: 10.1016/j.biopha.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 90.Chen YM, Fan WC, Tsai CM, et al. A phase II randomized trial of gefitinib alone or with tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. J Thorac Oncol. 2011;6:1110–6. doi: 10.1097/JTO.0b013e3182121c09. [DOI] [PubMed] [Google Scholar]
- 91.Giovannetti E, Zucali PA, Peters GJ, et al. Association of polymorphisms in AKT1 and EGFR with clinical outcome and toxicity in non-small cell lung cancer patients treated with gefitinib. Mol Cancer Ther. 2010;9:581–93. doi: 10.1158/1535-7163.MCT-09-0665. [DOI] [PubMed] [Google Scholar]
- 92.Inoue Y, Minegishi Y, Maemondo S, et al. A final results of a phase II study of first-line gefitinib for elderly patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations; NEJ 003 study. Ann Oncol. 2010;21:viii141. [Google Scholar]
- 93.Lee JS, Park K, Kim S-W, et al. A randomized phase III study of gefitinib (IRESSA™) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung. J Thorac Oncol. 2009;4:S283. (PRS.4) [Google Scholar]
- 94.Kim DW, Lee S-H, Lee JS, et al. A multicenter phase II study to evaluate the efficacy and safety of gefitinib as first-line treatment for Korean patients with advanced pulmonary adenocarcinoma harboring EGFR mutations. Lung Cancer. 2011;71:65–9. doi: 10.1016/j.lungcan.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Masago K, Fujia S, Togashi Y, et al. Clinicopathologic factors affecting the progression-free survival of patients with advanced non-small-cell lung cancer after gefitinib therapy. Clin Lung Cancer. 2011;12:56–61. doi: 10.3816/CLC.2011.n.008. [DOI] [PubMed] [Google Scholar]
- 96.Moiseyenko VM, Procenko SA, Levchenko EV, et al. High efficacy of first-line gefitinib in non-Asian patients with EGFR-mutated lung adenocarcinoma. Onkologie. 2010;33:231–8. doi: 10.1159/000302729. [DOI] [PubMed] [Google Scholar]
- 97.Park SH, Ha SY, Lee JI, et al. Epidermal growth factor receptor mutations and the clinical outcome in male smokers with squamous cell carcinoma of lung. J Korean Med Sci. 2009;24:448–52. doi: 10.3346/jkms.2009.24.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uruga H, Kishi K, Fujii T, et al. Efficacy of gefitinib for elderly patients with advanced non-small cell lung cancer harboring epidermal growth factor receptor gene mutations: a retrospective analysis. Intern Med. 2010;49:103–7. doi: 10.2169/internalmedicine.49.2531. [DOI] [PubMed] [Google Scholar]
- 99.Wu J-Y, Shih J-Y, Chen K-Y, et al. Gefitinib therapy in patients with advanced non-small cell lung cancer with or without testing for epidermal growth factor receptor (EGFR) mutations. Medicine. 2011;90:159–67. doi: 10.1097/MD.0b013e31821a16f4. [DOI] [PubMed] [Google Scholar]
- 100.Wu Y-L, Zhou C, Chen G, et al. First biomarker analyses from a phase III, randomised, open-label, first-line study of erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM) in Chinese patients (pts) with advanced non-small-cell lung cancer (NSCLC) with EGFR activating mutations (OPTIMAL, CTONG 0802) Ann Oncol. 2010;21:viii6. [Google Scholar]
- 101.Yamaguchi H, Soda H, Nakamura Y. Serum levels of surfactant protein D predict the anti-tumor activity of gefitinib in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2011;67:331–8. doi: 10.1007/s00280-010-1325-x. [DOI] [PubMed] [Google Scholar]
- 102.Yoshida K, Yatabe Y, Park J, et al. Clinical outcomes of advanced non-small cell lung cancer patients screened for epidermal growth factor receptor gene mutations. J Cancer Res Clin Oncol. 2010;136:527–35. doi: 10.1007/s00432-009-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 104.Lee KH, Han SW, Hwang PG, et al. Epidermal growth factor receptor mutations and response to chemotherapy in patients with non-small-cell lung cancer. Jpn J Clin Oncol. 2006;36:344–50. doi: 10.1093/jjco/hyl039. [DOI] [PubMed] [Google Scholar]
- 105.Tambo Y, Kasahara K, Sone T, et al. Prognostic and predictive impact of EGFR and K-ras mutation, and EGFR gene copy number in patients with advanced non-small cell lung cancer (NSCLC) who received first-line cytotoxic chemotherapy. J Clin Oncol. 2009;27:430s. [Google Scholar]
- 106.Kalikaki A, Koutsopoulos A, Hatzidaki D, et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer. 2010;69:110–5. doi: 10.1016/j.lungcan.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with non-small cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer. 2012;118:729–39. doi: 10.1002/cncr.26311. [DOI] [PubMed] [Google Scholar]
- 108.Lin C-C, Hsu H-H, Sun C-T, et al. Chemotherapy response in East Asian non-small cell lung cancer patients harboring wild-type or activating mutation of epidermal growth factor receptors. J Thorac Oncol. 2010;9:1424–9. doi: 10.1097/JTO.0b013e3181e9db73. [DOI] [PubMed] [Google Scholar]
- 109.Matsumoto S, Touge H, Kodani M. The difference in chemotherapeutic efficacy between advanced non-small cell lung cancers with and without EGFR mutations. J Thorac Oncol. 2011;6:S1230. [Google Scholar]
- 110.Yang JC-H, Shih J-Y, Su W-C, et al. A phase ii study of afatinib (BIBW 2992) in patients with adenocarcinoma of the lung and activating EGFR/HER1 Mutations (LUX-Lung 2) J Thorac Oncol. 2010;5:S376–7. [Google Scholar]
- 111.Rosell R, Massuti Sureda B, Costa C, et al. Concomitant actionable mutations and overall survival (OS) in EGFR-mutant non-small-cell lung cancer (NSCLC) patients (p) included in the EURTAC trial: EGFR L858R, EGFR T790M, TP53 R273H and EML4-ALK (V3) Ann Oncol. 2012;23:ixe22. (LBA31) [Google Scholar]
- 112.Douillard JY, Ostoros G, Cobo M, et al. Efficacy, safety and tolerability results from a phase IV, open-label, single arm study of 1st-line gefitinib in Caucasian patients (pts) with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) Lung Cancer. 2013;80:S31. [Google Scholar]
- 113.Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3337–44. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 115.De Pas T, Toffalorio F, Manzotti M, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6:1895–901. doi: 10.1097/JTO.0b013e318227e8c6. [DOI] [PubMed] [Google Scholar]
- 116.Klughammer B, Spleiss O. ‘Other’ (non-activating, non-T790M) EGFR mutations and their clinical implications collected from various Tarceva trials in NSCLC. Eur J Cancer. 2011;47:S20–1. [Google Scholar]
- 117.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 118.Wu M, Zhao J, Song SW, et al. EGFR mutations are associated with prognosis but not with the response to front-line chemotherapy in the Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2010;67:343–7. doi: 10.1016/j.lungcan.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 119.Mok T, Wu YL, Thongprasert S, et al. A randomized placebo-controlled phase III study of intercalated erlotinib with gemcitabine/platinum in first-line advanced non-small cell lung cancer (NSCLC): FASTACT-II. J Clin Oncol. 2012;30:Abstract 7519. [Google Scholar]


