Abstract
Tanshinone IIa is an effective monomer component of Danshen, which is a traditional Chinese medicine for activating blood circulation to dissipate blood stasis. Tanshinone IIa can effectively improve brain tissue ischemia/hypoxia injury. The present study established a rat model of spinal cord ischemia/reperfusion injury and intraperitoneally injected Tanshinone IIa, 0.5 hour prior to model establishment. Results showed that Tanshinone IIa promoted heat shock protein 70 and Bcl-2 protein expression, but inhibited Bax protein expression in the injured spinal cord after ischemia/reperfusion injury. Furthermore, Nissl staining indicated a reduction in nerve cell apoptosis and fewer pathological lesions in the presence of Tanshinone IIa, compared with positive control Danshen injection.
Keywords: Tanshinone IIa, Danshen, spinal ischemia/reperfusion injury, heat shock protein 70, Bcl-2, Bax, cell apoptosis, Chinese medicine, neural regeneration
Research Highlights
Tanshinone IIa reduced nerve cell apoptosis and effectively improved rat spinal ischemia/reperfusion injury by influencing heat shock protein 70, Bcl-2 and Bax expression.
Abbreviations HSP70, heat shock protein 70; ELISA, enzyme linked immunosorbent assay
INTRODUCTION
After ischemia/reperfusion injury, the spinal cord restores for a period of time, however function is greatly damaged, often resulting in irreversible, delayed death of spinal neurons[1]. Studies have shown that cell apoptosis is common in spinal nerve cells after spinal cord ischemia/reperfusion injury[3,4,5,6,7]. Heat shock protein 70 (HSP70) is a stress-protecting protein. Mitochondrial HSP70 may promote apoptosis by repairing or preventing degradation of cell apoptosis inhibitory protein, Bcl-2[8,9,10,11]. Bcl-2 and Bax are important cell apoptosis-regulatory genes. Bcl-2 inhibits apoptosis and Bax promotes apoptosis. The ratio of Bcl-2 to Bax controls the occurrence of apoptosis, whereby an increased ratio of Bcl-2 to Bax promotes cell survival and a decreased ratio promotes apoptosis[12,13,14].
In vitro studies have demonstrated that the required rate of Danshen injection is 65% to clear hydroxy radicals in the H2O2-Fe2+ system and 100% to clear superoxide anions in the xanthine-xanthine oxidase system[15]. Tanshinone IIa is an effective monomer component of Danshen and a representative liposoluble constituent. It participates in various biochemical events in organisms and exhibits bioactivities to antagonize oxidation and blood coagulation[16]. Bi et al[17] report that Tanshinone IIa rapidly distributes to tissues within 15 minutes after intravenous injection. Another study showed that Tanshinone IIa is slowly metabolized in vivo and remains detectable in tissues 20 hours after administration[18]. In addition, Tanshinone IIa has been shown to promote HSP70 expression, induce upregulation of Bcl-2 protein expression and inhibit Bax protein expression, thereby reducing nerve cell apoptosis and protecting the brain against ischemia/reperfusion injury[19,20].
Based on the important regulatory effects of HSP70, Bcl-2 and Bax in nerve cell apoptosis, the present study investigated the influence of Tanshinone IIa on apoptosis-related protein HSP70, Bcl-2 and Bax expression in spinal nerve cell apoptosis after ischemia/reperfusion injury, to explore the neuroprotective effect and mechanism of action for Tanshinone IIa.
RESULTS
Quantitative analysis of experimental animals
A total of 120 Sprague Dawley rats were used and randomly assigned to sham-surgery, model, Danshen (positive control) and Tanshinone IIa groups. The model, Danshen and Tanshinone IIa groups were subjected to spinal ischemia/reperfusion injury, and the Danshen and Tanshinone IIa groups were intraperitoneally injected with Danshen and sodium Tanshinone IIa sulfonate injection respectively, 0.5 hour prior to model establishment. Six rats were excluded due to failed model establishment or death. After supplementation, 120 rats were included in the final analysis, and six from each group were selected at 0.5, 1, 4, 8 and 12 hours after reperfusion for observation.
Tanshinone IIa improved spinal cord pathology of rats with spinal ischemia/reperfusion injury
Nissl staining showed no significant pathological changes in the spinal cord of the sham-surgery group. Spinal neuron volume was diminished or deformed with mild swelling at 0.5 and 1 hour post ischemia/reperfusion, accompanied by decreased Nissl bodies, but unchanged nuclei. At 4 hours, neuron morphologies were altered and some cells were swollen or broken, observed with an obscure outline, unclear boundary and disorder arrangement, Nissl bodies were significantly decreased, in some cases absent, or with light stain and karyopyknosis. At 8 and 12 hours, the number of neurons was gradually reduced and those remaining had an incomplete appearance with dissolved Nissl bodies in the cytoplasm. The boundary between the nuclei and cytoplasm was unclear, processes were decreased or absent and spaces were detected around the neurons. These pathological changes were significantly attenuated following intraperitoneal injection of Danshen and sodium Tanshinone IIa sulfonate injection. Moreover, the number of Nissl bodies was reduced to a greater extent in the Tanshinone IIa group compared with the Danshen group (Figure 1).
Figure 1.
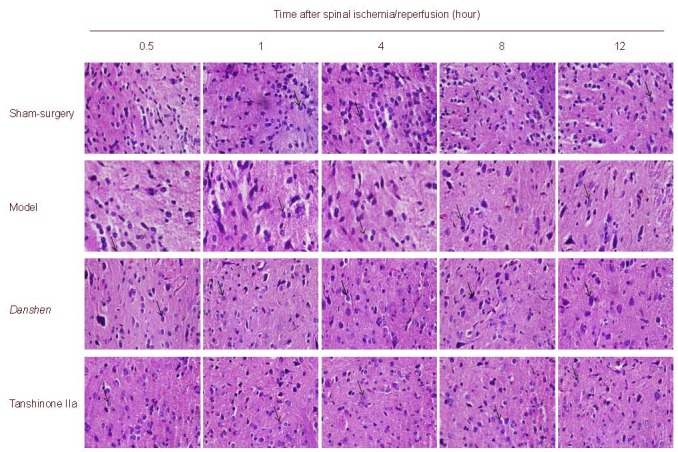
Spinal cord morphology (Nissl staining, light microscope, × 400).
At 0.5 and 1 hour after ischemia, the volume of some neurons was diminished or mildly deformed, and Nissl body number was slightly reduced, with nuclei remaining unchanged. With increasing time, pathological changes worsened. Danshen and sodium Tanshinone IIa sulfonate injection significantly improved neuronal morphology.
Influence of Tanshinone IIa on HSP70, Bcl-2 and Bax expression in the spinal cord of rats with spinal ischemia/reperfusion injury
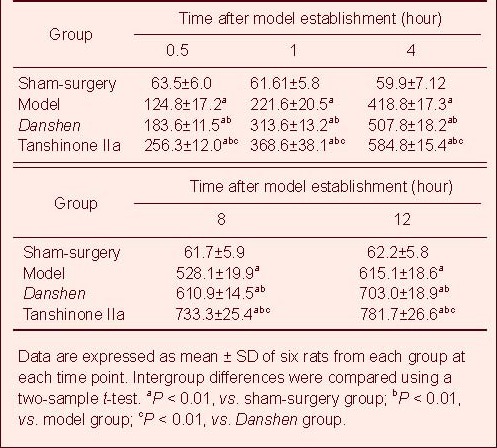
Enzyme linked immunosorbent assay (ELISA) showed low expression of HSP70 and Bcl-2 and no expression of Bax in the spinal cord of the sham-surgery group. At 0.5 hours post ischemia/reperfusion, HSP70, Bcl-2 and Bax expression were increased in the spinal cord compared with the sham group (P < 0.01), with gradual increase over time. HSP70, Bcl-2 and Bax expression were significantly greater in the Danshen and Tanshinone IIa groups compared with the model group (P < 0.01; Tables 1–3, Figure 2).
Table 1.
Heat shock protein 70 expression (μg/mg) in the spinal cord of rats with ischemia/reperfusion injury

Table 3.
Bax expression (μg/mg) in the spinal cord of rats with ischemia/reperfusion injury

Figure 2.

Bcl-2 and Bax expression in spinal cord of rats after ischemia/reperfusion for 12 hours (× 400). Arrows represent positive expression.
Table 2.
Bcl-2 expression (μg/mg) in the spinal cord of rats with ischemia/reperfusion injury
DISCUSSION
Under normal physiological conditions, Nissl bodies are abundant and large in nerve cells, reflecting their predominant function of synthesizing protein. However, following neuronal injury, the number of Nissl bodies is significantly reduced and in some cases absent[21]. Results from the present study showed no obvious pathological changes in the spinal cord of the sham-surgery group. Motor neurons displayed a complete appearance, Nissl bodies were abundant in the neuronal body with dark blue staining and irregular size, blue-stained axons were detected and nuclei were round, stained light blue with clear nucleoli. After spinal cord injury, neuron size was reduced, mild swelling of deformations was observed and Nissl bodies were dissolved, with unclear boundaries between the nucleus and cytoplasm. Axons were reduced or absent and spaces appeared around the neurons. Cell morphology was gradually restored and the number of Nissl bodies gradually increased after treatment with Danshen and Tanshinone IIa. In particular, the treatment effect was superior in the presence of Tanshinone IIa.
HSP70 can inhibit cell apoptosis[22]. HSP70 expression can be used to identify neuronal injury in the central nervous system and to evaluate efficacy of some prevention or treatment methods for central nervous system injury[23,24]. Studies show HSP70 can facilitate protein degradation and reduce activation of various proteases and nucleate endonuclease through ion channels inhibition of cell apoptosis[25]. Results from the present study showed that 0.5 hours after ischemia/reperfusion, HSP70, Bcl-2 and Bax expression were observed in the spinal cord and gradually increased with time, significantly higher than in the sham-surgery group. Moreover, HSP70, Bcl-2 and Bax expression in the spinal cord, following Danshen and Tanshinone IIa injection, were significantly higher than in the model group.
In conclusion, Tanshinone IIa may attenuate spinal ischemia/reperfusion injury by promoting HSP70 and Bcl-2 expression and downregulating Bax expression.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed in the Laboratory of College of Life Science, Fujian Normal University, China from December 2010 to June 2011.
Materials
Animals
A total of 126 Sprague Dawley rats, of clean grade, either female or male, aged 4 months, weighing 250 ± 10 g, were provided by the Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China (license No. 2007000508444). Animals were housed for adaptability for 1 week at 25 ± 2°C and fed with common food. All experiments were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[25].
Drugs
Tanshinone IIa injection was purchased from Shanghai No.1 Biochemical Pharmaceutical Co., Ltd., Shanghai, China (No. H19999195; 100213). The main component was sodium Tanshinone IIa sulfonate, with the chemical formula R-C6H4-SO3Na (R = C10–C13) and molecular weight 340–352. Danshen injection was purchased from Chiatai Qingchunbao Pharmaceutical Co., Ltd., Shanghai, China (No. Z33020177; 0911053) and stored at 4°C. 1 mL Danshen injection was equal to 1 g Danshen.
Methods
Establishment of spinal ischemia/reperfusion injury model
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 g/kg). Rats were fixed in the supine position on the sterile operating table. The middle of the abdomen was shaved, disinfected with iodophor, deiodinated with alcohol and a median-right incision was made at the abdomen. The abdominal aorta to the level of the renal artery was separated and the right renal artery at the proximal end was clamped with a Scoville-Lewis clip to occlude the abdominal aorta blood flow. The incision was sutured layer by layer and the abdominal cavity was temporarily closed. The artery was declamped to restore blood flow after 0.5 hour and the abdominal cavity was closed (Figure 3). Rats were observed using digital subtraction radiography (Siemens, Berlin, Germany) and the model was identified as successful with complete spinal ischemia[26]. The abdominal aorta to the level of the renal artery was separated alone in the sham-surgery group.
Figure 3.
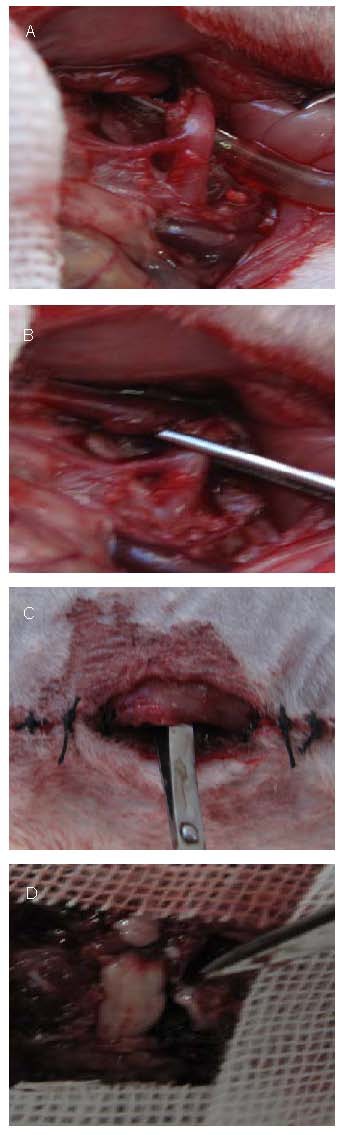
Establishment of spinal cord ischemia/reperfusion model.
(A) The abdominal aorta to the level of the renal artery was separated.
(B) The right renal artery at the proximal end was clamped.
(C)The abdominal cavity was temporarily closed.
(D) The spinous process and vertebral plate were removed, the nerve root was dissected to expose L2-5 segments.
Intraperitoneal injection of Danshen and Tanshinone IIa injection
Rat dosage was determined according to the following administration formula: rat dose = human dose × human conversion factor (36.8)/rat conversion factor (6.9)[27]. The Danshen and Tanshinone IIa groups were injected with 0.891 5 mL/kg Danshen and 3.565 2 mg/kg Tanshinone IIa injection respectively, 0.5 hours prior to model establishment.
Sampling
Rats were anesthetized and fixed in the prone position. The back was shaved and a median incision was made at the lumbar vertebra. Under sterile conditions, the bilateral sacrospinal muscles were cut and separated, and the spinous process and vertebral plate were removed. The nerve root was cut using a sharp blade to fully expose and harvest the spinal cord at L2-5 levels. The spinal cord was divided into two segments for Nissl staining and ELISA.
Nissl staining for morphology of spinal cord nerve cells
Spinal cord tissues were fixed in 4% paraformaldehyde for 24 hours, washed with distilled water for 4 hours to remove the paraformaldehyde solution, dehydrated, cleared and embedded. Samples were embedded by immersion in a mixture of Creosote, benzene and paraffin wax at 45°C, followed by twice immersion in paraffin wax at 54°C, for 2 hours each time. The embedded tissues were sectioned at a thickness of about 7 μm, adhered to gelatin-treated glass slides and incubated at 65°C overnight. Sections were dewaxed, mixed with 1% toluidine blue solution at 50°C, stained at 56°C and washed with water prior to differentiation with alcohol. Samples were then stained with eosin, dehydrated, cleared and mounted with neutral gum prior to observation using a light microscope (Suzhou Easymicro, Suzhou, China).
ELISA for HSP70, Bcl-2 and Bax expression in the spinal cord
Spinal cord tissues were prepared into a homogenate, diluted five times and incubated at 37°C for 30 minutes. Coated wells were washed for four times, mixed with 50 μL of enzyme conjugate (Wuhan Boster, Wuhan, China) and incubated at 37°C for 30 minutes. The wells were washed four times, mixed with 50 μL of chromogenic agent B solution (Wuhan Boster), shaken gently for 30 seconds and colorized in the dark at 37°C for 15 minutes. The reaction was terminated with stop buffer (Wuhan Boster). The blank well was zeroed. Absorbance of each well at 450 nm was determined using a microplate reader (BioTek, Winooski, VT, USA) within 15 minutes. The linear regression equation of the standard curve was calculated according to the standard sample concentration and corresponding absorbance. Sample concentration was calculated using the regression equation according to sample absorbance. Terminal concentration was then determined by multiplying the sample concentration by the dilution multiple, 5.
Statistical analysis
Data were expressed as mean ± SD and analyzed using SPSS 16.0 (SPSS, Chicago, IL, USA). Intergroup mean differences were compared using one-way analysis of variance and a two-sample t-test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Yanding Zhang, Yide Huang and Xuefen Hu, College of Life Science, Fujian Normal University, and Wenguang Zhang and Changzheng Li, Fujian University of Traditional Chinese Medicine, for their help.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30973765; New Century Excellent Talents Program, No. NECT-09-0013; the Foundation for Doctors, Ministry of Education, No. 20113519110001.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics.
(Edited by Zhao WH, Zhu LG/Su LL/Wang L)
REFERENCES
- [1].Zhao ZQ. Postconditioning in reperfusion injury: a status report. Cardiovasc Drugs Ther. 2010;24(3):265–279. doi: 10.1007/s10557-010-6240-1. [DOI] [PubMed] [Google Scholar]
- [2].Ning N, Dang X, Bai C, et al. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol. 2012;139(2):504–512. doi: 10.1016/j.jep.2011.11.040. [DOI] [PubMed] [Google Scholar]
- [3].Awad H, Ankeny DP, Guan Z, et al. A mouse model of ischemic spinal cord injury with delayed paralysis caused by aortic cross-clamping. Anesthesiology. 2010;113(4):880–891. doi: 10.1097/ALN.0b013e3181ec61ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jin XL, Zhao D. Effect of Ligustrum lucidum on apoptosis, Bcl-2 and Bax expression after spinal cord injury in rats. Zhongguo Shiyong Shenjing Jibing Zazhi. 2011;22:28–30. [Google Scholar]
- [5].Zhou LY, Liu XY, Liu DY. Effect of astragalus injection preconditioning on spinal cord function and neuronal apoptosis in the rat model of spinal cord ischemia- reperfusion injury. Zhongguo Zhongyi Gushing Ke Zazhi. 2011;10:7–9. [Google Scholar]
- [6].Liu XY, Wang HB, Deng CB, et al. Correlation between the expression of Bcl-2, Bax and neural cell apoptosis after chronic compression of cauda equina nerve in rats. Zhongguo Yike Daxue Xuebao. 2011;6:520–523. 527. [Google Scholar]
- [7].Yang X, Wang J, Zhou Y, et al. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. doi: 10.1016/j.canlet.2012.01.030. in press. [DOI] [PubMed] [Google Scholar]
- [8].Wang X, Wang C, Li B, et al. Protection of HSF1/HSP70 pathway on UVA-induced HaCaT cells apoptosis via inhibiting the activation of c-Jun N-terminal kinase. Wei Sheng Yan Jiu. 2012;41(1):40–45. [PubMed] [Google Scholar]
- [9].Moodley D, Mody GM, Chuturgoon AA. Initiation but no execution - modulation of peripheral blood lymphocyte apoptosis in rheumatoid arthritis - a potential role for heat shock protein 70. J Inflamm (Lond) 2011;8(1):30. doi: 10.1186/1476-9255-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rérole AL, Jego G, Garrido C. Hsp70: anti-apoptotic and tumorigenic protein. Methods Mol Biol. 2011;787:205–230. doi: 10.1007/978-1-61779-295-3_16. [DOI] [PubMed] [Google Scholar]
- [11].Lv HY, Chen QF. Bcl-2 family and neuronal apoptosis after cerebral ischemia/reperfusion. Zhongguo Xiandai Yiyao Zazhi. 2008;10(5):141–142. [Google Scholar]
- [12].Fan L, Wang K, Cheng B. Effects of buyang huanwu decoction on apoptosis of nervous cells and expressions of Bcl-2 and bax in the spinal cord of ischemia- reperfusion injury in rabbits. J Tradit Chin Med. 2006;26(2):153–156. [PubMed] [Google Scholar]
- [13].DU SC, Ge QM, Lin N, et al. ROS-mediated lipopolysaccharide-induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cell Mol Biol (Noisy-le-grand) 2012;(58 Suppl):OL1654–1659. [PubMed] [Google Scholar]
- [14].Qu X, Sun HX, Huang XL, et al. Effect of mukdenia rossii koidz, salvia and tetramethylpyrazine injection on hydroxyl radical. Zhongyuan Yikan. 2001;28(1):9–10. [Google Scholar]
- [15].Beijing: China Medical Science Press; 2010. Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, Part I. 2010th. [Google Scholar]
- [16].Hao H, Wang G, Cui N, et al. Pharmacokinetics, absorption and tissue distribution of tanshinone IIA solid dispersion. Planta Med. 2006;72(14):1311–1317. doi: 10.1055/s-2006-951698. [DOI] [PubMed] [Google Scholar]
- [17].Bi HC, Law FC, Zhong GP, et al. Study of tanshinone IIA tissue distribution in rat by liquid chromatography-tandem mass spectrometry method. Biomed Chromatogr. 2007;21(5):473–479. doi: 10.1002/bmc.778. [DOI] [PubMed] [Google Scholar]
- [18].Li H, Feng JL, Liu KX, et al. Effect of Tanshinone II A on expression of Bcl-2 and Bax after cerebral ischemic reperfusion injury in rats. Shizhen Guoyi Guoyao. 2009;20(1):82–84. [Google Scholar]
- [19].Wang Z, Gall JM, Bonegio RG, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79(8):861–870. doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xilinbaoleri , Xu WL, Bai JP. Neurons of beagle spinal cord showed by three stain methods after radiotherapy. Shiyong Yixue Zazhi. 2010;26(12):2112–2114. [Google Scholar]
- [21].Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223(1):163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- [22].Pastukhov IuF, Ekimova IV, Guzhova IV, et al. Content of chaperon Hsp70 in dopaminergic neurons of the black substance increases in proteasome dysfunstion. Ross Fiziol Zh Im I M Sechenova. 2011;97(7):649–660. [PubMed] [Google Scholar]
- [23].Fan SJ, Jiang H, Yang LJ, et al. Effects of adrenergic agents on stress-induced brain microstructural and immunochemical changes in adult male Wistar rats. Ann Anat. 2011;193(5):418–424. doi: 10.1016/j.aanat.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [24].Franklin TB, Krueger-Naug AM, Clarke DB, et al. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005;21(5):379–392. doi: 10.1080/02656730500069955. [DOI] [PubMed] [Google Scholar]
- [25].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [26].Zhang L, Liu ZB, Li CZ, et al. Establishment and identification of spinal cord ischemia/reperfusion injury models by vessel cast technique and digital subtraction angiography. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(40):8193–8195. [Google Scholar]
- [27].Shi Y, Mei SC. Beijing: China Agriculture Press; 2002. Practical Handbook of Medical Animal Experiments. [Google Scholar]


