Abstract
In the present study, a human neuroblastoma cell line (SH-SY5Y) and BV-2 microglia were treated with amyloid-β peptide (25–35), as a model of Alzheimer’s disease, to evaluate the protective effects of 10-3–10-8 g/mL Lingguizhugan decoction and to examine the underlying anti-inflammatory mechanism. Lingguizhugan decoction significantly enhanced the viability of SH-SY5Y cells with amyloid-β peptide-induced injury, and lowered levels of interleukin-1β, interleukin-6, tumor necrosis factor-α and nitric oxide in the culture supernatant of activated BV-2 microglia. The effects of 10-3 g/mL Lingguizhugan decoction were more significant. These results suggest that Lingguizhugan decoction can protect SH-SY5Y cells against amyloid-β peptide (25–35)-induced injury in a dose-dependent manner by inhibiting overexpression of inflammatory factors by activated microglia.
Keywords: Alzheimer’s disease, Lingguizhugan decoction, phlegm-warming and fluid-dispersing, inflammatory reaction, SH-SY5Y, BV-2
Research Highlights
-
(1)
Lingguizhugan decoction inhibits overexpression of interleukin-1β, interleukin-6, tumor necrosis factor-α and nitric oxide in activated BV-2 microglia in a dose-dependent manner.
-
(2)
Lingguizhugan decoction protects a human neuroblastoma cell line (SH-SY5Y) against amyloid-β peptide (25–35)-induced injury.
Abbreviations Aβ, amyloid-β; AD, Alzheimer’s disease; IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide
INTRODUCTION
Microglial activation caused by amyloid-β (Aβ) proteinosis and the resulting inflammatory reaction underlie the pathology of Alzheimer's disease (AD)[1]. Based on theories of traditional Chinese medicine, retention of phlegm and fluid plays an important role in the occurrence and development of AD[2,3,4].
Treatment with phlegm-warming and fluid-dispersing formulations shows remarkable efficacy in clinical practice[5,6,7]. Pharmacological studies show that phlegm and retained fluid play critical roles in the pathogenesis of inflammation[8].
Lingguizhugan decoction (LG) has its origins in Treatise on Cold Pathogenic and Miscellaneous Diseases[9]. As the basic prescription for phlegm and fluid retention, it has been widely applied in the clinical treatment of multiple diseases related to retention of phlegm and fluid[10]. In addition, pharmacological research has demonstrated that LG and its main components have good anti-inflammatory activities[11,12,13,14,15,16].
In the present study, we used a human neuroblastoma cell line, SH-SY5Y cells, to establish an AD cell culture model (induced by Aβ25-35), and we investigated the protective effects of different LG concentrations on Aβ25-35-induced damage in these cells.
Considerable evidence indicates that microglia (BV-2) participate in the inflammatory pathological process in neurodegenerative diseases[17]. Excessively activated microglia can produce neurotoxic factors, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, nitric oxide (NO), transforming growth factor-β and free radicals, as well as complement protein and other immune molecules[18]. These toxic substances further activate microglia, worsening injury and promoting neuronal death[19]. Thus, in the present study, we also explored the anti-inflammatory effects and mechanisms of action of LG on activated microglia.
RESULTS
Changes in SH-SY5Y cell morphology
Under the inverted phase-contrast microscope, cells in the normal group (SH-SY5Y cells) had spread and adhered without obvious changes in appearance.
After 24 hours, cells in the model group (25 μM Aβ25-35-treated SH-SY5Y cells) had shrunk, the intercellular spaces widened, and the number of round cells increased.
At 48 hours, a large number of cells in the model group were round and floating, with enhanced refractivity. The spreading and adherence of cells in each LG group increased, while the number of shrunken, round and floating cells was significantly less than in the model group.
LG enhances the viability of Aβ25-35-treated SH-SY5Y cells
MTT results showed that the viability of SH-SY5Y cells significantly decreased after 25 μM Aβ25-35 treatment (P < 0.01), but LG (10-3, 10-4, 10-5, 10-6 g/mL) significantly improved the viability of these cells (P < 0.05; Table 1, supplementary Figure 1 online).
Table 1.
Effect of Lingguizhugan decoction (LG) on the survival of SH-SY5Y cells
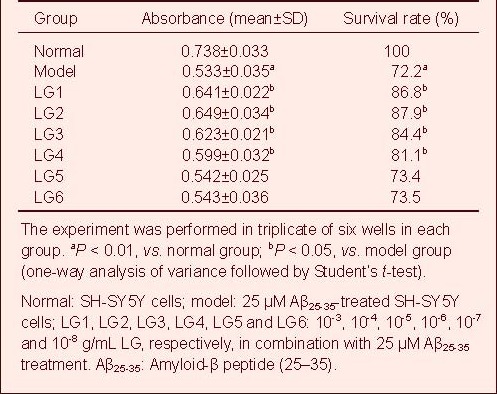
LG inhibits IL-1β expression in Aβ-activated microglia (BV-2)
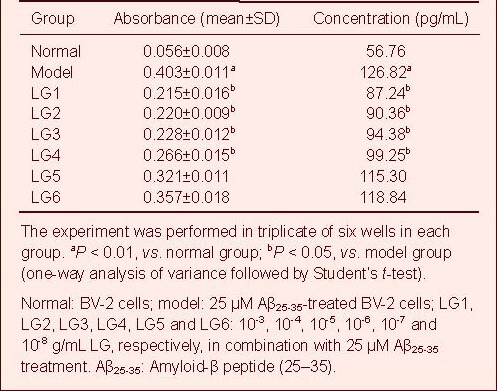
A standard curve was drawn and sample concentration was determined based on the standard curve. The IL-1β content in the cell culture supernatant in the model group (25 μM Aβ25-35-treated BV-2 cells) was significantly increased compared with the normal group (BV-2 cells), but was significantly reduced in all LG groups compared with the model group.
In particular, 10-3 and 10-4 g/mL LG significantly decreased IL-1β content (P < 0.01), followed by 10-5 and 10-6 g/mL LG (P < 0.05), while 10-7 and 10-8 g/mL LG slightly lowered IL-1β content (Table 2, supplementary Figure 2 online).
Table 2.
Effect of Lingguizhugan decoction (LG) on the release of interleukin-1β in BV-2 cells
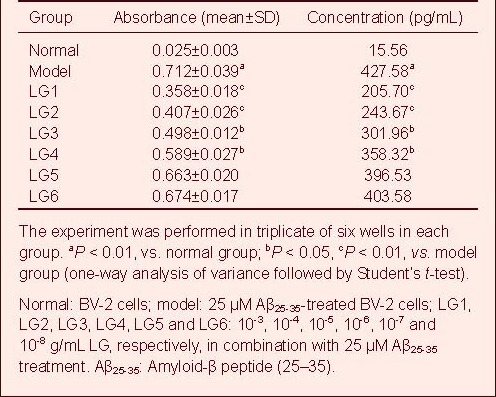
LG inhibits IL-6 expression in Aβ-activated BV-2 cells
A standard curve was drawn and sample concentration was determined based on the standard curve. The IL-6 content in the cell culture supernatant in the model group was significantly elevated compared with the normal group, but was significantly reduced in all LG groups compared with the model group.
In particular, 10-3 and 10-4 g/mL LG significantly decreased IL-6 content (P < 0.01), followed by 10-5 and 10-6 g/mL LG (P < 0.05), while 10-7 and 10-8 g/mL LG slightly lowered IL-6 content (Table 3, supplementary Figure 3 online).
Table 3.
Effect of Lingguizhugan decoction (LG) on release of interleukin-6 in BV-2 cells
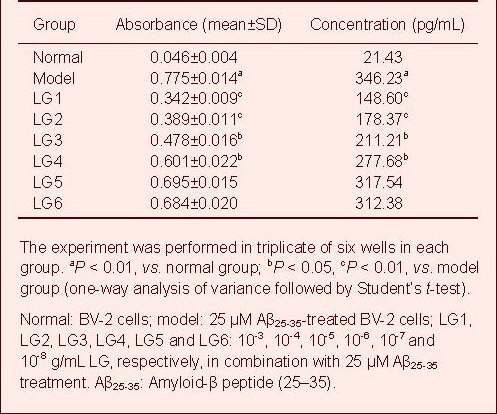
LG inhibits TNF-α expression in Aβ-activated BV-2 cells
A standard curve was drawn and sample concentration was determined based on the standard curve. The TNF-α content in the cell culture supernatant in the model group was significantly increased compared with the normal group, but was significantly reduced in all LG groups compared with the model group.
In particular, 10-3, 10-4 and 10-5 g/mL LG significantly decreased TNF-α content (P < 0.01), followed by 10-6 and 10-7 g/mL LG (P < 0.05), while 10-8 g/mL LG slightly lowered TNF-α content Table 4, supplementary Figure 4 online).
Table 4.
Effect of Lingguizhugan decoction (LG) on release of tumor necrosis factor-α in BV-2 cells
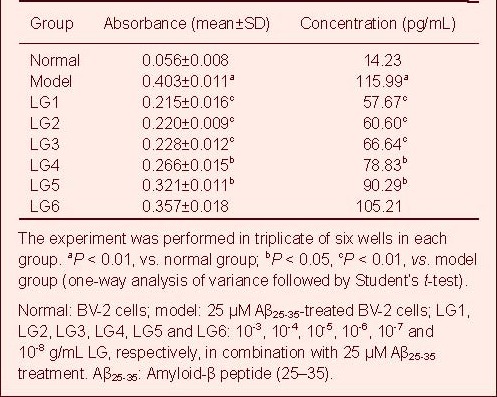
LG inhibits NO expression in Aβ-activated BV-2 cells
The volume of NO secreted by BV-2 cells in each group was quantified based on the standard curve. NO secretion by BV-2 cells in the model group was significantly increased compared with the normal group (P < 0.01), but was significantly reduced in the 10-3, 10-4, 10-5 and 10-6 g/mL LG groups compared with the model group (P < 0.05). However, the effects of 10-7 and 10-8 g/mL LG were not obvious (Table 5, supplementary Figure 5 online).
Table 5.
Effect of Lingguizhugan decoction (LG) on release of nitric oxide in BV-2 cells
DISCUSSION
Modern pharmacological research has shown that LG and its major components have good anti-inflammatory effects[20]. The main chemical components of Poria cocos are polysaccharide and three terpenoids. Studies have shown that pachyman and tuckahoe triterpene have good anti-inflammatory effects[21]. Liu[22] found that periapical inflammation was effectively alleviated by cinnamaldehyde. Largehead atractylodes rhizome has diuretic, anti-tumor, anti-diabetes and anti-aging effects. Huang et al[23] found that largehead atractylodes rhizome decoction can reduce serum levels of phlogogenic TNF-α in mouse. These observations indicate that largehead atractylodes rhizome decoction has anti-inflammatory effects.
The chief constituents of Radix Glycyrrhizae are triterpenoids (glycyrrihza glabra, i.e. glycyrrhizic acid salt, glycyrrhetic acid), flavonoids (licoflavone, isolicoflavone, glycyrrhizin) and licorice polysaccharide compounds. Qu et al[24] found that licorice decoction can significantly inhibit ear swelling in mice induced by dimethylbenzene, diminish egg white-induced foot swelling in rats, reduce peritoneal permeability and inhibit the proliferation of granuloma. Thus, licorice decoction has a good anti-inflammatory effect as well.
MTT assay is a routine method of analyzing the viability of mammalian cells, and is an index of metabolic activity. Results from the present study showed that after treatment with LG for 48 hours, the cell viability of each group was significantly enhanced compared with the model group, especially by LG at 10-3, 10-4, 10-5 and 10-6 g/mL. However, the effects of 10-7 and 10-8 g/mL LG were minimal.
In the present study, after Aβ induction, the levels of IL-1, IL-6 and TNF-α in the BV-2 supernatant were significantly increased. However, after treatment with different concentrations of LG, the secreted amounts of these three cytokines were reduced, most strongly by the higher concentration of the decoction. This shows that LG protects against cell injury induced by Aβ. Furthermore, the protection correlated positively with drug concentration.
In the pathological process of AD, activated microglia trigger and accelerate the immunological inflammatory reaction by releasing mediators of inflammation. Excessive release of IL-1β is the initial step in the AD inflammatory reaction. IL-6 also plays an important role in this process. It may induce protein aggregation in amyloid plaques. TNF-α is produced primarily by activated microglia. Excessive TNF-α release is toxic to neurons and neuroglial cells, and the factor cooperates with IL-1 to increase the production of IL-6[25,26,27,28,29].
In addition, activated microglia release NO[30], which is neurotoxic[31,32,33]. NO mediates and amplifies oxidative stress and the inflammatory reaction. This selectively damages neurons related to learning and memory, and promotes the formation of senile plaques, which further exacerbates AD pathology.
In the present study, we assessed changes in microglia-derived inflammatory factors, including IL-1β, IL-6, TNF-α and NO, induced by LG treatment. This was performed to evaluate the anti-inflammatory action of LG in AD. We found that LG inhibits the release by activated microglia of IL-1β, IL-6, TNF-α and NO. This indicates that LG is an anti-inflammatory agent and inhibits the release of inflammatory factors by activated microglia.
In Chinese medicine, the pathogenesis of AD is closely related to phlegm-fluid, and LG is the basic decoction to treat phlegm and fluid disease. In modern medicine, inflammatory mechanisms, triggered by Aβ-activated microglia, are considered crucial in AD etiology and pathogenesis. Pharmacological studies have shown that LG and its major components have good anti-inflammatory effects. Therefore, both traditional Chinese medicine and modern pharmacological research show that LG may have therapeutic potential in AD. However, few data are available. Therefore, in the present study, we propose the hypothesis that LG may have good therapeutic effect in AD, and its mechanism may be related to its anti-inflammatory effect. Using cell biology and molecular biology techniques, we examined the protective effect of LG in an AD cell culture model in vitro, and we investigated the immunological mechanisms to further the study of LG in AD prevention and treatment. Our study provides insight into the efficacy and mechanisms of action of traditional Chinese treatments for AD.
In conclusion, as a typical phlegm-warming and fluid-dispersing decoction, LG significantly protects neuronal cells from injury. This effect is mediated by its ability to inhibit the overexpression of IL-1β, TNF-α, IL-6, NO and other inflammatory factors released by activated microglia. Our research provides a new therapeutic strategy for AD. Further studies are needed to more completely clarify the effects and mechanisms of action of LG decoction in the treatment of AD.
MATERIALS AND METHODS
Design
A randomized, controlled cell biology study.
Time and setting
This experiment was conducted at the Experimental Center of Preclinical Medical College, Nanjing University of Traditional Chinese Medicine, China from March to July 2010.
Materials
Preparation of Aβ25-35
Aβ25-35 was purchased from Sigma (St. Louis, MO, USA). Aβ25-35, 1 mg, was added to 9 431 μL serum-free medium to prepare the 100 μM solution, and was filtered using a 0.22-μm filter membrane and incubated at 37°C for 3 days. The solution was aliquoted into 1-mL EP tubes, sealed with parafilm wrap, cryopreserved at -20°C, and thawed for subsequent use.
Preparation of LG
LG was composed of Poria cocos, Cassia Twig, largehead atractylodes rhizome and prepared Radix glycyrrhizae. All medicinal materials were purchased from Chinese Medicine Hall, Nanjing University of Traditional Chinese Medicine and appraised by the Drug Identification Department of the School of Traditional Chinese Materia Medica, Nanjing University of Traditional Chinese Medicine. Poria cocos was the dry sclerotium of one kind of epiphyte of Polyporaceae. Cassia Twig was the dry burgeon of Cinnamomum cassia of Lauraceae. Largehead atractylodes rhizome was the dry rhizome of Atractylodes Macroce Phala Koidzthe of the composite family. Prepared Radix Glycyrrhizae was the dry, processed root of Glycyrrhiza uralensis Fisch, a leguminous plant. According to the proportion in Treatise on Febrile Caused by Cold[9], Poria cocos:Cassia Twig:Bighead Atractylodes Rhizome: prepared Radix Glycyrrhizae = 4:3:2:2. Thus, 40 g Poria cocos, 30 g Cassia Twig, 20 g Bighead Atractylodes Rhizome and 20 g prepared Radix Glycyrrhizae were combined (110 g in total). The herbs were soaked in cold water for 30 minutes, 2–3 cm from the water surface. The medicine was decocted three times using a conventional method, filtered three times, and concentrated using a rotating evaporator at 60°C to obtain a 1 g/mL solution. After autoclave sterilization, the mixture was stored at 4°C.
Methods
Cell grouping and treatment
SH-SY5Y cells (China Center for Type Culture Collection, Wuhan, China) were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Hangzhou Sijiqing Biotechnology, Ltd., Hangzhou, China) in an incubator at 37°C, 5% CO2 and saturated humidity. The medium was changed every 2 days and the cells were passaged every 3–4 days. BV-2 cells (Cell Center of Basic Medicine, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, China) were cultured in high-glucose DMEM containing 10% FBS in a 37°C incubator at 5% CO2 and saturated humidity. The cells were passaged every 2–3 days. The starting culture was cultured for 24 hours, and the tissue culture plate was cleaned twice using PBS.
Cells were divided into normal, model and LG groups. Each group had 6 parallel wells (cell density of each type of cell was 1 × 105/mL, 100 μL per well). In the normal group, the DMEM culture medium was changed; in the model group, 25 μM Aβ25-35 was used, based on a previous study[34]; in the LG groups, 25 μM Aβ and LG at the final experimental concentration were added. The final concentration of LG ranged from 10-3–10-8 g/mL. This range was determined in a preliminary experiment. 1 mL LG (1 g/mL) was diluted in 100 mL high-glucose serum-free DMEM, and filtered and sterilized following centrifugation. Serum-free DMEM was used to dilute LG to concentrations of 1 × 10-3, 1 × 10-4, 1 × 10-5, 1 × 10-6, 1 × 10-7 and 1 × 10-8 g/mL. The six concentrations were designated LG1, LG2, LG3, LG4, LG5 and LG6, respectively.
MTT detection of SH-SY5Y cell viability
MTT assay was used to detect cell viability[35]. After 48 hours of administration, 20 μL MTT (Sigma) was added to every well and cultured for 4 hours. Then, 150 μL dimethyl sulfoxide was added to each well after discarding the supernatant, and the plate was shaken carefully to completely dissolve the formazan product (the bluish violet crystal of the MTT reaction). The absorbance value (A) at 490 nm was measured. Cell viability = Aexperimental group/Anormal group × 100%. Every experiment was performed in triplicate.
Enzyme linked immunosorbent assay (ELISA) detection of inflammatory factor expression in BV-2 cells
BV-2 cells in the logarithmic growth phase were cultured in 96-well microtiter plates (1 × 105 cells/mL), with 100 μL in each well. When the cells adhered, they were treated with LG at different concentrations (LG1, LG2, LG3, LG4, LG5, LG6), in addition to Aβ. The upper portion of the medium was collected to determine the concentration of IL-1β, IL-6 and TNF-α by ELISA (ELISA kit was purchased from Beijing Sizhengbai Biotech Co., Ltd.)[36].
ELISA detection of NO secretion in BV-2 cells
The cells, cultured for 24 hours, were treated with drugs, and 100 μL supernatant was collected from each well and transferred to another 96-well microtiter plate, mixed with 100 μL Griess[37] reagent (Sigma) for 10 minutes, and A545 was measured for ELISA (Beijing Sizhengbai Biotech Co., Ltd.). The concentration of NO was calculated based on the standard curve[38].
Statistical analysis
Data were expressed as mean ± SD and analyzed with SPSS 16.0 for windows (SPSS, Chicago, IL, USA). Comparison among groups was performed with one-way analysis of variance, and comparison between two groups was performed with Student's t-test. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Baozhi Shan from Shanghai Scientific & Technical Publishers, China, for manuscript revision and technical help.
Footnotes
Funding: This study was financially sponsored by Graduate Student Research and Innovation Program of Jiangsu Province, No. CX09B_267Z.
Conflicts of interest: None declared.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Wu HX, Wang Z/Su LL/Wang L)
REFERENCES
- [1].Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons Higher than previously reported. JAMA. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- [2].Fu RJ. Diagnose, differentiation of symptoms and signs for classification of syndrome and curative effect evaluation standard. Zhongyi Zazhi. 1991;32(2):121. [Google Scholar]
- [3].Zhang L, Ji LJ. Discuss of the relationship between spleen, stomach in TCM and invasion of Alzheimer's disease. Fujian Zhongyiyao. 2007;38(4):51. [Google Scholar]
- [4].Zhao WY, Chen R. Treat Alzheimer's disease from spleen. Zhongyiyao Xuekan. 2005;23(9):1665–1666. [Google Scholar]
- [5].Qian RJ. The superficial view of diagnosis and treatment based on spleen for dementia after apoplexy. Zhonghua Zhongyiyao Xuekan. 2007;25(7):1345–1346. [Google Scholar]
- [6].Tang XM. Turbid pathogenic factor and its pathogenic mechanism. Liaoning Zhongyi Zazhi. 2006;33(11):1416–1418. [Google Scholar]
- [7].Wang SP, Wang WZ, Li SM. Phlegm and blood stasis as basic pathogenesis of vascular dementia. Zhongguo Zhongyi Jichu Yixue Zazhi. 2004;10(10):1–2. [Google Scholar]
- [8].Zhang J, Pang J, Chen QQ. Different influence of brain tissue lipid peroxidation in Alzheimer's disease mouse to dispense prescriptions: according to phlegmatic theory or renal deficiency theory. Liaoning Zhongyiyao Daxue Xuebao. 2008;10(9):151–152. [Google Scholar]
- [9].Xiong MQ. Beijing: China Press of Traditional Chinese Medicine; 2007. Theory on Exogenous Febrile Disease. [Google Scholar]
- [10].Yang P, Shao QF. Clinical application of Lingguizhugan decoction. Xiandai Zhongyiyao. 2009;29(3):63–64. [Google Scholar]
- [11].Huang JL, Long ZJ, Wu HQ, et al. Effect of Lingguizhugan decoction on IL-1β, TNF-α and PGE-2 in joint fluid of rats with adjuvant arthritis. Zhongguo Zhongyiyao Keji. 2004;11(3):75–77. [Google Scholar]
- [12].Fang HY, Huang JL, Sang FF. Effects of Lingguizhugan decoction on levels of angiotensin II, endothelin-1, tumor necrosis factor-α and interleukin-1β in rats with chronic heart failure. Anhui Zhongyi Xueyuan Xuebao. 2010;29(2):53–55. [Google Scholar]
- [13].Hou AJ, Peng SP, Xiang R. Anti-inflammatory effect of poriacocos polysaccharide. Zhongyao Yaoli yu Linchuang. 2003;19(3):15–16. [Google Scholar]
- [14].Wang DL, Chen WD, Xu XX. Anti-inflammatory effect of triterpene acids from poriacocos. Anhui Yiyao. 2009;13(9):1021–1023. [Google Scholar]
- [15].Xu SJ, Shen YJ, Xie YH. Experimental study on the anti-inflammation effect of volatile oil in ramulus cinnamomi. Zhongyao Xinyao yu Linchuang Yaoli. 2007;18(3):186–189. [Google Scholar]
- [16].Dong HY, Dong YaL, He LC. Studies on constituents and anti-inflammatory activity of rhizoma atractylodis macrocephalae. Zhongguo Yaoxue Zazhi. 2007;42(7):1055–1059. [Google Scholar]
- [17].Kim MJ, Seong AR, Yoo JY, et al. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol Nutr Food Res. 2011;55(12):1798–1808. doi: 10.1002/mnfr.201100262. [DOI] [PubMed] [Google Scholar]
- [18].Cui Y, Wu J, Jung SC, et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010;33(11):1814–1821. doi: 10.1248/bpb.33.1814. [DOI] [PubMed] [Google Scholar]
- [19].Svensson C, Fernaeus SZ, Part K, et al. LPS-induced iNOS expression in Bv-2 cells is suppressed by an oxidative mechanism acting on the JNK pathway--a potential role for neuroprotection. Brain Res. 2010;1322:1–7. doi: 10.1016/j.brainres.2010.01.082. [DOI] [PubMed] [Google Scholar]
- [20].Lu S, Xu YY, Mu YN, et al. Experimengtal effect of Guizhi Fuzi Decoction on the levels of interleukin 1β, prostaglandin E2 in adjuvant-induced arthritis rats. Shiyong Zhongyi Neike Zazhi. 2008;22(11):24–25. [Google Scholar]
- [21].Elman MJ, Aiello LP, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu HY. Effect of cinnamal on periapical periodontitis in rat. Zhongguo Liaoyang Yixue. 2009;18(3):193–195. [Google Scholar]
- [23].Huang YY, Ding XY, Sun W. Effect of Baizhu decoction used externally on serum content of TNF-α in mice with auricle infection. Beijing Zhongyiyao Daxue Xuebao. 2005;28(6):57–58. [Google Scholar]
- [24].Qu XM, Jin ZT, Shang YH, et al. Research on the effect of Licorice root on the anti-inflammation. Shiyong Yaowu yu Linchuang. 2005;8(5):14–16. [Google Scholar]
- [25].McGeer PL, Walker DG, Pitas RE, et al. Apolipoprotein E4 (ApoE4) but not ApoE3 or ApoE2 potentiates beta-amyloid protein activation of complement in vitro. Brain Res. 1997;749(1):135–138. doi: 10.1016/s0006-8993(96)01324-8. [DOI] [PubMed] [Google Scholar]
- [26].Casal C, Serratosa J, Tusell JM. Relationship between beta-AP peptide aggregation and microglial activation. Brain Res. 2002;928(1-2):76–84. doi: 10.1016/s0006-8993(01)03362-5. [DOI] [PubMed] [Google Scholar]
- [27].Volmar CH, Ait-Ghezala G, Ait-Ghezala G, et al. The granulocyte macrophage colony stimulating factor (GM-CSF) regulates amyloid beta (Abeta) production. Cytokine. 2008;42(3):336–344. doi: 10.1016/j.cyto.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [28].Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13(4):359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- [29].Vehmas AK, Kawas CH, Stewart WF, et al. Immune reactive cells in senile plaques and cognitive decline in Alzheimer's disease. Neurobiol Aging. 2003;24(2):321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- [30].Xiang Z, Ho L, Yemul S, et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 2002;10(5-6):271–278. doi: 10.3727/000000002783992352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Webster SD, Yang AJ, Margol L, et al. Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol. 2000;161(1):127–138. doi: 10.1006/exnr.1999.7260. [DOI] [PubMed] [Google Scholar]
- [32].Shen Y, Lue L, Yang L, et al. Complement activation by neurofibrillary tangles in Alzheimer's disease. Neurosci Lett. 2001;305(3):165–168. doi: 10.1016/s0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- [33].Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer's disease beta-peptide. J Exp Med. 1998;188(3):431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang XH, Yu HL, Xiao R, et al. Neurotoxicity of beta-amyloid peptide 31-35 and 25-35 to cultured rat cortical neurons. Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43(12):1081–1085. [PubMed] [Google Scholar]
- [35].Zhou SL, Qu YN, Zhang J, et al. Comparison between SRB way and MTT way applying to count cells in cells culture. Zhongguo Xiandai Yixue Zazhi. 2005;15(17):2615–2620. [Google Scholar]
- [36].Ljungström I, Engvall E, Ruitenberg EJ. Proceedings: ELISA, enzyme linked immunosorbent assay - a new technique for sero-diagnosis of trichinosis. Parasitology. 1974;69(2):xxiv. [PubMed] [Google Scholar]
- [37].Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- [38].Zhang Q, Wang LP, Yang LH. Check out standard solution by means of standard curve. Xiandai Celiang yu Shiyanshi Guanli. 2009;17(1):12–13. [Google Scholar]


