Abstract
This study used electroacupuncture at Renzhong (DU26) and Baihui (DU20) in a rat model of cerebral ischemia/reperfusion injury. Neurological deficit scores, western blotting, and reverse transcription-PCR results demonstrated that electroacupuncture markedly reduced neurological deficits, decreased corpus striatum aquaporin-4 protein and mRNA expression, and relieved damage to the blood-brain barrier in a rat model of cerebral ischemia/reperfusion injury. These results suggest that electroacupuncture most likely protects the blood-brain barrier by regulating aquaporin-4 expression following cerebral ischemia/reperfusion injury.
Keywords: electroacupuncture, cerebral ischemia/reperfusion, blood-brain barrier, aquaporin-4, brain edema, rat, Renzhong (DU26), Baihui (DU20), brain injury, regeneration, neural regeneration
Research Highlights
-
(1)
Electroacupuncture at Renzhong (DU26) and Baihui (DU20) significantly reduced neurological deficits in rats with cerebral ischemia/reperfusion injury.
-
(2)
Electroacupuncture at Renzhong and Baihui diminished aquaporin-4 expression in the rat corpus striatum following cerebral ischemia/reperfusion injury.
-
(3)
Electroacupuncture at Renzhong and Baihui relieved injury to the blood-brain barrier in rats following cerebral ischemia/reperfusion injury.
-
(4)
Electroacupuncture most likely protected the blood-brain barrier from cerebral ischemia/ reperfusion injury by regulating aquaporin-4 expression.
Abbreviation BBB, blood-brain barrier
INTRODUCTION
Cerebral ischemia/reperfusion injury causes damage to the blood-brain barrier (BBB), increases vascular permeability and extravasation of plasma proteins from capillaries, increases fluid in the intercellular space, and induces vasogenic brain edema, resulting in brain injury[1,2] and cell death. Aquaporin-4 content is highest in the brain[3,4], and is mainly located on the surface and end-feet of astrocytes surrounding blood vessels, i.e., both sides of the BBB, suggesting that aquaporin-4 plays an important role in maintaining BBB integrity and in the pathophysiological changes of vasogenic brain edema[5,6]. An increase in aquaporin-4 expression may aggravate injury to endothelial cells and tight junctions, promote BBB destruction, and lead to water entering brain tissues, resulting in an increase in brain water content and the formation of brain edema[7]. Acupuncture has been shown to reduce brain injury and BBB permeability, to ameliorate microcirculation, and to protect the BBB[8,9]. Previous studies have predominantly focused on the morphology and ultrastructure of the BBB following acupuncture, but have not analyzed the protective mechanisms involved. Few studies have focused on how acupuncture exerts its protective effects and its role in regulating aquaporin-4 expression. Therefore, this study established a rat model of focal cerebral ischemia/reperfusion injury and investigated the relationship of BBB permeability and aquaporin-4 expression following acupuncture. We aimed to provide scientific evidence for electroacupuncture in the treatment of brain edema and brain ischemia.
RESULTS
Quantitative analysis of experimental animals
Sprague-Dawley rats (n = 256) were randomly assigned to control group (n = 64), model group (n = 96) and electroacupuncture group (n = 96). Rat models of cerebral ischemia/reperfusion injury were induced in the model and electroacupuncture groups. Following 5 minutes of ischemia, electroacupuncture was administered at Renzhong (DU26) and Baihui (DU20) in rats from the electroacupuncture group. Each group was subdivided into five subgroups, and each rat was sacrificed at 6, 12, 24, 48 and 72 hours following reperfusion, with 4–6 rats at each time point. Three rats died during anesthetic administration, and nine rats died from infection or other unknown reasons. Therefore, a total of 244 rats were included in the final analysis, comprising 88 rats in the model group, 92 rats in the electroacupuncture group and 64 rats in the control group.
Electroacupuncture markedly improved neurological function in rats following cerebral ischemia/reperfusion injury
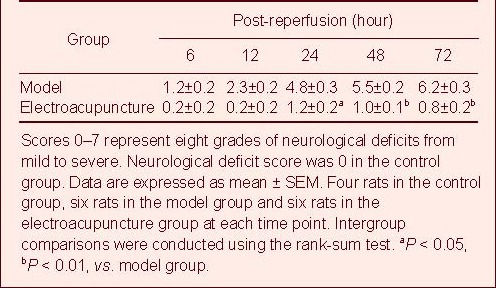
With prolonged ischemia/reperfusion times, neurological deficit scores were gradually aggravated and were worst at 72 hours after reperfusion, which was consistent with the symptoms of acute brain ischemia[10]. However, electroacupuncture markedly reduced neurological deficits and brain edema in rats following cerebral ischemia/reperfusion injury (Table 1).
Table 1.
Effects of electroacupuncture on neurological function in rats with cerebral ischemia/reperfusion injury
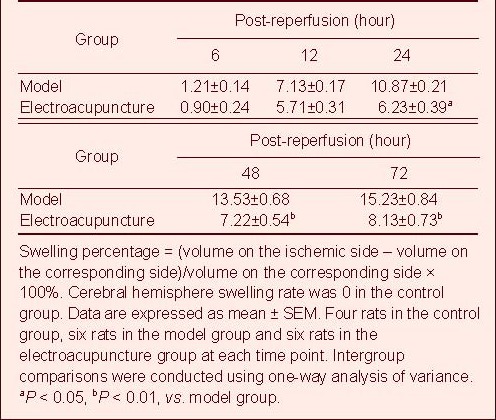
Electroacupuncture improved cerebral hemisphere swelling in rats following cerebral ischemia/reperfusion injury
The cerebral hemisphere swelled at 6 hours following reperfusion. Swelling gradually became more severe and peaked at 72 hours. Cerebral hemisphere swelling significantly reduced on the ischemic side following electroacupuncture treatment (P < 0.05; Table 2, supplementary Figure 1 online).
Table 2.
Effects of electroacupuncture on cerebral hemisphere swelling rate (%) in rats with cerebral ischemia/reperfusion injury
Electroacupuncture effectively suppressed aquaporin-4 expression in the rat corpus striatum following cerebral ischemia/reperfusion injury
At 6–72 hours following ischemia/reperfusion, aquaporin-4 expression increased in the rat corpus striatum on the cerebral ischemia/reperfusion side when compared with the control group. Moreover, aquaporin-4 expression increased with prolonged reperfusion times (P < 0.05 or P < 0.01). Electroacupuncture reduced aquaporin-4 expression in the rat corpus striatum following cerebral ischemia/reperfusion (P < 0.05 or P < 0.01). Electroacupuncture effectively suppressed aquaporin-4 protein expression (Figure 1).
Figure 1.
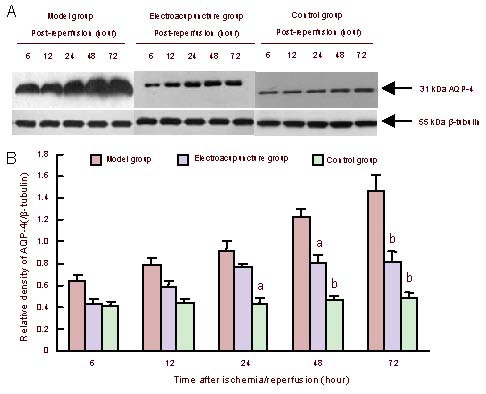
Effect of electroacupuncture effects on aquaporin-4 (AQP-4) expression in the rat corpus striatum following cerebral ischemia/reperfusion.
(A) Western blot assay of aquaporin-4 expression.
(B) Quantitative results of aquaporin-4 expression. Data are expressed as mean ± SEM. Four rats in the control group, six rats in the model group and six rats in the electroacupuncture group at each time point. Intergroup comparisons were conducted using one-way analysis of variance. aP < 0.05, bP < 0.01, vs. model group.
Electroacupuncture effectively inhibited aquaporin-4 mRNA expression in the rat corpus striatum following cerebral ischemia/reperfusion injury
Aquaporin-4 mRNA expression was altered in the rat right corpus striatum depending on the reperfusion time. Intergroup differences were identical to western blotting experiments (Figure 2).
Figure 2.
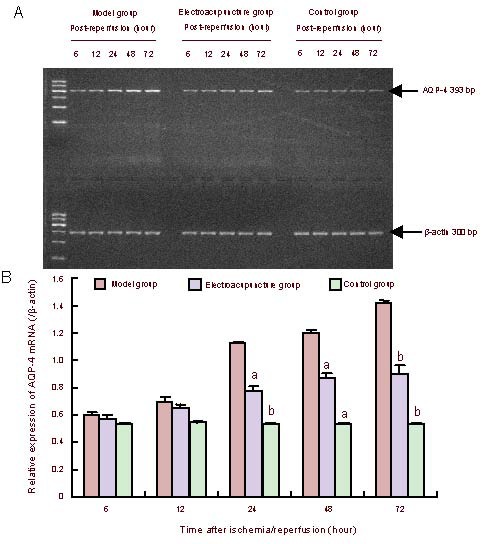
Aquaporin-4 (AQP-4) mRNA expression in the rat corpus striatum.
(A) PCR of aquaporin-4 mRNA expression.
(B) Quantitative results of aquaporin-4 mRNA expression. The ratio of target band to internal reference gray value served as the protein expression intensity. Data are expressed as mean ± SEM. Four rats in the control group, six rats in the model group and six rats in the electroacupuncture group at each time point. Intergroup comparisons were conducted using one-way analysis of variance. aP < 0.05, bP < 0.01, vs. model group.
Aquaporin-4 expression in astrocytes in the rat corpus striatum
Laser confocal microscopy revealed that aquaporin-4 and glial fibrillary acidic protein expression were detectable in the rat right corpus striatum. Glial fibrillary acidic protein served as a specific marker of astrocytes[11]. Aquaporin-4 expression was visible on the end-feet of astrocytes (Figure 3).
Figure 3.
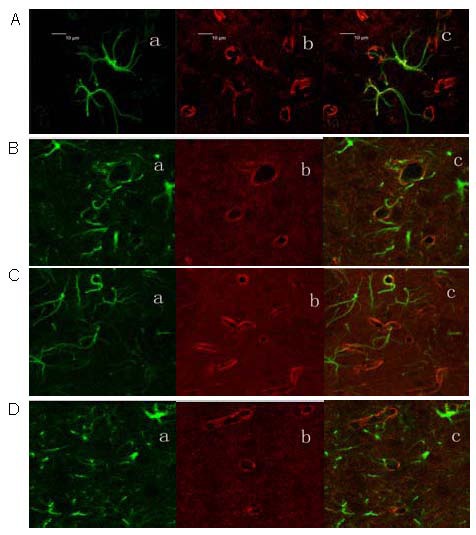
Coexpression of aquaporin-4 and glial fibrillary acidic protein in the rat corpus striatum at 24 hours following cerebral ischemia/reperfusion (laser confocal microscopy, × 400).
Green represents glial fibrillary acidic protein (Fluorescein label, a), red represents aquaporin-4 (Rhodamine label, b), and yellow represents coexpression (c).
(A) Control group; (B) electroacupuncture group; (C, D) model group.
DISCUSSION
Numerous studies have confirmed that aquaporin-4 is strongly associated with the BBB[12,13,14]. The BBB is composed of astrocyte foot processes, tight junctions and endothelial cells. Blood capillaries are surrounded by glial cell foot processes forming a glial limiting membrane, which accounts for 85–99% of the capillary surface area[15,16,17]. Aquaporin-4 was densely distributed on glial cell foot processes, and played an important role in edema formation and cellular swelling[18]. Taniguchi et al [19] found that aquaporin-4 mRNA expression increased at 1 day following focal cerebral ischemia/reperfusion, was most obvious at 3 days, and then gradually reduced at 7 days, which showed a parallel relationship to edema formation and extinction, which are identical to this study.
Monitoring of cerebral blood flow using transcranial Doppler sonography confirmed successful establishment of the rat cerebral ischemia/reperfusion model. Brain edema, measured using cresyl violet staining instead of the dry/wet weight method, and reverse transcription- PCR revealed that aquaporin-4 mRNA expression gradually increased over time, and that vasogenic brain edema, damage to the BBB and increased permeability occurred in the model group. The present study observed that electroacupuncture reduced aquaporin-4 expression and astrocyte foot process swelling inhibited BBB permeability, and relieved injury to the BBB.
Because astrocyte foot processes are a part of the BBB[20] and aquaporin-4 was extensively distributed on glial cell end-feet, electroacupuncture reduced the swelling of astrocyte foot processes by suppressing aquaporin-4 expression, resulting in the protection of the BBB and a reduction in brain edema. Changes in aquaporin-4 levels markedly affected the structure and function of the BBB. Regulation of aquaporin-4 levels under these pathological conditions may provide a new target for the prevention and treatment of BBB injury[21].
In summary, early use of electroacupuncture for the treatment of cerebral ischemia/reperfusion injury can regulate water balance and reduce injury to the BBB by suppressing aquaporin-4 expression. Therefore, electroacupuncture results in a reduction in brain injury, and the prevention and treatment of brain edema.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
Experiments were performed in the Affiliated Hospital of Nanjing University of Chinese Medicine and School of Basic Medical Sciences, Fudan University in China from March 2006 to January 2012.
Materials
Clean, healthy, male Sprague-Dawley rats (n = 256) aged 2 months and weighing 215–230 g were supplied by the Shanghai Experimental Animal Center, Chinese Academy of Sciences (license No. SCXK (Hu) 2007-0005). All rats were housed at 22–25°C in a 12-hour light/dark cycle and were allowed free access to food and water. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[22].
Methods
Establishment of focal cerebral ischemia/reperfusion injury model
In accordance with a previously published method[23], following 8 hours of preoperative fasting, the rats were intraperitoneally anesthetized with 10% (v/v) chloral hydrate (0.36 mL/100 g). A median incision was made on the neck. The right external carotid artery, common carotid artery, internal carotid artery and pterygopalatine artery were occluded by an arteriole clamp. A small cut was made on the right external carotid artery, and a 0.18 mm-diameter 4/0 nylon thread was inserted into the internal carotid artery through the external carotid artery, and then moved into the initial part of the middle cerebral artery. When resistance was felt and cerebral blood flow decreased to 20% of the basal value, model establishment was deemed successful. Cerebral blood flow was monitored using transcranial Doppler sonography (Perimed Co., Jarfalla, Sweden). Following 1 hour of ischemia, the nylon thread was withdrawn to the external carotid artery, i.e., the beginning of reperfusion.
Electroacupuncture stimulation
Using a G6805-II electroacupuncture apparatus (Shanghai Medical Electronic Machine Co., Ltd., Shanghai, China), electroacupuncture was administered at Renzhong (1 mm below the nasal tip, in the middle of the nasolabial fold) and Baihui (in the middle of parietal bone) in rats following 5 minutes of ischemia, with sparse waves at 3.85 Hz, for 1.28 seconds, and dense waves at 6.25 Hz for 2.08 seconds. The strength was 0.8–1.0 mA for 30 minutes.
Neurological deficit scores in rats with brain injury
Neurological deficits were scored in a single-blind design at 6, 12, 24, 48 and 72 hours following reperfusion. There were eight grades (0–7 scores). Scoring criteria: a score of 0, no asymmetrical activities; a score of 1, left forepaw cannot completely extend when lifting tail; a score of 2, left forepaw disability; a score of 3, left forepaw tightly closed to the chest wall; a score of 4, turning left when free running; a score of 5, left forepaw makes an action of pushing back; a score of 6, rotation surrounding original point (supplementary Video 1 online); a score of 7, the left limb cannot support the body[24].
Brain specimen perfusion fixation and tissue section preparation
At 6, 12, 24, 48 and 72 hours following reperfusion, saline and 4% (w/v) paraformaldehyde were perfused through the left ventricle. The brain tissues were obtained by decapitation, fixed in sucrose and sliced into 30 μm-thick coronal sections using a freezing microtome (Leica, Solms, Germany), and then stored at −20 °C.
Cresyl violet staining for measuring brain edema
Following 6, 12, 24, 48 and 72 hours of reperfusion, cresyl violet staining was performed[25]. One brain section was selected out of every 13 sections from each rat; in total 18 sections were obtained. Areas from the ischemic side and corresponding side of each section were measured using an image analysis and management system (Q5701W, Leica) and its software. Brain edema was measured in accordance with the trapezoid formula as follows:
V = 1/2(S1 + S2 + S2 +…+ Sn-1 + Sn-1 + Sn) × 30 × number of interval sections (n = 18). (Vischemia side− Vcorresponding side)/Vcorresponding side × 100% refers to the percentage of brain hemisphere swelling on the ischemic side induced by brain edema.
Western blotting for aquaporin-4 protein expression in the rat corpus striatum
The right corpus striatum was obtained[25], treated with protein lysate and protease inhibitor, kept in an ice bath, homogenized by ultrasound, and centrifuged at a high speed. The supernatant was boiled at 100°C for 3–5 minutes. Protein content was measured using the Bradford method. Protein was electrophoresed on a 10% (w/v) SDS-polyacrylamide gel, transferred to a polyvinylidene fluoride membrane, and blocked in phosphate buffered saline containing 5% (w/v) skim milk powder at 4°C overnight. The specimens were incubated in rabbit anti-aquaporin-4 polyclonal antibody (1: 2 000; Chemicon, Temecula, CA, USA) and mouse anti-β-tubulin antibody (Invitrogen, Carlsbad, CA, USA) at room temperature for 2 hours. Membranes were then incubated in horseradish peroxidase goat anti-rabbit secondary antibody (1: 4 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and horseradish peroxidase goat anti-mouse secondary antibody (1: 2 000; Invitrogen) at room temperature for 1 hour, followed by development using the enhanced chemiluminescence fluorescence system (Amersham, Buckinghamshire, UK). The gray value was measured using gel analysis software (Amersham). Protein sample expression was determined by calculating the gray value ratio of the target band to the internal reference.
Reverse transcription-PCR of aquaporin-4 mRNA expression in the rat corpus striatum
Total RNA was extracted from the corpus striatum using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. The aquaporin-4 primer sequence was synthesized by the Invitrogen Company: upstream: 5’-TCA GCA TCG CCA AGT CTG TC-3’, downstream: 5’-GCG CCT ATG ATT GGT CCA AC-3’, with a fragment length of 393 bp. In accordance with the Promega AMV reverse transcription kit (Promega, Madison, WI, USA), reverse transcribed cDNA species were amplified by PCR, at an annealing temperature of 58°C, 22 cycles. PCR products were measured by electrophoresis on a 2.5% (w/v) agarose gel. The gray value was determined using the Syngene SYDR-1990 system (Amersham). The gray value ratio of the target band to β-actin served as target mRNA expression.
Laser confocal microscopy of aquaporin-4 and glial fibrillary acidic protein in the rat corpus striatum
The rat corpus striatum was sliced into sections, washed, blocked, and then incubated in rabbit anti-aquaporin-4 polyclonal antibody (1:100) and mouse anti-rat glial fibrillary acidic protein antibody (1:200; Chemicon) diluted in PBS containing 1% (v/v) goat serum and 0.5% (v/v) Triton X-100 at 37°C for 1 hour, and then at 4°C overnight. Subsequently, the above-mentioned specimens were incubated in Rhodamine-labeled donkey anti-rabbit IgG (1:500; Rockland, ME, USA) and Fluorescein-labeled goat anti-mouse IgG (1:500; Rockland) IgG diluents at 37°C for 1 hour (this procedure and the following processes were performed in the dark). After washing, sections were blocked with glycerol, stored at 4°C, wrapped with aluminum foil in the dark, and then observed using a laser confocal microscope (Leica).
Statistical analysis
Data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA) and expressed as mean ± SEM. Intergroup comparisons were conducted using one-way analysis of variance or the rank-sum test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Professor Jingchun Guo and Professor Gencheng Wu from the School of Basic Medical Sciences, Fudan University in China for providing study design and technical support.
Footnotes
Funding: This project was funded by the National Natural Science Foundation of China (Youth), No. 81001556.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Nanjing University of Chinese Medicine, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Cheng HY, Wei JZ/Qiu Y/Wang L)
REFERENCES
- [1].Begley DJ. Understanding and circumventing the blood- brain barrier. Acta Paediatr Suppl. 2003;92(443):83–91. doi: 10.1111/j.1651-2227.2003.tb00226.x. [DOI] [PubMed] [Google Scholar]
- [2].Zou W, Sun XW, Yu XP, et al. Research progress on blood-brain barrier and cerebral ischemia-reperfusion injury. Zhonghua Zhongyiyao Xuekan. 2009;27(3):466–469. [Google Scholar]
- [3].Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- [4].Amiry-Moghaddam M, Hoddevik EH, Ottersen OP. Aquaporins: multifarious roles in brain. Neuroscience. 2010;168(4):859–861. doi: 10.1016/j.neuroscience.2010.04.071. [DOI] [PubMed] [Google Scholar]
- [5].Aoki K, Uchihara T, Tsuchiya K, et al. Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol. 2003;106(2):121–124. doi: 10.1007/s00401-003-0709-y. [DOI] [PubMed] [Google Scholar]
- [6].Ribeiro Mde C, Hirt L, Bogousslavsky J, et al. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83(7):1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- [7].Peng YJ, Wang HS, Sun JH, et al. The relationship between aquaporin-4 and brain edema. Linchuang he Shiyan Yixue Zazhi. 2012;11(1):69–70. [Google Scholar]
- [8].Wang SQ. Wuhan: Hubei University of Chinese Medicine; 2009. The Protective effect of electro-acupuncture plus moxibustion on acute cerebral ischemia-reperfusion injury in rats’ BBB. [Google Scholar]
- [9].Liu XN, Li YZ, Sun C. The effect of acupuncturing on ultrastructure of BBB and rheology of iCVD in experimental rabbits. Mudanjiang Yixueyuan Xuebao. 2002;23(2):1–4. [Google Scholar]
- [10].Wu J. Beijing: People's Medical Publishing House; 2005. Neurology. [Google Scholar]
- [11].Zilles K, Hajós F, Kálmán M, et al. Mapping of glial fibrillary acidic protein-immunoreactivity in the rat forebrain and mesencephalon by computerized image analysis. J Comp Neurol. 1991;308(3):340–355. doi: 10.1002/cne.903080303. [DOI] [PubMed] [Google Scholar]
- [12].Saadoun S, Papadopoulos MC, Krishna S. Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: immunohistochemical case review. J Clin Pathol. 2003;56(12):972–975. doi: 10.1136/jcp.56.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nico B, Frigeri A, Nicchia GP, et al. Role of aquaporin-4 water channel in the development and integrity of the blood-brain barrier. J Cell Sci. 2001;114(Pt 7):1297–1307. doi: 10.1242/jcs.114.7.1297. [DOI] [PubMed] [Google Scholar]
- [14].Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [15].Venero JL, Vizuete ML, Machado A, et al. Aquaporins in the central nervous system. Prog Neurobiol. 2001;63(3):321–336. doi: 10.1016/s0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- [16].Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- [17].Yoneda K, Yamamoto N, Asai K, et al. Regulation of aquaporin-4 expression in astrocytes. Brain Res Mol Brain Res. 2001;89(1-2):94–102. doi: 10.1016/s0169-328x(01)00067-5. [DOI] [PubMed] [Google Scholar]
- [18].Papadopoulos MC, Manley GT, Krishna S, et al. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- [19].Taniguchi M, Yamashita T, Kumura E, et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78(1-2):131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- [20].Golden PL, Pollack GM. Blood-brain barrier efflux transport. J Pharm Sci. 2003;92(9):1739–1753. doi: 10.1002/jps.10424. [DOI] [PubMed] [Google Scholar]
- [21].Yukutake Y, Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2010;168(4):885–891. doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- [22].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [23].Peng YJ, Chen L, Guo JC, et al. Improvement of methods for rat model of focal cerebral ischemia reperfusion. Liaoning Zhongyiyao Daxue Xuebao. 2010;12(11):48–50. [Google Scholar]
- [24].Peng YJ, Zhou F, Gu J, et al. Regulative effect of electroacupuncture on aquaporin-4 in rats with focal cerebral ischemia/reperfusion. Zhenci Yanjiu. 2007;32(2):3–6. [PubMed] [Google Scholar]
- [25].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


