Abstract
In this study, rabbit models of optic nerve injury were reproduced by the clamp method. After modeling, rabbit models were given one injection of 50 ng recombinant human ciliary neurotrophic factor into the vitreous body and/or intragastric injection of 4 g/kg compound light granules containing Radix Angelicae Sinensis and Raidix Paeoniae Alba at 4 days after modeling, once per day for 30 consecutive days. After administration, the animals were sacrificed and the intraorbital optic nerve was harvested. Hematoxylin-eosin staining revealed that the injured optic nerve was thinner and optic nerve fibers were irregular. After treatment with recombinant human ciliary neurotrophic factor, the arrangement of optic nerve fibers was disordered but they were not markedly thinner. After treatment with compound light granules, the arrangement of optic nerve fibers was slightly disordered and their structure was intact. After combined treatment with recombinant human ciliary neurotrophic factor and compound light granules, the arrangement of optic nerve fibers was slightly disordered and the degree of injury was less than after either treatment alone. Results of tensile mechanical testing of the optic nerve showed that the tensile elastic limit strain, elastic limit stress, maximum stress and maximum strain of the injured optic nerve were significantly lower than the normal optic nerve. After treatment with recombinant human ciliary neurotrophic factor and/or compound light granules, the tensile elastic limit strain, elastic limit stress, maximum stress and maximum strain of the injured optic nerve were significantly increased, especially after the combined treatment. These experimental findings indicate that compound light granules and ciliary neurotrophic factor can alleviate optic nerve injury at the histological and biochemical levels, and the combined treatment is more effective than either treatment alone.
Keywords: optic nerve injury, ciliary neurotrophic factor, compound light granules, mechanical characteristics, tissue morphology, retinal ganglial cells, stress, strain, biomechanics, traditional Chinese medicine, neural regeneration
Research Highlights
-
(1)
The therapeutic effect of the combined treatment of compound light granules and ciliary neurotrophic factor for optic nerve injury was observed using biomechanical and histological examinations.
-
(2)
The stress and strain of the optic nerve were assessed in longitudinal tensile testing, and the optic nerve stress-strain relationship was established using regression analysis and quantitative analysis was performed.
-
(3)
After optic nerve injury, the tensile elastic limit strain, elastic limit stress, maximum stress, and maximum strain were significantly reduced, and the tensile-bearing capacity was reduced.
-
(4)
Compound light particles and ciliary neurotrophic factor can improve the morphology of the injured optic nerve, increase tensile mechanical index and resistance capacities towards loading and deformation, with the combined treatment being more effective than either alone.
Abbreviations RGCs, retinal ganglion cells; CNTF, ciliary neurotrophic factor
INTRODUCTION
The optic nerve is composed of retinal ganglion cells (RGCs), axons and glia. Damage can occur to any part of the optic nerve, causing partial or complete loss of visual function. Therefore, the protection of optic nerves is one of the main focuses of research in the field of modern visual science[1]. Increasing attention has been paid to the diagnosis and treatment of optic nerve injury, and the protection of optic nerves[2,3,4,5,6,7,8,9,10]. A number of treatments have been explored for the treatment of optic nerve injury, including the administration of various antioxidants, nerve growth factor, apoptosis suppressing gene therapy, stem cell transplantation and autologous peripheral nerve bridging. Although some curative effects have been obtained, these therapies have not been applied in clinical practice[11]. Hall et al [12] used the antioxidant structure of vitamin E to replace the weak antioxidant amino side chain in Lazaroids steroid to synthesize U-78517F, a compound which has strong antioxidant properties and similar pharmacological actions to vitamin E. Furthermore, U-78517F has been shown to promote recovery following optic nerve injury. Watanabe et al [13] demonstrated that several neurotrophic (nerve growth factor, ciliary neurotrophic factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor) and growth factors (fibroblast growth factor) significantly promoted the survival of RGCs in vitro and after optic nerve transection injury. Cui et al [14] reported that neurotrophic factor increases the expression of retinal growth associated protein-43 after optic nerve crush injury in Sprague- Dawley (SD) rats. Optic nerve crush injury was reproduced in SD rats at a distance of 2 mm from the eyeball and neurotrophic factor was microinjected into the vitreous body through the sclera. According to the studies of Liu et al[15], neural stem cells, isolated from the hippocampus of SD embryonic rats, can improve the survival rate of RGCs and protect injured RGCs following transplantation into the vitreous body at the site of optic nerve injury in rats after cell culture. In addition, Yi et al [16] investigated changes in morphology and Bcl-2/Bax expression in the retina after optic nerve contusion in rats. They found that the number of RGCs decreased significantly after injury, especially within the first 2 weeks; while Bcl-2 and Bax expression increased to varying degrees following injury, with changes in Bax occurring later than changes in Bcl-2. The expressions of both Bcl-2 and Bax increased initially post-injury and subsequently decreased. Furthermore, Bcl-2/Bax expression correlated with the number of surviving RGCs. Thus, the reduction in RGC numbers after optic nerve injury is one of the main pathological causes of visual function impairment following optic nerve injury, and both Bcl-2 and Bax play an important role in the mechanism behind RGC death.
Optic nerve injury is mainly treated by drugs in the early stages. Experimental and clinical studies in traumatic optic nerve injury have shown that non-glucocorticoid steroids, glucocorticoid steroids, vitamin E, excitatory amino acid antagonists, thromboxane receptor antagonist, gangliosides, calcium channel blockers and vitamins can all inhibit the formation and activities of lipid peroxides, ameliorate vasogenic edema, promote tissue repair and functional reconstruction following optic nerve injury. However, drug treatments have certain adverse reactions and complications, some of which may even have toxic effects[17]. Taoka et al [18] found that after optic nerve injury, vitamin E levels were reduced in the body. Vitamin E helps prevent the formation of free radicals, while also functions as a scavenger in vivo and effectively alleviates ischemic injury after optic nerve injury, reduces damage and promotes optic nerve repair. However, oral administration of vitamin E cannot cross the blood brain barrier effectively, thus limiting its therapeutic benefits in scavenging and preventing the generation of free radicals. Nimodipine, a calcium channel blocker, was also shown to play a protective role in optic neuritis of acute experimental autoimmune encephalomyelitic rats, as reported by Xu et al [19]. Other groups, such as Ding et al [20], have further observed optic canal decompression combined with monosialotetrahexosylganglioside (GM-1) in the treatment of traumatic optic nerve injury. Results showed that there were no significant differences in preoperative general and traumatic conditions between single operation or combined treatment groups. Among nine cases in the single decompression group, there was no improvement in two, effective treatment in four, highly effective in two, and excellent in one case, giving a total efficiency rate of 77%. In comparison, among the 11 cases in the combined treatment group, no improvement was seen in two cases, effective improvement in three, highly effective in four, and excellent in two, with a total efficiency rate of 81%. The degree of recovery of visional acuity in the combined treatment group was better than the single operation group. Optic nerve decompression can release the compression on the optic nerve by making slices in the bone and reduce hematoma. Furthermore, cutting the optic nerve sheath and common tendinous ring can provide enough space for the damaged optic nerve fibers to promote functional recovery of the optic nerve. In addition, optic canal decompression combined with GM-1 can significantly promote the repair of damaged optic nerves and the improvement of the patients’ visual acuity.
Traditional Chinese medicine has been suggested to be more effective than western medicine in the treatment of optic nerve injury in its therapeutic effects and prevention of adverse reactions[1]. Increasing evidence has emerged involving the use of traditional Chinese medicine for the treatment of optic nerve injury[21,22,23]. Wang et al [24] reported the effects of Buyanghuanwu decoction on the expression of malondialdehyde and superoxide dismutase in the retina after experimental optic nerve injury. Results found that Buyanghuanwu decoction significantly inhibited the increase of malondialdehyde and decreased the activity of superoxide dismutase at 5, 14 and 28 days after treatment. The effect of Fuming medicine on axoplasmic flow after traction optic nerve damage was also observed[25], with 3H-leucine and horseradish peroxidase being used as a tracer. After 30 days of treatment with Fuming medicine, axoplasmic flow of injured optic nerve significantly improved under autoradiography and electron microscopy.
According to the studies of Zhu et al [26], compound light granule (Fuguang pellet) up-regulated the expression of the anti-apoptotic gene Bcl-2 and down-regulated the expression of the pro-apoptotic gene Bax after optic nerve crush injury in rabbits, while also alleviating the injury. The majority of studies to date have confirmed the therapeutic effects of using traditional Chinese medicine or neurotrophic factor for the treatment of optic nerve injury in animal models through biological and morphological investigations; however, the biomechanical indicators are not well defined. The biomechanical index is important for determining neurological impairment following brain and peripheral nerve injury for correct drug therapy.
This study aimed to verify the effects of ciliary neurotrophic factor (CNTF) and/or compound light granules in the treatment of optic nerve injury in experimental rabbit models by biomechanical and morphological analyses, in a broader attempt to provide biomechanical basis for clinical practice.
RESULTS
Quantitative analysis of experimental animals
Fifty healthy rabbits were randomly divided into five groups: normal control; model; CNTF; compound light granule; and CNTF + compound light granule groups. The interventions and outcome measures in each group are shown in Table 1. There were 20 optic nerve specimens prepared in each group, of which 15 were used for biomechanical analysis via tensile test and the remaining five were used for histological observation. All 50 rabbits were involved in the final analysis.
Table 1.
Grouping, intervention and main outcome measures
Effect of CNTF and compound light granules on the pathological changes in longitudinal sections of injured optic nerve in rabbits
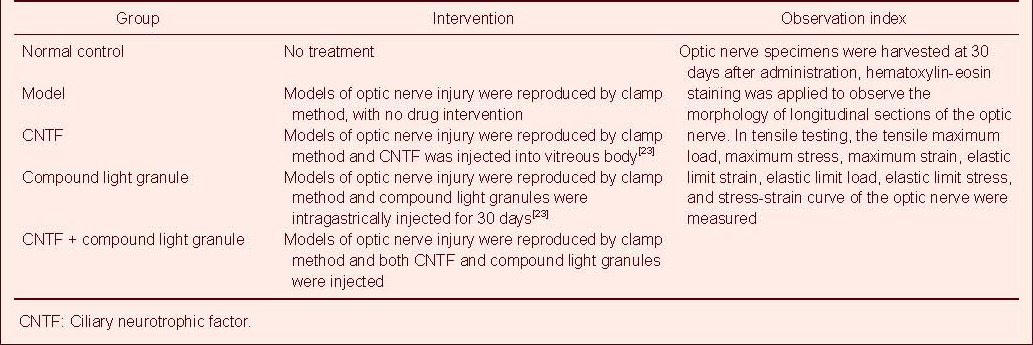
The morphological changes in the longitudinal profiles of the optic nerve were observed by hematoxylin-eosin staining and optical microscopy. In normal control group, optic nerve fibers were arranged in a regular and parallel manner, the staining was uniform, axons and other contents were clearly seen and the glia were of similar sizes (Figure 1).
Figure 1.
Morphology of a longitudinal section of the normal optic nerve in rabbits (hematoxylin-eosin staining, × 400).
Fibers are densely arranged in parallel rows, with intact structure, uniform staining, and similar size and arrangement of glial cells (red arrow).
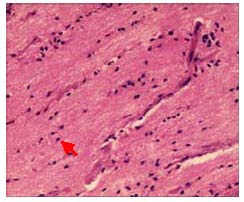
In the model group, optic nerve fibers were thin, tortuous, irregularly arrange, and the nuclei were not uniform (Figure 2).
Figure 2.
Pathological change in the longitudinal sections of the optic nerve in rabbits with optic nerve injury (hematoxylin-eosin staining, × 400).
Optic nerve fiber structure became disorganized, nerve fibers and glial cells degenerated and became necrotic, and dissolved nuclei were occasionally observed (red arrow).
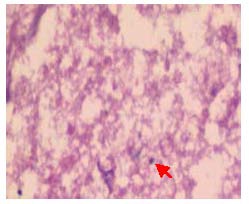
In the CNTF group, optic nerve fibers were disordered, and the optic nerve appeared healthier and the degree of injury was lighter than the model group (Figure 3).
Figure 3.
Pathological changes in the longitudinal sections of the optic nerve in rabbits after treatment with ciliary neurotrophic factor (hematoxylin-eosin staining, × 400).
Optic nerve fibers arranged in disorder, the structure was visible, fiber bundles were not continuous, glial nuclei were increased, with some irregular nuclei, a small number of nuclei were dissolved and there were occasional nuclear fragments (red arrow).
In the compound light granule group, a portion of optic nerve fibers were in a disordered arrangement, there was no thinning of the optic nerve and glial nuclei were not uniform (Figure 4).
Figure 4.
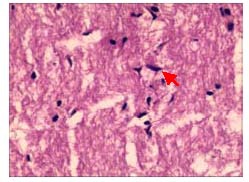
Pathological changes in the longitudinal sections of the optic nerve in rabbits after treatment with compound light granules (hematoxylin-eosin staining, × 400).
The structure of optic nerve fibers was visible; partial optic nerve fibers arranged irregularly, there was no thinning of the optic nerve, and glial nuclei were partially uneven (red arrow).
In the CNTF + compound light granule group, optic nerve fibers were disordered, glial nuclei were significantly increased and were mostly in an ordered arrangement (a small amount were still irregular), there was no thinning of the optic nerve and the degree of optic nerve injury was lighter than the CNTF and compound light granule groups (Figure 5).
Figure 5.
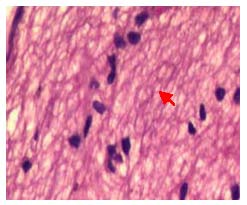
Pathological changes in the longitudinal sections of the optic nerve in rabbits after treatment with ciliary neurotrophic factor and compound light granules (hematoxylin-eosin staining, × 400).
Optic nerve fibers arranged in disorder, fiber bundles were continuous (red arrow), glial nuclei were mostly regular, with a few irregular nuclei being observed.
Effect of CNTF and complex light granules on tensile mechanical indexes of injured optic nerve in rabbits
To compare and analyze the tensile mechanical properties of specimens in each group, a tensile test was performed with an electronic universal testing machine. The results are shown in Tables 2–6.
Table 2.
Tensile mechanical index of normal rabbit optic nerve in tensile testing
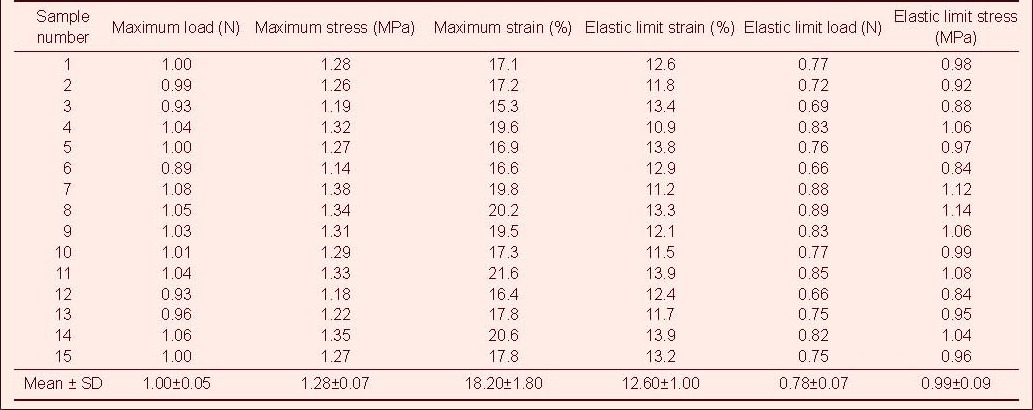
Table 6.
Tensile mechanical index of injured rabbit optic nerve after ciliary neurotrophic factor and compound light granule treatment in tensile testing
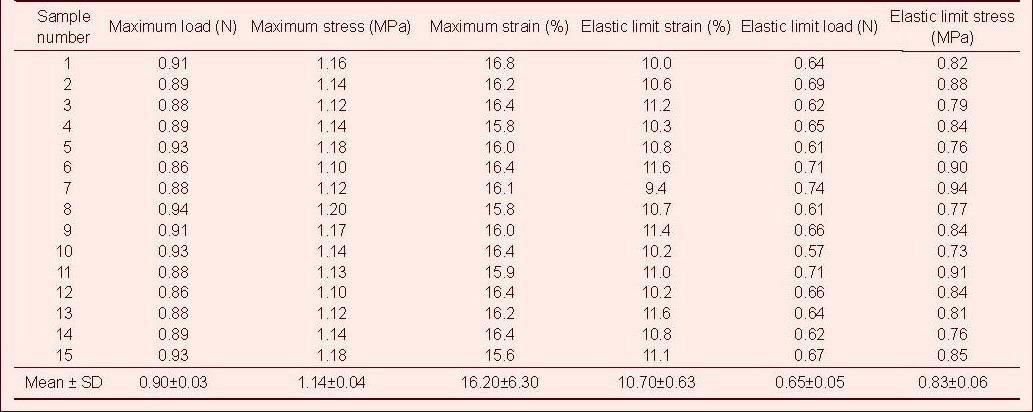
Table 3.
Tensile mechanical index of injured rabbit optic nerve in tensile testing
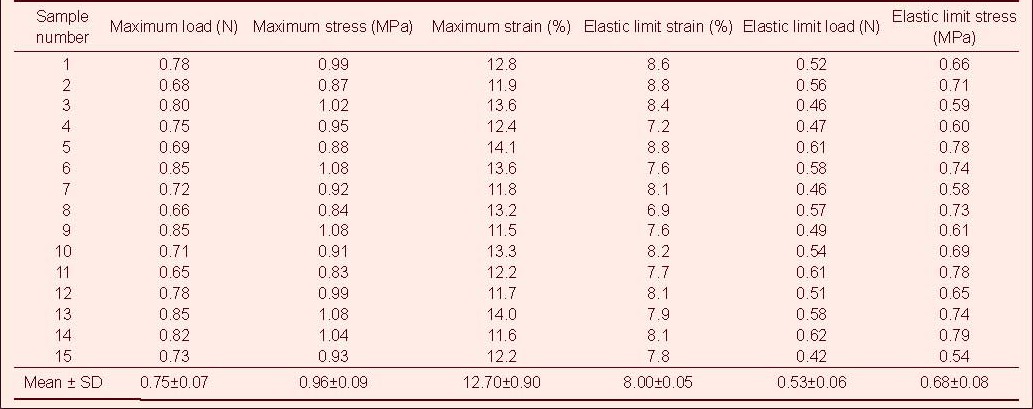
Table 4.
Tensile mechanical index of injured rabbit optic nerve after ciliary neurotrophic factor treatment in tensile testing
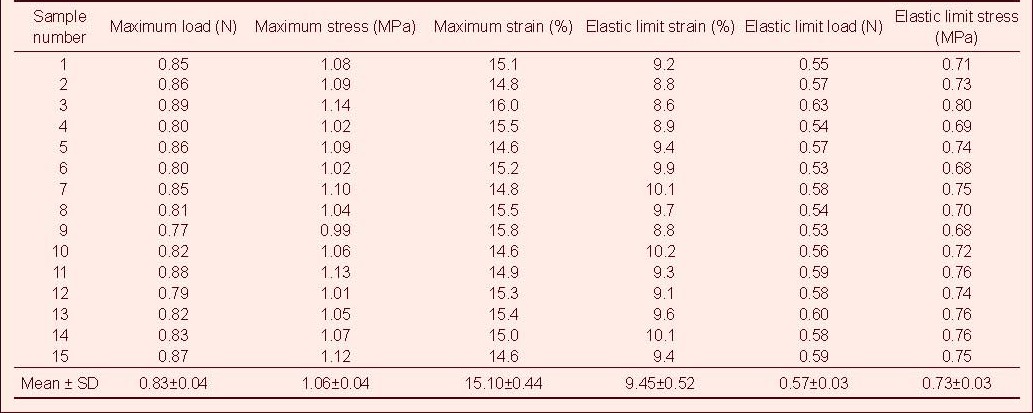
Table 5.
Tensile mechanical index of injured rabbit optic nerve after compound light granule treatment in tensile testing
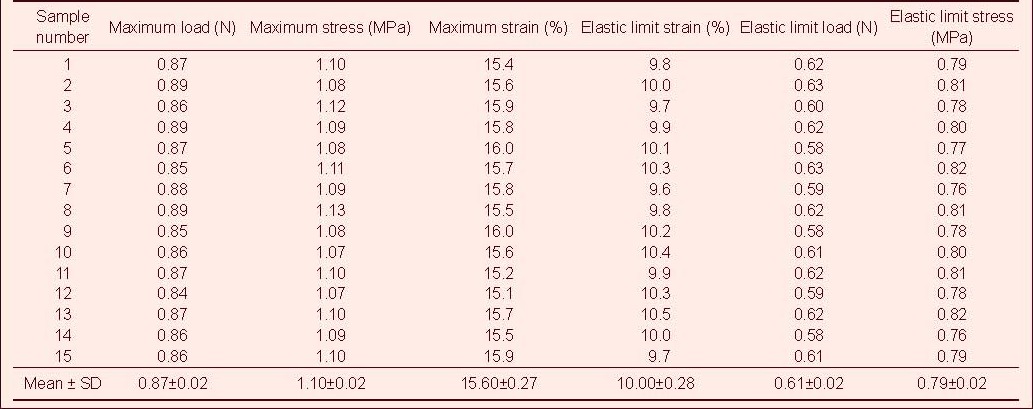
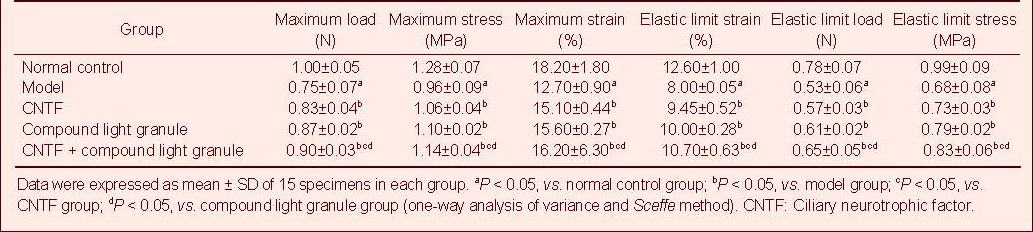
Tensile test showed that the maximum tensile load, stress and strain, and the elastic limit load, stress and strain of the optic nerve in the normal control group were significantly higher than those in the model, CNTF, compound light granule and CNTF + compound light granule groups (P < 0.05). Compared with model group, the above indexes were increased in the CNTF, compound light granule and CNTF + compound light granule groups, with the most significant difference in the CNTF + compound light granule group (P < 0.05; Table 7).
Table 7.
Effect of CNTF and compound light granules on tensile mechanical indexes of injured rabbit optic nerve
Effect of CNTF and complex light granule on the stress-strain curve of the optic nerve in rabbits
Tensile test data for specimens in each group (n = 15) were subjected to curve fitting analysis and the obtained stress-strain curves are shown in Figures 6–10. We could see that the normal optic nerve had the strongest tensile bearing capacity, followed by the injured optic nerve in the CNTF + compound light granule group, the injured optic nerve in the compound light granule group, and the injured optic nerve in the CNTF group. The injured optic nerve in the model group without any treatment had the weakest tensile bearing capacity.
Figure 6.
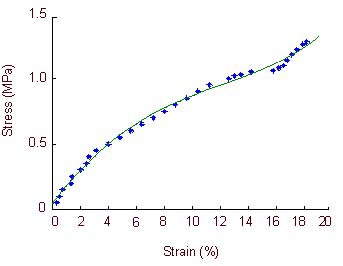
Stress-strain curve of the optic nerve in the normal control group.
In normal rabbits, the stress-strain curve is exponential when optic nerve strain is 0–3.8%, is directly proportional when optic nerve strain is 3.8–12.6%, and returns to an exponential relationship when optic strain is 12.6–15.8%. Large deformations occur in the specimens when the optic nerve strain is 15.8–18.2%, when the specimens lose their load-bearing capacity.
Figure 10.
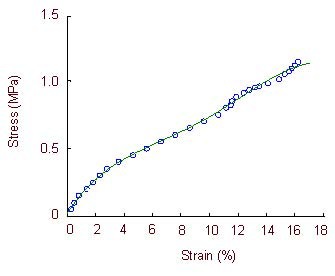
Stress-strain curve of the optic nerve in the ciliary neurotrophic factor + compound light granule group.
The stress-strain curve is exponential when optic nerve strain is –2.8%, and is directly proportional when optic nerve strain is 2.8–10.7%. Large deformations occur in the specimens when the optic nerve strain is 12.8–16.2%, when specimens lose their load-bearing capacity.
Figure 7.
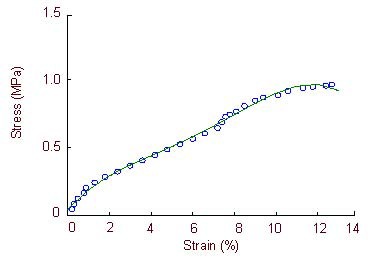
Stress-strain curve of the optic nerve in the model group.
The stress-strain curve is exponential when optic nerve strain is 0–1.8% and 8.0–10.4%. Large deformations occur in the specimens when the optic nerve strain is 10.4–12.7%, when specimens lose their load-bearing capacity.
Figure 8.
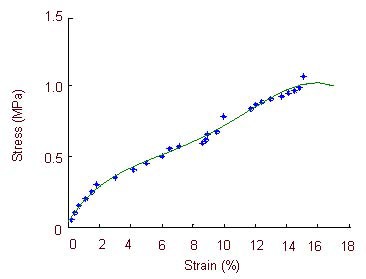
Stress-strain curve of the optic nerve in the ciliary neurotrophic factor group.
The stress-strain curve is exponential when optic nerve strain is 0–2.2% and 9.45–11.7%. Large deformations occur in the specimens when the optic nerve strain is 11.7–15.1%, when specimens lose their load-bearing capacity.
Figure 9.
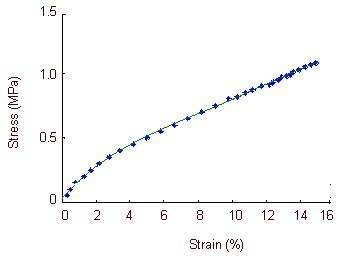
Stress-strain curve of the optic nerve in the compound light granule group.
The stress-strain curve is exponential when optic nerve strain is 0–2.5%, is directly proportional when optic nerve strain is 2.5–10.2%, and returns to an exponential relationship when optic strain is 10.2–12.2%. Large deformations occur in the specimens when the optic nerve strain is 12.2–15.6%, when specimens lose their load-bearing capacity.
Establishment of stress-strain functional correlation in the optic nerve of rabbits after CNTF and compound light granule treatment
The stress-strain function correlation was determined from the stress and strain data using a regression analysis method. The results are as follows: Normal control group:
δ(ε) = 0.003 9e5 − 0.034 8e4 + 0.174e3 − 0.037 4e2 Model group:
δ(ε) = 0.047 1e5 + 0.044 7e4 + 0.196e3 + 0.041 2e2 CNTF group:
δ(ε) = 0.004 6e5 − 0.001 9e4 + 0.039e3 − 0.041 7e2
Compound light granule group:
δ(ε) = 0.001 1e5 − 0.015 1e4 + 0.149 3e3 − 0.027 0e2
CNTF+ compound light granule group:
δ(ε) = 0.001 7e5 - 0.021 4e4 + 0.162e3 + 0.019 2e2 δ: stress, ε: strain, e: exponential function.
DISCUSSION
Establishing animal models of optic nerve injury is fundamental for nerve injury research.
The optic nerve of rabbits has a similar structure to the human optic nerve, which makes them a good model for studying optic nerve injury. According to a previous report[26], establishing an optic nerve injury model 2 mm behind the eyeball using a reverse vascular clamp is a good method for avoiding damage to the blood circulation. In this study, this method was reproduced in our animal models of optic nerve injury at a specific site and time point by the same experienced eye doctor, and tensile tests and optical microscopy were used to confirm the success of modeling.
The relationship between the stress-strain curves of the specimens tested was exponential when stress was initiated, following which it became linear, returned to being exponential, and finally increased quickly with stress. The stress-strain curve was nonlinear as continuous force may cause the specimen fibers to extend until fracture. The change in the relationship between the stress-strain was similar among groups, but the stress-strain value decreased significantly in the model group, and decreased slightly in the CNTF, compound light granule, and CNTF + compound light granule groups, with the largest decline in the CNTF + compound light granule group.
The physiological functions of the optic nerve require an intact structure of nerve fibers, which imparts a certain resistance to load and deformation. Any resection or partial injury of nerve fibers may cause a loss in structural integrity, leading to a full or partial loss of optic nerve resistance to load, thereby resulting in deformation. Under a light microscope, the optic nerve fibers in the model group appeared to be thinning and were irregularly arranged; some fibers were beaded and traveled parallel to the longitudinal axis of optic nerve, but the majority of nerve fibers deviated from the track and had a disordered structure. There were an increasing number of glial cells near the center, while vacuolar degeneration was observed near the margin, where there were very few cells present. The resistance capacities to loading and deformation were obviously reduced.
As a neurotrophic factor, CNTF plays an important role in neural activity. CNTF can promote the survival of a variety of neural cells and is important for nervous system development, differentiation and restoration after clamp injury[21]. CNTF promotes RGC survival and growth in vitro, significantly delays RGC death after optic nerve crush injury, and improves early survival rate[27,28]. Cui et al [29] reported that CNTF could increase the RGC survival after axons were resected and obviously promote cell growth into the sciatic nerve. The present study found that optic nerve fibers were disorderly arranged in the CNTF group; glial nuclei were increased and irregularly arranged; a small amount of cell vacuoles were visible; nuclei were fragmented; and optic nerve fibers formed a circuit, with occasional nuclear fragments, but there was no thinning of the optic nerve and the degree of injury was lower than the model group. CNTF treatment may alter the morphology of optic nerve in animals with optic nerve injury and enhance the resistance towards loading and deformation. All evidence supports that CNTF has good potential for the treatment of optic nerve injury.
Ou et al [30] previously reported the clinical efficacy of Fuguang particles for the treatment of central serous chorioretinopathy, vitreous opacities, optic atrophy and other eye diseases.
Compound light granules contains a variety of anti-inflammatory components, such as Chinese Angelica, which has immune activity and improves mononuclear phagocytes[31,32]; total glucosides of White peony Alba, which has anti-inflammatory and immunomodulatory effects, and can be directly applied to macrophages where it inhibits the release of prostaglandin E2 and other bioactive substances and suppresses the leukotriene B4, which has a variety of biological activities and a very strong capacity for leukocyte chemotaxis and aggregation[33,34].
Compound light granule is an effective treatment for traumatic optic nerve injury through its antioxidant and anti-inflammatory activities. Clinical treatment mainly focuses on activating blood circulation to dissipate blood stasis, relieving the depressed liver, and nourishing the liver and kidney, all of which are the main effects of compound light granule treatment. In this study, optic nerve fiber arrangement was disordered in the compound light granules group, glial nuclei were increased, with most nuclei being regularly arranged (a few were irregular); a small amount of cell vacuolization was visible; nuclear debris was present with occasional fragments being visible; there was no significant thinning of the optic nerve; and the resistance to loading and deformation was increased. This indicates that compound light granules are effective at repairing the injured optic nerve. The most significant reparative effect of the injured optic nerve after CNTF + compound light granule treatment confirmed that the combined actions of CNTF and compound light granules are optimal for treatment of optic nerve injury, as this treatment obviously improves the morphology of the injured optic nerve and increases the resistance to loading and deformation.
Previous treatment for optic nerve injury in animals has mainly consisted of the administration of CNTF or compound light granules alone[14,26,30]. In this study, we used the combined treatment of CNTF and compound light granules and verified their therapeutic effect by tensile mechanical testing and microstructural analysis.
During the experiments, all optic nerve specimens were sampled and stored under the same conditions. Tensile tests were performed at the same time and the experimental temperature and speed were consistent. The morphological observations were performed at the same site in all specimens, thus avoiding data error. Because of limited sample size and individual differences of animals, our experimental data may have discrepancies, and further studies are required to enlarge the sample size.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment and biomechanical study.
Time and setting
Experiments were performed from June 2011 to April 2012 in the Mechanics Experiment Center of Jilin University, China.
Materials
Experimental animals
Fifty healthy, male Japanese big-ear rabbits, aged 5 months and weighing 2.5–2.8 kg, were provided by the Changchun Hi-tech Medical Animal Experiment Center, China (license No. SCXK (Ji) 2003-0004). Prior to experiments, rabbits were anesthetized with 10% chloral hydrate (35 mg/kg) via intraperitoneal injection, and there were no lesions in the eye or ocular fundus upon examination. Animals were housed at 18–25°C, in a relative humidity of 55–70%, with fresh air flow and natural light. Animals were allowed free access to food and water. All experimental protocols were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[35].
Medicine
Compound light granules were prepared by Tuobing Pharmaceutical Factory of Shantou Special Economic Region, China. The ingredients include Radix Angelicae Sinensis 120 g, white peony root 160 g, Tuckahoe 200 g, White Atractylodes Rhizome 250 g, Danshen Root 120 g, Chinese thorowax root 200 g, Rhizoma Dioscoreae 160 g, Rehmannia Dride Rhizome 200 g, Fructus Lycii 250 g, Magnetite 160 g, Fructus Gardeniae 120 g, Rhizoma Cimicifugae 120 g, Fructus Schisandrae 120 g, and Radix Glycytthizae 80 g.
Methods
Establishment of optic nerve injury models
The model of optic nerve injury was reproduced in rabbits according to a previously described method[26]. In brief, rabbits were anesthetized with 0.1% urethane (5 mg/kg) through ear vein injection and fixed on the working table. The rabbit eye top lateral orbital skin was cut, exposing the supraorbital margin and orbital wall. The orbital wall bone plate on both sides was removed (depth 7–8 mm, width 6 mm), the posterior eyeball bulbar fascia was cut open and bluntly resected along the superior rectus muscle to the eyeball, thus exposing the retrobulbar optic nerve. About 5 mm of the optic nerve was freed 3 mm away from the posterior pole. The optic nerve was clamped by the same senior doctor in the Department of Ophthalmology for all animals, using a mosquito clamp (type J31020, specification 12.5 cm; Shanghai Medical Equipment (Group) Co., Ltd., Shanghai, China) for 5 seconds (Figure 11). The operative field was rinsed with gentamicin solution twice and sutured with 10-0 nylon sutures (Nike Qingdao Medical Material Co., Ltd., Qingdao, Shandong Province, China) layer by layer. The modeling operations were performed with care to avoid damaging the central retinal vascular system and the conjunctival sac was postoperatively coated with erythromycin eye ointment. Loss of response of the damaged eye to direct light and the presentation of mydriasis, with no retinal hemorrhage and vascular occlusion in the ocular fundus were defined as successful model establishment.
Figure 11.
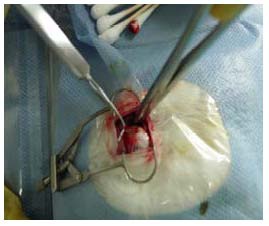
Establishment of optic nerve injury models.
Freeing of the retrobulbar optic nerve and clamping of the optic nerve with a mosquito forcep.
Drug intervention
Immediately after modeling, rabbits in the CNTF group were injected with 50 ng recombinant human CNTF (Sigma, St. Louis, MO, USA) into the vitreous body using a syringe[14]. In the CNTF + compound light granule group, models were given vitreous body injection of 50 ng CNTF[14], 4 days later, intragastric injection of compound light granules (4 g/kg) once per day, for 30 consecutive days[26]. The compound light granule group was given only compound light granules for 30 days. The normal control and model groups were not treated.
Harvesting specimens
After compound light granules administration, rabbits were sacrificed using the ear vein insufflation method and the optic nerve was exposed. The intraorbital segment of the optic nerve was removed with an operation microscope (type S88, Carl Zeiss Company, Yarra, Germany) and stored in normal saline at 4°C.
Biomechanical analysis of the rabbit optic nerve by tensile testing
This study used the automatic control electronic universal testing machine (Figure 12; Changchun Testing Machine Research Institute, Changchun, Jilin Province, China) for tensile testing. The length and diameter of optic nerve specimens were measured according to a previously described method[36] (Changchun Third Optical Instrument Factory, Changchun, Jilin Province, China). The tensile specimens were 10 mm long and the diameter of specimens was 0.98–1.02 mm in the normal control group, 0.96–0.99 mm in the model group, 0.98–1.01 mm in the compound light granules group, 0.97–1.02 mm in the CNTF group and 0.98–1.02 mm in the CNTF + compound light granules group. Each optic nerve specimen was preset by repeated loading for 10 times[35]. Experimental temperature simulated normal human temperature at 36.5 ± 1°C. All optic nerve specimens were subjected to tensile testing at a speed of 2 mm/min. During the testing process all specimens were sprayed with physiological saline to maintain humidity. On completion of the experiment, the stress, strain and stress-strain curve were automatically calculated using computer software.
Figure 12.

Electronic universal testing machine for measuring the length and diameter of each optic nerve specimen.
Light microscopic observation of the longitudinal structure of the rabbit optic nerve
One intraorbital optic nerve in each group was selected and fixed in 10% neutral formalin solution for 24 hours. After conventional gradient ethanol dehydration, xylene clearing and paraffin embedding, specimens were cut into continuous longitudinal sections, with a thickness of 3 μm, and wax-mounted on slides coated with protein glycerin. Following this, they were subjected to the following: thermostat roasting; conventional dewaxing; hematoxylin staining for 3 minutes, followed by tap water rinsing; 1% hydrochloric acid differentiation, followed again by tap water rinsing; weak ammonia treatment to return blue; tap water rinsing; eosin staining for 2 minutes; conventional gradient ethanol dehydration, xylene clearing to make samples transparent; and neutral gum mounting. Morphological changes in the optic nerve were observed under a BX51 optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
Quantitative data were expressed as mean ± SD and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Data difference between groups was compared with one-way analysis of variance and Sceffe method. A difference of P < 0.05 was considered statistically significant. The stress-strain functional relationship of each specimen was established by regression analysis.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Animal Ethics Committee of China-Japan Union Hospital of Jilin University, China.
(Edited by Yang L, Luan XP/Yang Y/Wang L)
REFERENCES
- [1].Sheng YM, Meng XL. Research progress of the optic nerve protective effect of Chinese medicine. Yiyao Daobao. 2007;26(10):1191–1193. [Google Scholar]
- [2].Kamradt MC, Lu M, Werner ME, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280(12):11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- [3].Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006;133(5):813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- [4].Munemasa Y, Kwong JM, Caprioli J, et al. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50(8):3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- [5].Ghosh JG, Houck SA, Doneanu CE, et al. The beta4-beta8 groove is an ATP-interactive site in the alpha crystallin core domain of the small heat shock protein, human alphaB crystallin. J Mol Biol. 2006;364(3):364–375. doi: 10.1016/j.jmb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [6].Chowdary TK, Raman B, Ramakrishna T, et al. Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem J. 2004;381(Pt 2):379–387. doi: 10.1042/BJ20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol. 2008;246(7):1029–1046. doi: 10.1007/s00417-008-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilhelm H. Traumatic optic neuropathy. Laryngorhinootologie. 2009;88(3):194–207. doi: 10.1055/s-0029-1192010. [DOI] [PubMed] [Google Scholar]
- [10].Wang JC, Cui J, Jia J, et al. The changes of glutamate concentrations in retinal and it's toxic effects with injured optic nerve in rabbits. Nao yu Shenjing Jibing Zazhi. 2010;18(3):213–215. [Google Scholar]
- [11].You SW, So KF, Yip HK. Axonal regeneration of retinal ganglion cells depending on the distance of axotomy in adult hamsters. Invest Ophthalmol Vis Sci. 2000;41(10):3165–3170. [PubMed] [Google Scholar]
- [12].Hall ED, Yonkers PA, Andrus PK, et al. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9(Suppl 2):S425–442. [PubMed] [Google Scholar]
- [13].Watanabe M, Sawai H, Fukuda Y. Survival of axotomized retinal ganglion cells in adult mammals. Clin Neurosci. 1997;4(5):233–239. [PubMed] [Google Scholar]
- [14].Cui ZL, Wang L, Hu D, et al. Neurotrophic factors promoting the expression of retinal growth associated protein-43 in retina after crush injuries of the optic nerve. Yanke Xin Jinzhan. 2003;23(2):76–78. [Google Scholar]
- [15].Liu HR, Yang CH, Gao JW, et al. Protective effect of neural stem cell transplantation on retinal ganglion cells of rats with optic nerve injury. Zhongguo Weiqinxi Shenjing Waike Zazhi. 2006;11(5):32–35. [Google Scholar]
- [16].Yi SH, Wu HS, Deng WL, et al. The change of morphology and Bax/Bcl-2 of retina after optic nerve crush in rat. Yan Waishang Zhiye Yanbing Zazhi. 2006;28(5):321–324. [Google Scholar]
- [17].Zhao CL, Fang Y. New progress of drug treatment in optic nerve damage. Linchuang Yanke Zazhi. 2000;8(5):387–389. [Google Scholar]
- [18].Taoka Y, Ikata T, Fukuzawa K. Influence of dietary vitamin E deficiency on compression injury of rat spinal cord. J Nutr Sci Vitaminol (Tokyo) 1990;36(3):217–226. doi: 10.3177/jnsv.36.217. [DOI] [PubMed] [Google Scholar]
- [19].Xu N. Chongqing: Chongqing Yike Daxue; 2007. An experimental study of effects of nimodipine on optic neuritis of acute experimental autoimmune encephalomyelitis in rat. [Google Scholar]
- [20].Ding ZY, Zhang JM, Shi J, et al. Optic canal decompression combined with GM-1 in the treatment of traumatic optic nerve injury. Shiyong Linchuang Yiyao Zazhi. 2002;16(1):39–41. [Google Scholar]
- [21].Liu DJ, Lan CJ, Gan YY. Studys on the nertoprotective effects of rigeron brevicapas hand mass on the optic nerve of chronic glaucoma model in rats. Guoji Yanke Zazhi. 2011;11(7):1154–1156. [Google Scholar]
- [22].Wang ZP. Observation on clinical application of compound Anisodine for patients with optic atrophy disease. Huli Yanjiu. 2010;24(18):1657. [Google Scholar]
- [23].Zhang YS. Effect of Chinese medicine Fuming Tablet on different kinds of primary open angle glaucoma optic nerve damage. Zhongguo Yaowu yu Linchuang. 2012;12(2):256–257. [Google Scholar]
- [24].Wang YJ, Tong JA. Effect of BYHWD on the expression of malondialdehyde and superoxide dismutase in retina after experimental optic nerve injury. Xiandai Zhongyiyao. 2012;32(2):75–76. [Google Scholar]
- [25].Xu L, Xia DZ. Effect of Fuming medicine on axoplasmic flow after traction optic nerve damage. Zhongguo Shiyong Yanke Zazhi. 1999;17(11):654–656. [Google Scholar]
- [26].Zhu J, Jiang W, Huang L, et al. Effect of fuguang pellet on Bcl-2 and Bax expression after optic nerve crush in rabbits. Guoji Yanke Zazhi. 2011;11(5):791–794. [Google Scholar]
- [27].Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- [28].Lehwalder D, Jeffrey PL, Unsicker K. Survival of purified embryonic chickretinal ganglion cells in the presence of neurotrophic factors. J Neurosci Res. 1989;24(2):329–337. doi: 10.1002/jnr.490240225. [DOI] [PubMed] [Google Scholar]
- [29].Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11(18):3999–4002. doi: 10.1097/00001756-200012180-00019. [DOI] [PubMed] [Google Scholar]
- [30].Ou RS, Zhang WL, Cui JZ. Clinical efficacy of Fuguang particles for the treatment of central serous chorioretinopathy, vitreous opacities, optic atrophy and other eye diseases. Zhongguo Minkang Yixue. 2007;19(2):109. [Google Scholar]
- [31].Wang YP, Zhu BD. The research progress of pharmacology of angelica polysaccharide. Zhongxiyi Jiehe Zazhi. 1991;11(1):61–63. [PubMed] [Google Scholar]
- [32].Li MF, Mei QB, Ren J, et al. Effect of Angelica polysaccharide on immune function in mice. Disi Junyi Daxue Xuebao. 1987;8(6):422–423. [Google Scholar]
- [33].Li J, Chen MZ, Xu SY, et al. Effect of TGP on rat peritoneal macrophages produce prostaglandin E2 and its mechanism. Zhongguo Yaolixue Tongbao. 1994;10(4):267–270. [Google Scholar]
- [34].Li J, Zhao WZ, Chen MZ, et al. Effect of TGP on rat peritoneal macrophages to generate the leukotriene B4. Zhongguo Yaolixue Tongbao. 1992;8(1):36–38. [Google Scholar]
- [35].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [36].Liu GY, Zhang Q, Jin Y, et al. Autogous nerve anastomosis versus humanamniotic membrane anastomosis A rheological comparison following simulated sciatic nerve injury. Neural Regen Res. 2011;6(31):2424–2428. [Google Scholar]


