Abstract
Levels of UVB radiation (UVB) and mean temperatures have increased substantially over recent decades in many regions of the world. Both stressors independently can compromise immune function, disease resistance and fitness in fish. The impact of UVB can also be exacerbated by interactions with environmental temperatures. In this paper, we test the hypothesis that UVB and temperature act synergistically to influence patterns of energy consumption and susceptibility to disease. We exposed mosquitofish, Gambusia holbrooki, to a factorial design of low and high UVB levels and low (18°C) and high (25°C) temperatures. The combination of high UVB and high temperature interacted synergistically to suppress metabolism and exacerbate infection intensity by the fish pathogen whitespot (Ichtyhophthirius multifiliis). Given the rapid changes in the thermal environment globally, the interaction between UVB and temperatures on energy use and disease resistance could pose significant problems for aquatic animal health in the context of both pre-existing and emerging diseases.
Keywords: UV radiation, infection, climate change, temperature, synergistic interaction, disease
1. Introduction
UVB radiation (UVB, 295–320 nm) impairs cellular function, and its impact manifests at all levels of biological organization [1,2]. Increased UVB levels can have detrimental effects on growth rates, development, locomotor performance and survival in both vertebrate and invertebrate species [3–6]. UVB also suppresses immune function and impairs resistance to pathogens, which can influence population persistence [7–9].
The impact of UVB can be exacerbated by interactions with other environmental drivers [3,6,10–12]. Temperature, in particular, can modulate the effects of UVB; for example, simultaneous exposure of amphibian embryos to low temperatures and high UV radiation levels caused a synergistic increase in mortality [6]. The potential of such an interaction is of particular concern in the context of climate warming [1,2]. High UVB can impair the immune system [9,10], and high temperature can increase pathogen loads [13]. Many vertebrate species are constrained by pathogen loads [14], and a synergistic interaction between UVB and increasing temperatures on disease resistance could have important consequences. Conversely, the efficacy of immune responses [15] and UVB-induced damage repair rates may increase with temperature [16], so that the interaction between UVB and temperature may be synergistic or antagonistic.
We investigated whether UVB and environmental temperatures interact to increase the susceptibility of mosquitofish (Gambusia holbrooki) to infection by the ectoparasite whitespot (Ichtyhophthirius multifiliis). We hypothesized that fish exposed to high UVB and low temperatures would have a greater susceptibility to whitespot than fish from the higher temperatures.
2. Material and methods
(a). Study animals and treatments
Adult eastern mosquitofish, G. holbrooki (mass = 0.087 ± 0.02 g, mean ± s.d.), were collected from the University of Queensland, St Lucia campus and exposed to a factorial combination of two UVB treatments (low UVB and high UVB) and two temperature treatments (low, 18°C and high, 25°C) such that there were four experimental treatments with 10 replicate tanks in each. Each tank consisted of a 2 l plastic container with 700 ml of aged water (aerated continuously) and 15 fish. Treatment temperatures reflect the maximum and minimum water temperatures recorded 5–10 cm below the water surface at the collection site over a 24 h period, and the thermal range for the infective phase of the whitespot pathogen.
All fish were exposed to a 12 h L : 12 h D light cycle generated by four 40 W full spectrum (UVB, UVA and visible wavelengths) fluorescent tubes (Repti Glo 5.0, Exo Terra, Montreal, Canada; UVB irradiance = 0.23 ± 0.05 W m−2) for 10 h d−1 with an additional pulse of high UV for 2 h in the middle of the day (4 × Repti Glo 10.0 tubes; Exo Terra; UVB irradiance = 0.47 ± 0.1 W m−2; electronic supplementary material, figure S1 and table S1). In the low UVB treatments, fluorescent lights were shielded with UVB retardant film (Energy Control Window Film, Handihomes, Australia; peak UVB irradiance = 0.05 ± 0.02 W m−2, baseline UVB = 0.01 ± 0.01 W m−2). The peak irradiance of UVB generated by our high UV lighting regime was equivalent to a UV index of approximately 1.7 (see electronic supplementary material). Fish were fed daily with goldfish flakes. Fish were exposed to experimental treatments for 10 days, after which the UV lights were switched off. Fish remained in thermal treatments until the end of the whitespot exposure period.
(b). Pathogen challenge
Whitespot (I. multifiliis) theronts were harvested from infected black mollys (Poecilia sphenops) (see electronic supplementary material). Following the 10 day UVB exposure, approximately 1300 live theronts per fish were added to half of the tanks in each treatment. Fish were maintained in the absence of UV for an additional 8 days to allow the whitespot parasites to develop until they were visible. Fish were then euthanased (AQUI-S, New Zealand; 175 mg l−1) and both lateral surfaces photographed using a Nikon digital camera attached to a Carl Zeiss stereo dissecting microscope. The number of infected fish and the number of whitespot lesions per fish were quantified using SigmaScanPro (SyStat Software, California, USA).
(c). Metabolic rate
Resting metabolic rate (RMR) was measured immediately after UVB exposure as an indicator of the energetic cost of responding to UVB radiation (e.g. repair of UVB-induced damage). RMR was determined as the rate of oxygen consumption. Fish were sealed into individual 20 ml plastic syringes containing air-saturated tank water and an oxygen-sensitive sensor spot (5 mm, PSt3; Presens, Regensburg, Germany). Temperature-compensated measures of dissolved oxygen concentration in the syringes were made after 20–30 min, depending on water temperature, with an optical fibre cable (PreSens) connected to a Fibox 3 oxygen meter (PreSens). Oxygen uptake rate was calculated using the equation:
where 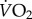 is oxygen consumption rate (ml O2 h−1); ma, slope of O2 consumption by the fish over the trial (ml h−1); mc, slope of O2 consumption in a control (blank) respirometer over the trial (ml h−1) and V, volume of water in the respirometer (l). Body mass was considered as a covariate in the statistical analysis.
is oxygen consumption rate (ml O2 h−1); ma, slope of O2 consumption by the fish over the trial (ml h−1); mc, slope of O2 consumption in a control (blank) respirometer over the trial (ml h−1) and V, volume of water in the respirometer (l). Body mass was considered as a covariate in the statistical analysis.
(d). Statistical analysis
All statistical analyses were conducted using R [17]. The number of fish infected with whitespot (prevalence) was compared between the treatments using binomial logistic regression with UV, temperature and the UV × temperature interaction as fixed factors (stats package [17]). We performed a non-parametric bootstrap analysis of the coefficients of the binomial generalized linear model (10 000 replicates) and calculated bootstrap standard errors and 95% confidence intervals for the model parameters using the BCa method [18] in the boot package [19]. We used linear mixed effects models [20,21] to examine the effects of experimental treatments on metabolic rates and whitespot infection intensity. UV level, temperature and the UV × temperature interaction were considered as fixed factors and tank as a random factor. Body mass was considered as a covariate in the analysis of metabolic rate.
3. Results
(a). Metabolic rate
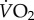 was influenced by body mass (F1,92.3 = 40.37, p < 0.0001), but there was no difference in fish masses across treatments (one-way ANOVA; F3,91 = 1.02, p = 0.39). There was a significant interaction between temperature and UVB exposure level (F1,44 = 4.43, p < 0.05). At 25°C metabolic rates were 22% lower in the high UVB treatment group compared with the low UVB treatment group (figure 1).
was influenced by body mass (F1,92.3 = 40.37, p < 0.0001), but there was no difference in fish masses across treatments (one-way ANOVA; F3,91 = 1.02, p = 0.39). There was a significant interaction between temperature and UVB exposure level (F1,44 = 4.43, p < 0.05). At 25°C metabolic rates were 22% lower in the high UVB treatment group compared with the low UVB treatment group (figure 1).
Figure 1.
The effects of UVB and temperature on metabolic rate of G. holbrooki. There was no difference in body mass between treatments (p = 0.39). Treatments with the same lower case letter are not significantly different. (Online version in colour.)
(b). Whitespot infection
Whitespot infection prevalence was high in all treatment groups (approx. 80–100%; figure 2a). There was no effect of temperature (β = 18.68, error = 3.93, confidence intervals = −0.67, 20.80) or UVB (β = −0.46, error = 4.56, confidence intervals = −2.46, 1.3) or their interaction (β = −18.58, error = 4.48, confidence intervals = −20.80, 0.94) on infection prevalence. An interaction between high UVB and high temperature (F1,115 = 56.63; p < 0.0001; figure 2b) shows that this combination caused a threefold increase in whitespot infection intensity.
Figure 2.
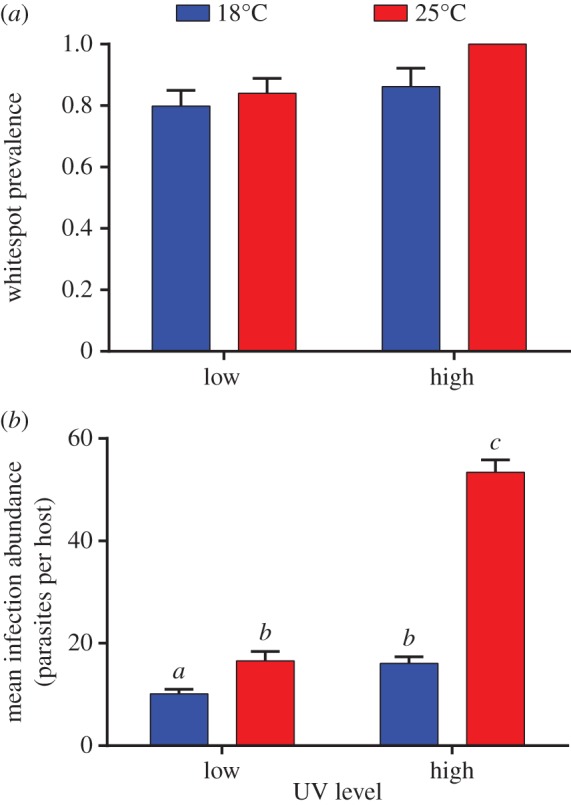
The effects of UVB and temperature on (a) the prevalence and (b) the intensity of infection with whitespot, I. multifiliis, in G. holbrooki. Treatments with the same lower case letter are not significantly different. (Online version in colour.)
4. Discussion
We show that UVB interacts with temperature to exacerbate deleterious impacts on animal health that lead to reduced disease resistance in mosquitofish. Fish in the high temperature, high UVB treatment experienced a large increase in whitespot infection intensity relative to other treatment groups, suggesting that the detrimental effects of UVB are magnified when multiple stressors (high temperature and high UVB) are combined. Juvenile salmon exposed to high water temperatures and increased levels of UVB experienced reduced innate immune function [22], suggesting these stressors promote pathogen susceptibility in fish through their effect on the immune system.
Interactions between high temperatures and high UVB suppressed RMR by 22% in G. holbrooki. A similar effect of UVB on RMR has been demonstrated in amphibian larvae [3]. Lower RMR may signify an accumulation of damage to important structural or metabolic components of cells which could depress metabolism and preclude repair [23]. Hence there may be an allocation trade-off when energy production is constrained by temperature and the high UVB levels. Under these circumstances, the capacity to sustain immune function may be compromised, leading to increased disease susceptibility. Damage to mitochondria [24] could also decrease maximal metabolic rates and thereby metabolic scope, which may explain observed reductions in activity levels in the presence of UVB [3].
These findings have important implications for aquatic disease ecology in the context of global climate change [25]. While multiple stressors can influence the susceptibility to diseases in hosts, they may also influence disease progression by affecting the infectivity of parasites and pathogens. A greater appreciation of the effects of multiple stressors at both host and pathogen levels is important for our understanding of future disease risks.
Ethics statement
This research was approved by the University of Queensland Animal Welfare Unit (permit number SBS/022/10).
Data accessibility
Raw data files can be accessed from the Dryad data repository (doi:10.5061/dryad.74b31).
Supplementary Material
Acknowledgements
The authors thank Dr Simon Blomberg for statistical advice.
Funding statement
This research was supported by an Australian Research Council Discovery grant to C.E.F. and F.S. (DP140102773).
References
- 1.Williamson CE, et al. 2014. Solar ultraviolet radiation in a changing climate. Nat. Clim. Change 4, 434–441. ( 10.1038/NCLIMATE2225) [DOI] [Google Scholar]
- 2.Hader DP, Helbling EW, Williamson CE, Worrest RC. 2011. Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 10, 242–260. ( 10.1016/S0169-5347(02)00014-9) [DOI] [PubMed] [Google Scholar]
- 3.Alton LA, White CR, Wilson RS, Franklin CE. 2011. The energetic cost of exposure to UV radiation for tadpoles is greater when they live with predators. Funct. Ecol. 26, 93–103. ( 10.1111/j.1365-2435.2011.01900.x) [DOI] [Google Scholar]
- 4.Jokinen EI, Salo HM, Markkula SE, Immonen AK, Aaltonen TM. 2001. Ultraviolet B irradiation modulates the immune system of fish (Rutilus rutilus, Cyprinidae) part III: lymphocytes. Photochem. Photobiol. 73, 505–512. ( 10.1562/0031-8655(2001)0730505UBIMTI2.0.CO2) [DOI] [PubMed] [Google Scholar]
- 5.Macias G, Marco A, Blaustein AR. 2007. Combined exposure to ambient UVB radiation and nitrite negatively affects survival of amphibian early life stages. Sci. Total Environ. 385, 55–65. ( 10.1016/j.scitotenv.2007.06.016) [DOI] [PubMed] [Google Scholar]
- 6.van Uitregt VO, Wilson RS, Franklin CE. 2007. Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Glob. Change Biol. 13, 1114–1121. ( 10.1111/j.1365-2486.2007.01353.x) [DOI] [Google Scholar]
- 7.Jokinen IE, Markkula ES, Salo HM, Kuhn P, Nikoskelainen S, Arts MT, Browman HI. 2008. Exposure to increased ambient ultraviolet B radiation has negative effects on growth, condition and immune function of juvenile Atlantic salmon (Salmo salar). Photochem. Photobiol. 84, 1265–1271. ( 10.1111/j.1751-1097.2008.00358.x) [DOI] [PubMed] [Google Scholar]
- 8.Markkula SE, Salo HM, Immonen AK, Jokinen EM. 2005. Effects of short- and long-term ultraviolet B irradiation on the immune system of the common carp (Cyprinus carpio). Photochem. Photobiol. 81, 595–602. ( 10.1562/2004-07-13-RA-231.1) [DOI] [PubMed] [Google Scholar]
- 9.Salo HM, Jokinen EI, Markkula SE, Aaltonen TM, Penttila HT. 2000. Comparative effects of UVA and UVB irradiation on the immune system of fish. J. Photochem. Photobiol. B 56, 154–162. ( 10.1016/S1011-1344(00)00072-5) [DOI] [PubMed] [Google Scholar]
- 10.Bancroft BA, Baker NJ, Blaustein AR. 2008. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 22, 987–996. ( 10.1111/j.1523-1739.2008.00966.x) [DOI] [PubMed] [Google Scholar]
- 11.Searle CL, Belden LK, Bancroft BA, Han BA, Biga LM, Blaustein AR. 2010. Experimental examination of the effects of ultraviolet-B radiation in combination with other stressors on frog larvae. Oecologia 162, 237–245. ( 10.1007/s00442-009-1440-8) [DOI] [PubMed] [Google Scholar]
- 12.Storfer A. 2003. Amphibian declines: future directions. Diversity Distrib. 9, 151–163. ( 10.1046/j.1472-4642.2003.00014.x) [DOI] [Google Scholar]
- 13.Karvonen A, Rintamaki P, Jokela J, Valtonen ET. 2010. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 40, 1483–1488. ( 10.1016/j.ijpara.2010.04.015) [DOI] [PubMed] [Google Scholar]
- 14.Andreou D, Arkush KD, Guégan J-F, Gozlan RE. 2012. Introduced pathogens and native freshwater biodiversity: a case study of Sphaerothecum destruens. PLoS ONE 7, e36998 ( 10.1371/journal.pone.0036998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait NN. 1969. The effect of temperature on the immune response in cold-blooded vertebrates. Physiol. Zool. 42, 29–35. ( 10.2307/30152463) [DOI] [Google Scholar]
- 16.MacFadyen EJ, Williamson CE, Grad G, Lowery M, Jeffrey WH, Mitchell DL. 2004. Molecular response to climate change: temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria. Glob. Change Biol. 10, 408–416. ( 10.1111/j.1529-8817.2003.00750.x) [DOI] [Google Scholar]
- 17.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://wwwR-projectorg/) [Google Scholar]
- 18.Efron B, Tibshirani RJ. 1993. An introduction to the bootstrap. New York, NY: Chapman and Hall. [Google Scholar]
- 19.Canty A, Ripley B. 2014. boot: Bootstrap R (S-Plus) functions. R package version 1.3–11. http://cran.r-project.org/web/packages/boot/index.html.
- 20.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1–6 http://CRAN.R-project.org/package=lme4.
- 21.Kuznetsova A, Brockhoff PB, Christensen RHB.2013. lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 20–3. http://CRANR-projectorg/package=lmerTest .
- 22.Jokinen IE, Salo HM, Markkula E, Rikalainen K, Arts MT, Browman HI. 2011. Additive effects of enhanced ambient ultraviolet B radiation and increased temperature on immune function, growth and physiological condition of juvenile (parr) Atlantic salmon, Salmo salar. Fish Shellfish Immunol. 30, 102–108. ( 10.1016/j.fsi.2010.09.017) [DOI] [PubMed] [Google Scholar]
- 23.Fischer JM, Fields PA, Pryzbylkowski PG, Nicolai JL, Neale PJ. 2006. Sublethal exposure to UV radiation affects respiration rates of the freshwater cladoceran Daphnia catawba. Photochem. Photobiol. 82, 547–550. ( 10.1562/2005-08-30-RA-664) [DOI] [PubMed] [Google Scholar]
- 24.Cline SD. 2012. Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim. Biophys. Acta 1819, 979–991. ( 10.1016/j.bbagrm.2012.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overholt EP, Hall SR, Williamson CE, Meikle CK, Duffy MA, Cáceres CE. 2012. Solar radiation decreases parasitism in Daphnia. Ecol. Lett. 15, 47–54. ( 10.1111/j.1461-0248.2011.01707.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data files can be accessed from the Dryad data repository (doi:10.5061/dryad.74b31).


