Abstract
Fossil mesostigmatid mites (Acari: Parasitiformes: Mesostigmata) are extremely rare, and specimens from only nine families, including four named species, have been described so far. A new record of Myrmozercon sp. described here from Eocene (ca 44–49 Myr) Baltic amber represents the first—and so far only—fossil example of the derived, extant family Laelapidae. Significantly, modern species of this genus are habitually myrmecophilous and the fossil mite described here is preserved attached to the head of the dolichoderine ant Ctenobethylus goepperti (Mayr, 1868). It thus offers the oldest unequivocal evidence for an ecological association between mesostigmatid mites and social insects in the order Hymenoptera.
Keywords: Arachnida, Parasitiformes, Hymenoptera, myrmecophily, fossil record
1. Introduction
Mesostigmatid mites (Acari: Parasitiformes) are a diverse group with over 11 400 living species [1]. These small, eyeless, sometimes fast-moving arachnids are a typical component of soil and leaf-litter faunas. Many are predators on other small organisms, but a range of lifestyles and habitats have been recorded, including trends towards parasitism and other intimate associations with larger arthropods [2] or vertebrates [3,4]. Although species-rich today, the fossil record of Mesostigmata is surprisingly sparse [5, table 1] with only 14 published records (all Cenozoic) of which four comprise named species. A reason for this may be that—in contrast to acariform mites—few lineages of Mesostigmata have colonized the surfaces of trees, reducing the opportunity for them to become fossilized in tree resin as amber. Recently, fossils belonging to the Uropodina clade were described from the Rovno [6] and Baltic [5] ambers, respectively (both Eocene, ca 44–49 Ma). The mites in question were discovered through their attachment as phoretic ‘stowaways’ on larger arthropods, in these examples beetles (Coleoptera: Cryptophagidae and Cerambycidae). The latter authors suggested that the rarity of fossil mesostigmatids is probably an artefact, and that further discoveries could be made by searching for mites in phoretic (or parasitic) association with other inclusions trapped in amber.
Here, we document a new mesostigmatid mite from Baltic amber attached to the head of an ant (figures 1 and 2), the latter assignable to the species Ctenobethylus goepperti (Mayr, 1868) (Hymenoptera: Formicidae: Dolichoderinae). This is by far the most abundant ant species found in Baltic amber (ca 50% of all ants [7]). We can assign this unique mite specimen to Myrmozercon Berlese, 1902; a genus in the family Laelapidae whose living representatives are typically myrmecophilous, i.e. strongly associated with ants. In a wider context, this remarkable discovery offers the oldest evidence for an intimate (probably parasitic) relationship between mesostigmatic mites and social insects within the order Hymenoptera. This fossilized behaviour foreshadows the emergence of other species that attack hymenopterans; such as Tropilaelaps clareae Delfinado and Baker, 1961 (Laelapidae) or Varroa destructor Anderson & Trueman, 2000 from the closely related family Varroidae—mites that are of commercial importance today as pests of honeybees [8,9].
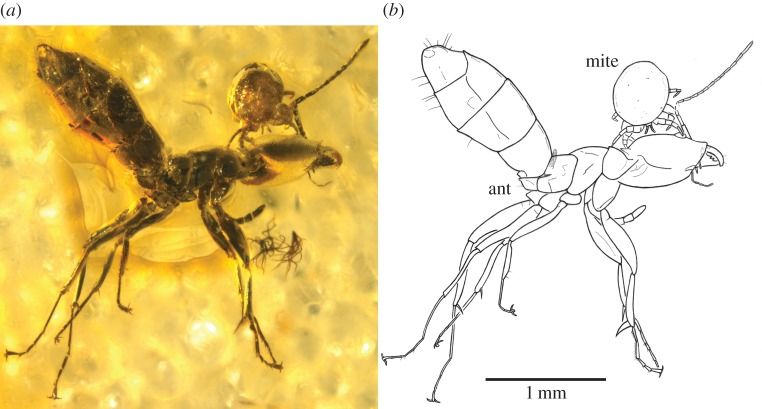
Figure 1.
F2534/BB/CJW, Myrmozercon sp. (Mesostigmata: Laelapidae) from Eocene Baltic amber attached to the dolichoderine ant Ctenobethylus goepperti (Mayr, 1868). (a) Overview. (b) Interpretative drawing. (Online version in colour.)
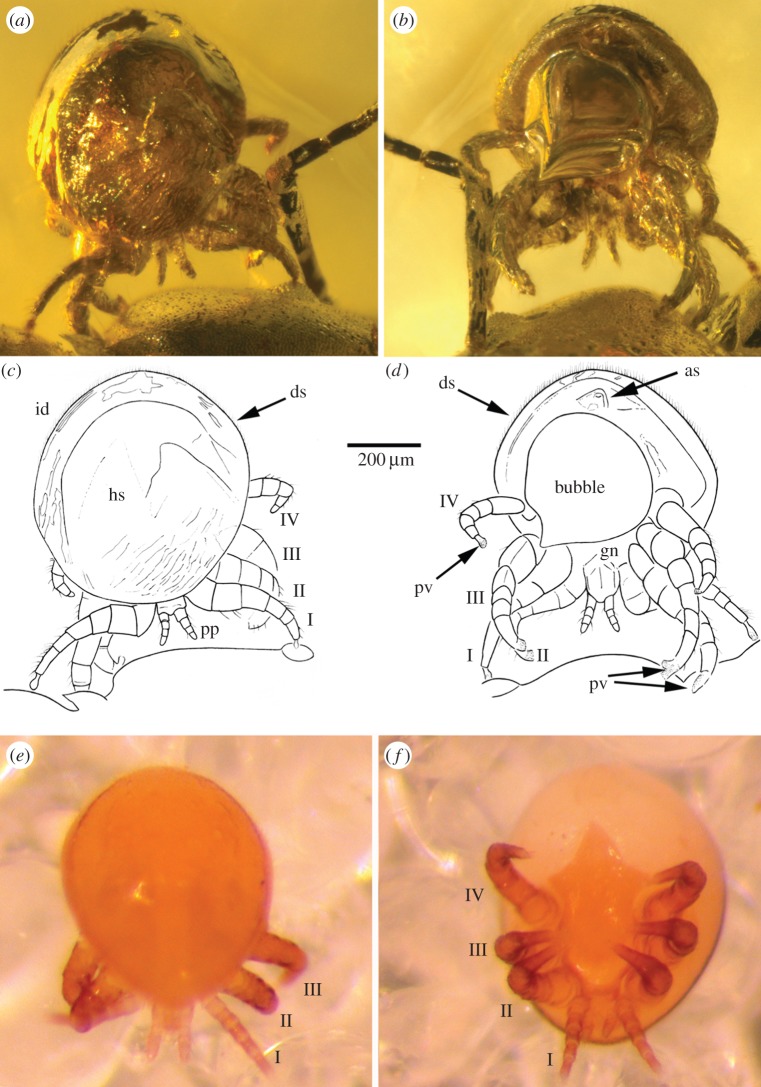
Figure 2.
Details of the fossil mite in (a) dorsal view and (b) ventral view, together with respective interpretative drawings (c,d) and comparative dorso-ventral images (e,f) of the living species Myrmozercon brevipes Berlese, 1902. as, anal shield; ds, dorsal setae; gn, gnathosoma; hs, holodorsal shield; id, idiosoma; pp, pedipalp; pv, pulvillus; legs numbered I–IV. (Online version in colour.)
2. Material and methods
The new amber specimen derives from the Jörg Wunderlich collection and bears the repository number F2534/BB/CJW. It will be transferred at a later date to the Senckenberg Institute Frankfurt/Main or one of its satellite institutions (J. Wunderlich 2012, personal communication). The ant and mite are preserved in a thin, rectangular piece of clear yellow amber with dimensions of ca 20 × 14 mm. A bubble partially obscures the ventral surface of the mite. A precise locality for the specimen is not available, but much of the recently collected Baltic amber stems from the Kaliningrad region of the Russian Baltic coast. Baltic amber is thought to have been deposited in a warm forest environment and is conventionally dated at Eocene (Lutetian), or about 44–49 Ma, but could be slightly younger [10]. For a recent overview of this amber deposit and its geological setting, see Weitschat & Wichard [11]. Specimens were studied and drawn under a Leica stereomicroscope and Zeiss Axioscope compound microscope, both with a camera lucida attachment. The specimens were photographed via the Leica Application Suite software which enabled stacks of images at various focal depths to be combined into a final picture.
3. Systematic palaeontology
Parasitiformes Reuter, 1909
Mesostigmata Canestrini, 1891
Monogynaspida Camin & Gorirossi, 1955
Gamasina Kramer, 1881
Dermanyssiae Evans & Till, 1979
Dermanyssoidea Kolenati, 1859
Laelapidae Berlese, 1892
Genus Myrmozercon Berlese, 1902
= Myrmonyssyus Berlese, 1903
= Parabisternalis Ueckermann & Loots, 1995
Type species. Myrmozercon brevipes Berlese, 1902.
Myrmozercon sp.
(a). Material
F2534/BB/CJW. Baltic amber. Palaeogene: Eocene: Lutetian.
(b). Description
Dorsal idiosoma convex, rounded to oval in outline (length 700 µm; width 650 µm); holodorsal shield present. Surface of holodorsal shield smooth; some wrinkles visible, but larger and deeper sculptural pattern not visible. Long dorsal setae not visible; probable dorsal setae short, particularly around posterior idiosomal margin, and hypertrichous. Sternal, genital and most of ventral region obscured but subtriangular anal shield visible. All legs short, stubby (lengths ca 420 µm or less, leg IV notably smaller), terminating in fan-shaped pulvillus; tarsus I ca 2× length tibia I. Gnathosoma ca 240 µm long (in ventral view); chelicerae equivocal, but palp distinct, short (100 µm), linear, tarsus obscure.
(c). Remarks
Unfortunately, important characters for differentiating known Myrmozercon such as the details of the dorsal shield, sternal and genital shields and their setation, and the chelicerae are not visible in the fossil specimen and preclude meaningful comparisons with living species. However, our knowledge of extant species of Mesostigmata that live on ants strongly supports the assignment of this fossil to Myrmozercon. Particular characters that support this assignment include: the similar body shape and size; stubby legs with mostly short, broad segments; all tarsi with well-developed, pad-like ambulacra; anal shield, and the palpal tibia apparently encompassing the tarsus (figure 2a–f). Ant-associated antennophorine Mesostigmata (Aenictequoidea, Anennophoroidea) also exhibit hypertrichy, but lack ambulacra on legs I and differ from this fossil in many other details. Other gamasine ant-associates also differ in general habitus and in particular characters including having ambulacra with strong claws, a well-developed palpal tarsus, and lacking extensive hypertrichy.
4. Discussion
For recent accounts of Myrmozercon, including a redescription of the type species, see [12–16]. Living representatives of this genus are true myrmecophiles. They are typically found in ant nests or on worker or alate ants where they are thought to be parasitic, rather than commensal, although this has not yet been demonstrated experimentally [13]. Twenty-four extant species of Myrmozercon—many originally described under the junior synonym Myrmonyssyus Berlese, 1903—are known, mostly from a single species of ant host (see comments in [13]). Some associations are unknown; M. robustisetae was described from a termite nest [16] but appears to be misplaced in the genus Myrmozercon [15]. Previous associations have been reported from a wide variety of ant genera in three subfamilies: Dolichoderinae (Iridomyrmex, Tapinoma); Formicinae (Camponotus, Myrmecocystus, Polyrhachis); Myrmicinae (Aphenogaster, Crematogaster, Messor, Tetramorium). Extant species of Myrmozercon with dense hypertrichy are associated with the dolichoderine genera Iridomyrmex and Tapinoma. Interestingly, the Baltic amber ant Ctenobethylus is very similar to Iridomyrmex, such that it was synonymized under this modern genus for 20 years before being re-instated as a distinct, extinct genus [17].
Our present discovery of a Myrmozercon mite in amber attached to an ant is significant for a number of reasons. It represents the first—and so far only—example of the derived mesostigmatid family Laelapidae, and its superfamily Dermanyssoidea, in the fossil record. Second, there has only been one other published record of ants in amber being parasitized by mites. Wheeler mentioned workers of the formicine Lasius schiefferdeckeri Mayr, 1868 with ‘gamasid’ (= mesostigmatid) mites attached to their hind tibiae [18, pp. 21–22]. That said, his original (fig. 58) drawing evidently shows a larval parasitengonid mite; an acariform group that are commonly discovered parasitizing other amber arthropods. Third, in modern ecosystems, there is considerable interest in those Mesostigmata that attack social insects [19] and, in particular, honeybees (see above). We demonstrate here that, at least for ants, ecological relationships of this nature can be traced back almost 50 Myr.
Indeed, various types of association between mesostigmatid mites and ants have been recorded in the literature. In some of these, mites express explicit morphological adaptations. A good example would be the ‘holdfast’ mechanism in the Trichocylliba subgenera (Acari: Uropodina), within which different degrees of adaptation can be observed. The genus Circocylliba has only very strong claws to attach itself to the ant's body. By contrast, in the other subgenera (Coxequesoma and Antennequesoma), the idiosoma margin is curved and helps attach the mites to the legs or the antenna (see [20] and references therein). Other types of ant association involve mites—such as our Myrmozercon here—in which specific morphological adaptations are not apparent, and the mite simply holds itself in place using its legs. Myrmozercon also lacks claws on the legs and has instead a suction-like pulvillus, also visible in the fossil (figure 2d, pv), but these features are not unique to this genus and can be found in other mites living on insects as well.
As in our amber piece, living Myrmozercon species can sometimes be found attached to the head of their ant host [15]; a specific behaviour that we can now trace back to at least the Eocene. The high host specificity noted above—i.e. one Myrmozercon species typically attaches itself to only one ant species—may be important if other fossil ants are discovered carrying mesostigmatids, the prediction being that different amber ants should host different Myrmozercon species. The high frequency of Ctenobethylus among all Baltic amber ant specimens may allow for a more precise survey of this specific association with Myrmozercon. As mentioned above, amber, being fossilized tree resin, tends to trap arboreal faunal elements more often than ground-dwelling ones, and there is at least one case of a modern Myrmozercon species being associated with an arboreal ant [12]. A number of living species from other genera of the family Laelapidae can also be found on ants [15], and our research further shows that it may be worth checking specimens of other social insects preserved in amber for associated (parasitiform) mite inclusions. It is conceivable that we shall be able to recover further palaeontological data documenting the origins and evolution of these important host–parasite relationships.
Acknowledgement
We thank Jörg Wunderlich (Hirschberg) for making material from his collection available and Ise Schmitz-Kössendrup (Berlin) and the reviewers for helpful comments.
References
- 1.Beaulieu F, Dowling APG, Klompen H, de Moraes GJ, Walter DE. 2011. Superorder Parasitiformes Reuter, 1909. In Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness (ed Zhang Z-Q.). Zootaxa 3148, 123–128. [Google Scholar]
- 2.Hunter PE, Rosario RMT. 1988. Associations of Mesostigmata with other arthropods. Annu. Rev. Entomol. 33, 393–417. ( 10.1146/annurev.en.33.010188.002141) [DOI] [Google Scholar]
- 3.Walter DE, Proctor HC. 1999. Mites. Ecology, evolution and behaviour. Wallingford, UK: CABI Publishing. [Google Scholar]
- 4.Lindquist EE, Krantz GW, Walter DE. 2009. Order Mesostigmata. In A manual of acarology (eds Krantz GW, Walter DE.), pp. 124–231, 3rd edn Lubbock, TZ: Texas Tech University Press. [Google Scholar]
- 5.Dunlop JA, Kontschán J, Zwanzig M. 2013. Fossil mesostigmatid mites (Mesostigmata: Gamasina, Microgyniina, Uropodina), associated with longhorn beetles (Coleoptera: Cerambycidae) in Baltic amber. Naturwissenschaften 100, 337–344. ( 10.1007/s00114-013-1031-8) [DOI] [PubMed] [Google Scholar]
- 6.Lyubarsky GY, Perkovsky EE. 2012. The first Eocene species of the genus Cryptophagus (Coleoptera, Clavicornia, Cryptophagidae). Vest. Zool. 46, e36–e40. ( 10.2478/v10058-012-0007-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dlussky GM, Rasnitsyn AP. 2009. Ants (Insecta: Vespida: Formicidae) in the Upper Eocene amber of Central and Eastern Europe. Paleontol. J. 43, 1024–1042. ( 10.1134/S0031030109090056) [DOI] [Google Scholar]
- 8.Sammataro D, Gerson U, Needham G. 2000. Parasitic mites of honey bees: life history, implications and impact. Ann. Rev. Entomol. 45, 519–548. ( 10.1146/annurev.ento.45.1.519) [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. J. Invert. Pathol. 103, 96–119. ( 10.1016/j.jip.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 10.Standke G. 1998. Die Tertiärprofile der Samländischen Bernsteinküste bei Rauschen. Schrift. Geowiss. 7, 93–133. [Google Scholar]
- 11.Weitschat W, Wichard W. 2010. Baltic amber. In Biodiversity of fossils in amber from the major world deposits (ed. Penney D.), pp. 80–115. Manchester, UK: Siri Scientific Press. [Google Scholar]
- 12.Walter DE. 2003. A new mite from an arboreal ant (Formicidae: Polyrhachis sp.): Myrmozercon iainkayi n. sp. (Mesostigmata: Laelapidae). Int. J. Acarol. 29, 81–85. ( 10.1080/01647950308684325) [DOI] [Google Scholar]
- 13.Shaw MD, Seeman OD. 2009. Two new species of Myrmozercon (Acari: Laelapidae) from Australian ants (Hymenoptera: Formicidae). Zootaxa 2025, 43–55. [Google Scholar]
- 14.Ghafarian A, Joharchi O, Jalalizand A, Jalaeian M. 2013. A new species of Myrmozercon Berlese (Acari, Mesostigmata, Laelapidae) associated with ant from Iran. ZooKeys 272, 21–28. ( 10.3897/zookeys.272.4404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joharchi O, Moradi M. 2013. Review of the genus Myrmozercon Berlese (Acari: Laelapidae), with description of two new species from Iran. Zootaxa 3686, 244–254. ( 10.11646/zootaxa.3686.2.6) [DOI] [PubMed] [Google Scholar]
- 16.Rosario RMT, Hunter PE. 1988. The genus Myrmozercon Berlese, with descriptions of two new species (Acari: Mesostigmata: Laelapidae). J. Parasitol. 74, 466–470. ( 10.2307/3282057) [DOI] [Google Scholar]
- 17.Dlussky GM. 1997. Genera of ants (Hymenoptera: Formicidae) from Baltic amber. Paleontol. J. 31, 616–627. [Google Scholar]
- 18.Wheeler WM. 1915. The ants of the Baltic amber. Schrift. Phys. Ökonom. Ges. Königsberg 55, 1–142. [Google Scholar]
- 19.Eickwort GC. 1990. Associations of mites with social insects. Ann. Rev. Entomol. 35, 469–488. ( 10.1146/annurev.en.35.010190.002345) [DOI] [Google Scholar]
- 20.Elzinga RJ. 1982. The genus Antennequesoma (Acari: Uropodina) and descriptions of four new species. Acarologia 23, 319–325. [Google Scholar]