Abstract
During human walking, perturbations to the upper body can be partly corrected by placing the foot appropriately on the next step. Here, we infer aspects of such foot placement dynamics using step-to-step variability over hundreds of steps of steady-state walking data. In particular, we infer dependence of the ‘next’ foot position on upper body state at different phases during the ‘current’ step. We show that a linear function of the hip position and velocity state (approximating the body center of mass state) during mid-stance explains over 80% of the next lateral foot position variance, consistent with (but not proving) lateral stabilization using foot placement. This linear function implies that a rightward pelvic deviation during a left stance results in a larger step width and smaller step length than average on the next foot placement. The absolute position on the treadmill does not add significant information about the next foot relative to current stance foot over that already available in the pelvis position and velocity. Such walking dynamics inference with steady-state data may allow diagnostics of stability and inform biomimetic exoskeleton or robot design.
Keywords: biomechanics, foot placement, walking, stability, control, dynamics
1. Introduction
Humans use active neuromuscular control to stabilize their top-heavy bodies during walking [1]. Mathematical models [1–3] suggest the effectiveness of appropriate foot placement to avoid falling forward or sideways: a person falling rightwards could produce a corrective leftward force effectively by placing the next foot to the right of its usual position. Experiments involving external mechanical [3] and visual perturbations [4] have found some evidence for such foot placement dynamics, likely due to both active control and passive dynamics. In this article, we infer plausible foot placement dynamics without such external perturbations, using natural step-to-step variability in steady-state walking data; see figure 1a for foot placement variability. Such natural variability might contain stabilizing responses to internal muscle and sensory noise [5–7]. Exploiting such variability [5–7], we infer how the foot position depends on the state of the upper body (pelvis) state during the previous step, explaining a large fraction of the random-looking variability in figure 1a.
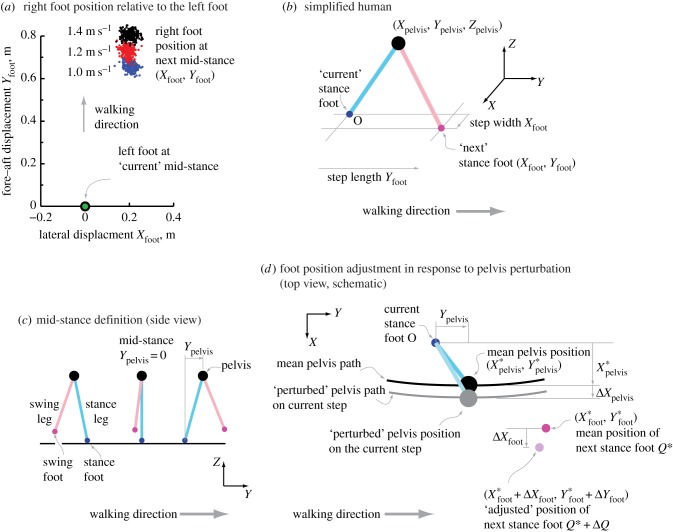
Figure 1.
Describing walking motion with pelvis and foot positions. (a) The right-foot position at mid-stance relative to the previous left-foot stance position, shown for a single subject, for each of about 265 strides at 1 m s−1 (blue dots), 1.2 m s−1 (red) and 1.4 m s−1 (black). (b) Human walking motion represented by pelvis position and two foot positions. (c) Mid-stance is defined as when the swing foot has the same fore–aft position as the pelvis. The stance foot position is assumed fixed at the foot position at mid-stance. (d) A schematic showing a deviation from the average pelvis trajectory and a corresponding change in the next stance foot position.
2. Material and methods
(a). Experimental
Subjects gave informed consent. Subjects (N = 10, eight male, two female, age 27.50 ± 5.10 years, height 1.74 ± 0.11 m, body mass 76.8 ± 14.2 kg; mean ± s.d.) walked on a treadmill for 5 min each at three constant speeds: at 1.0, 1.2 and 1.4 m s−1 for five subjects and at 0.98, 1.25 and 1.43 m s−1 for the rest, averaging 265 strides per trial (s.d. 36). Walking motions were recorded using a marker-based motion capture system (Vicon T20, position error < 0.3 mm), with four markers on each foot including an ankle marker and three markers on the upper pelvic region, close to the level of the body center of mass (see the electronic supplementary material for marker locations). No forces were measured.
(b). Describing the walking motion with pelvis and foot positions
For simplicity, we represent human walking in three dimensions by three salient points (figure 1b): one for the ‘pelvis’, a weighted sum of the three markers approximating the body centre of mass (see the electronic supplementary material), and one for each foot (ankle). Here, Y is fore–aft, X is lateral (sideways and rightwards) and Z is vertical. These axes do not rotate relative to the ground. Figure 1b shows the pelvis position (Xpelvis, Ypelvis, Zpelvis) and the position of the ‘next’ stance foot (Xfoot, Yfoot, 0), measured relative to the position of the ‘current’ stance foot (origin O). We define ‘mid-stance’ of each stance phase as when the pelvis has the same fore–aft position as the stance foot (figure 1c). During each stance phase, the current stance foot (origin O) is considered fixed at its mid-stance value and travels with the treadmill belt. Thus, mid-stance is Ypelvis = 0. We normalize the ‘distance from mid-stance’ Ypelvis by the trial's mean stride length Dstride to obtain ϕ = Ypelvis/Dstride, a proxy for the ‘phase’ of the system along the gait cycle. If ϕ = 0 is a left mid-stance, the next right mid-stance is around ϕ = +0.5. The swing foot is the foot contralateral to the current stance foot (figure 1c) and eventually becomes the next stance foot.
(c). Inferring foot placement dynamics
During each step, the pelvis state P(ϕ) as a function of distance from mid-stance ϕ is
noting equivalence of Ypelvis and ϕ. The next (contralateral) stance foot position is Q = (Xfoot, Yfoot). For each trial, P*(ϕ) and Q* are averages of these quantities over all strides.
Given pelvis state P(ϕ), we wish to predict the next foot position Q (figure 1d). We seek a linear relation between the deviation from the mean pelvis state 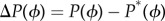 and the deviation from the mean foot position ΔQ = Q − Q*
and the deviation from the mean foot position ΔQ = Q − Q*
| 2.1 |
Here, J(ϕ) is a 2 × 5 matrix, the partial derivative (sensitivity or Jacobian or regression coefficients matrix) of the foot position with respect to the pelvis state P(ϕ), for example, the (1, 1) element of J(ϕ) is 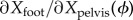 . The elements have units consistent with displacements in metres and time in seconds. Each trial of steady human walking gives P(ϕ) and Q for hundreds of strides. We estimate J(ϕ) by ordinary least squares (electronic supplementary material).
. The elements have units consistent with displacements in metres and time in seconds. Each trial of steady human walking gives P(ϕ) and Q for hundreds of strides. We estimate J(ϕ) by ordinary least squares (electronic supplementary material).
We also obtained linear relations between other sets of input and output variables, for instance, including additional input variables such as swing foot position and velocity (denoted R(ϕ)) or the absolute position of the person on the treadmill, to see whether these variables improve predictive power (see the electronic supplementary material).
3. Results
(a). Step in the direction of the fall
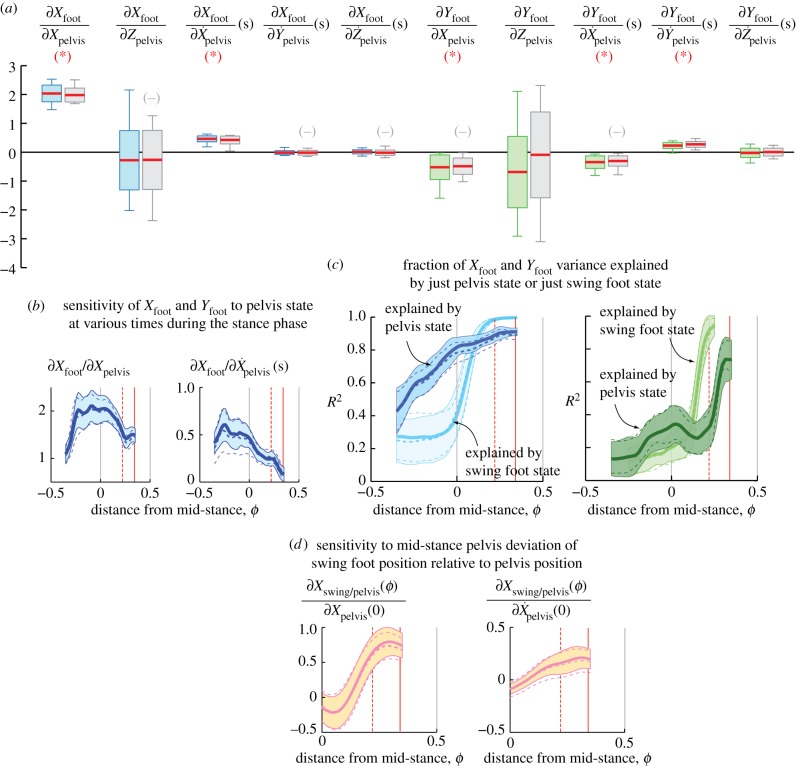
Figure 2a shows the estimated elements of the matrix J(0), relating the mid-stance pelvis state P(0) to the next foot position (Xfoot, Yfoot), pooled over subjects and speeds. The J(0) elements did not show significant speed dependence (electronic supplementary material, figure S1). Five out of 10 elements of J(0) are significantly different from zero, with their 95% CI bounded away from zero. Surrogate data analysis (electronic supplementary material, figures S2 and S3) performed by shuffling the input data sequence for given output sequence showed that these non-zero coefficients were significant at p = 0.05.
Figure 2.
Foot placement dynamics. (a) The estimated partial derivative J(0) of the foot position with respect to pelvis state at mid-stance P(0); box-plot shows mean across subjects and trials (red midline), standard deviation over all trials (boxes) and 95% interval (whiskers, 2.5 to 97.5 percentile). Colour shading indicates left to right transitions and grey indicates right to left. A negative sign (−) indicates display of the sign-reversed coefficients for some right to left transitions; red asterisks indicate coefficients statistically different from zero (p < 0.05). (b) Sensitivity of foot position on selected pelvis states at different phases ϕ; mean (thick solid line) ± s.d. (coloured band) are shown for left to right transitions; see the electronic supplementary material, figure S4. (c) Fraction of foot position variance explained by phase-dependent pelvis state (dark colours) or the phase-dependent swing foot state (light colours). (d) The sensitivity of the swing foot relative to the pelvis, in response to mid-stance pelvis deviations. In panels (b–d), red dashed (ϕ = 0.22) and solid (ϕ = 0.34) lines roughly indicate, respectively, moments just before heel-strike and just after push-off, with double stance between; solid lines and filled bands are for left to right transitions and dashed lines are for right to left transitions.
Using only the significantly non-zero J(0) elements, we find that the sideways foot position Xfoot is mainly affected by sideways pelvis position and velocity
| 3.1 |
Thus, in response to an extra rightward pelvis deviation (falling rightwards), the subject will step more to the right than usual, thereby stepping in the direction of the fall. Specifically, equation (3.1) predicts that deviations of 1 cm and 1 cm s−1 in rightward hip position and speed, respectively, will result in a 2.45 cm rightward deviation of the next stance foot. Equation (3.1) applies to transitions from left to right stance or vice versa.
Analogously, the forward foot position Yfoot is
| 3.2 |
This expression corresponds to transitions from left to right stance phases: rightward pelvis perturbations during left mid-stance imply shorter right steps; larger forward speeds imply longer steps. For right to left transitions, we switch ‘left’ and ‘right’ in the previous statement; the equation is similar except for sign changes due to ‘leftward’ being the ‘negative’ X-direction: 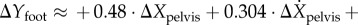
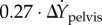 . These coefficients show approximate left–right mirror-symmetry of walking.
. These coefficients show approximate left–right mirror-symmetry of walking.
(b). Dependence of coefficients on distance to mid-stance
Figure 2b shows that the estimated J(ϕ) varies systematically with distance ϕ from mid-stance. In particular, the coefficients 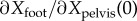 and
and 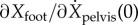 decrease with increase in ϕ beyond mid-stance. The electronic supplementary material has detailed depictions of these ϕ-dependent regression results for various input–output pairs: from pelvis state P(ϕ) to Q (electronic supplementary material, figure S4), from swing foot state R(ϕ) to Q (electronic supplementary material, figure S5) and from (P(ϕ), R(ϕ)) to Q (electronic supplementary material, figure S6). The phase-dependent linear relations (figure 2b; electronic supplementary material, figure S4) could provide a phase-dependent target for the foot position.
decrease with increase in ϕ beyond mid-stance. The electronic supplementary material has detailed depictions of these ϕ-dependent regression results for various input–output pairs: from pelvis state P(ϕ) to Q (electronic supplementary material, figure S4), from swing foot state R(ϕ) to Q (electronic supplementary material, figure S5) and from (P(ϕ), R(ϕ)) to Q (electronic supplementary material, figure S6). The phase-dependent linear relations (figure 2b; electronic supplementary material, figure S4) could provide a phase-dependent target for the foot position.
(c). Mid-stance pelvis state explains sideways foot position
The pelvis state at mid-stance explains about 81% of the next Xfoot variance (R2 values, figure 2b), almost entirely due to variables in equation (3.1). The pelvis state just before heel-strike (ϕ = 0.22) can explain a higher fraction (89%) of the Xfoot variance. The mid-stance pelvis state can also explain 61% of the variance of sideways foot position relative to pelvis at heel-strike. Mid-stance pelvis state explains about 33% of Yfoot variance.
Notably, mid-stance pelvis state explains Xfoot variance better than the mid-stance swing foot state (81% versus 40%, figure 2b). Adding mid-stance swing foot state to the pelvis state as a regressor does not give us any more than 81% variance explanation (electronic supplementary material, figure S6). Thus, at mid-stance, the pelvis ‘knows’ much more about the future foot position than the foot itself. The swing foot becomes the next stance foot position and so, eventually overtakes the pelvis state's predictive ability (figure 2b). Assuming linearity, these trends suggest that most swing foot deviation typically happens after mid-stance.
Regressions from mid-stances of even earlier steps find that the Jacobian coefficients and the foot position variance fraction explained both approach zero (electronic supplementary material, figure S10). The Xfoot variance explained is 13% instead of 81% if we used the previous ipsilateral mid-stance pelvis state.
(d). Relative motion of swing foot and pelvis
For instance, the swing foot position relative to pelvis is (Xswing/pelvis, Yswing/pelvis), where Xswing/pelvis = (Xswing − Xpelvis), etc. Figure 2d shows that the partial derivatives of the swing-foot–pelvis separation with respect to sideways deviations are positive by heel-strike (electronic supplementary material, figure S8). Thus, while a rightward pelvis deviation at mid-stance will result in further rightward pelvis deviation between mid-stance and heel-strike, it will result in an even greater rightward deviation of the next foot position; thus, we can rule out the foot placement deviation being entirely due to the pelvis deviation with the foot being rigidly carried by the pelvis.
(e). Station-keeping: effect of position on treadmill
A treadmill's finite dimensions may affect walking dynamics: humans may wish to avoid the treadmill's edge (electronic supplementary material, figure S11). However, including the person's position on the treadmill as a regressor in addition to the pelvis state explains only 2–5% more foot position variance (electronic supplementary material, figure S9). The subjects' position on the treadmill drifts slowly and is not as well controlled as limb states relative to the body, perhaps because station-keeping is less important than not falling. These results were not affected by the substantial treadmill width differences between subjects 1–5 and 6–10 (widths 0.92 m versus 0.51 m; electronic supplementary material, figure S1).
4. Discussion
By fitting linear relations to variability in steady-state walking data, we have inferred foot placement dynamics consistent with the idea that the foot positions change in the perturbation direction. These foot position dependencies could be due to active feedback control in response to pelvis state deviations, or due to passive dynamics and feed-forward control. Thus, most conservatively, our results are only about the pelvis state's (i.e. approximate centre of mass state's) predictive ability of the next foot position. Nevertheless, we have shown that what seems ‘random variability’ in figure 1a can be explained by a simple linear function of pelvis state deviations far in advance of heel-strike. Also, the foot position dependence on state at different gait phases ϕ (figure 2b; electronic supplementary material, S4 and S5) provides a more general description of foot position dynamics, which could be used in a foot-position controller, say, in an exoskeleton for walking assistance.
Equations (3.1) and (3.2) are formal data-derived versions of those previously proposed [1,3]. By showing that all terms except the sideways position and velocity terms drop out of equation (3.1), our results support the assumptions in the so-called extrapolated centre of mass model [3], similar to our simple linear models but not allowing for phase-dependence and arbitrary regression; further study is necessary to distinguish which model is better. Stride length reduction in equation (3.2) may be analogous to shorter stride times in perturbation experiments [3].
Fitting a controlled mechanics-based model to the data may explain the systematic trends in regressed coefficients J(ϕ). Perturbation experiments could test whether inferred relations from steady-state data can reliably predict consequences of external perturbations. Performing external perturbations [3,4] and inferring the subsequent inputs to the leg muscles (from electromyography or inverse dynamics) can delineate the relative importance of feedback and feed-forward control.
Detailed inference of human walking controller could inform diagnoses of human stability and the design of biomimetic assistive exoskeletons and walking robots. Future work on such controller inference could also consider overground experiments, longer term correlations and planning, more degrees of freedom, fits to a muscle-driven mechanics-based model and nonlinear controller models.
Supplementary Material
Ethics statement
The protocol was approved by OSU's Institutional Review Board.
Data accessibility
Data is available through Dryad (doi:10.5061/dryad.5kh00).
Funding statement
This work was supported in part by NSF grant no. 1254842.
References
- 1.Bauby CE, Kuo AD. 2000. Active control of lateral balance in human walking. J. Biomech. 33, 1433–1440. ( 10.1016/S0021-9290(00)00101-9) [DOI] [PubMed] [Google Scholar]
- 2.Redfern MS, Schumann T. 1994. A model of foot placement during gait. J. Biomech. 27, 1339–1346. ( 10.1016/0021-9290(94)90043-4) [DOI] [PubMed] [Google Scholar]
- 3.Hof AL, Vermerris SM, Gjaltema WA. 2010. Balance responses to lateral perturbations in human treadmill walking. J. Exp. Biol. 213, 2655–2664. ( 10.1242/jeb.042572) [DOI] [PubMed] [Google Scholar]
- 4.O'Connor SM, Kuo AD. 2009. Direction-dependent control of balance during walking and standing. J. Neurophysiol. 102, 1411 ( 10.1152/jn.00131.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurmuzlu Y, Basdogan C. 1994. On the measurement of dynamic stability of human locomotion. J. Biomech. Eng. 116, 30–36. ( 10.1115/1.2895701) [DOI] [PubMed] [Google Scholar]
- 6.Dingwell JB, Cusumano JP. 2001. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. J. Biomech. Eng. 123, 27–32. ( 10.1115/1.1336798) [DOI] [PubMed] [Google Scholar]
- 7.Revzen S, Guckenheimer JM. 2012. Finding the dimension of slow dynamics in a rhythmic system. J. R. Soc. Interface 9, 957–971. ( 10.1098/rsif.2011.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available through Dryad (doi:10.5061/dryad.5kh00).