Abstract
The health benefits of diets containing rich sources of long-chain omega-3 polyunsaturated fatty acids (n-3 LC-PUFA) are well documented and include reductions in the risk of several diseases typical of Western societies. The dietary intake of n-3 LC-PUFA has also been linked to fertility, and there is abundant evidence that a range of ejaculate traits linked to fertility in humans, livestock and other animals depend on an adequate intake of n-3 LC-PUFA from dietary sources. However, relatively few studies have explored how n-3 LC-PUFA influence reproductive fitness, particularly in the context of sexual selection. Here, we show that experimental reduction in the level of n-3 LC-PUFA in the diet of guppies (Poecilia reticulata) depresses a male's share of paternity when sperm compete for fertilization, confirming that the currently observed trend for reduced n-3 LC-PUFA in western diets has important implications for individual reproductive fitness.
Keywords: condition dependent, sexual selection, ejaculate quality, fertilization
1. Introduction
As with many traits subject to sexual selection, ejaculates can be conspicuously variable within individual species (e.g. [1–3]). While the basis for such variation remains contentious (e.g. [4]), recent work exploring the role of condition dependence in sperm traits suggests that variation among individual males in the acquisition and/or allocation of resources may constitute a considerable source of variance in ejaculate quality in polyandrous species [5–8].
The manipulation of resource availability, particularly through nutrient supplementation, offers a useful way to test for condition dependence in ejaculate traits. Accordingly, several studies have shown that males fed nutritionally enriched diets produce higher-quality ejaculates compared with those fed low-quality diets [5–9], and one study has reported significant effects of diet quality in regulating the outcome of sperm competition [5]. Among the key nutrients known to influence ejaculate quality, long-chain omega-3 polyunsaturated fatty acids (n-3 LC-PUFA; namely eicosapentaenoic acid and docosahexaenoic acid) play a critical role in determining the structural properties of spermatozoa, with concomitant effects on male fertility [10–12]. Animals cannot synthesize PUFA de novo, and their ability to bioconvert dietary C18 PUFA to LC-PUFA is limited. Animals must therefore obtain n-3 LC-PUFA from dietary sources. The experimental manipulation of dietary n-3 LC-PUFA levels therefore offers a useful way to test their effects on ejaculate ‘fitness’, and ultimately in sperm competitiveness. Despite their importance in regulating ejaculate traits [13,14], only a single study has considered fatty acids explicitly in the context of postcopulatory sexual selection [15], and no study has investigated the link between n-3 LC-PUFA intake and sperm competitiveness.
In this study, we use the guppy Poecilia reticulata, a highly polyandrous livebearing fish known to exhibit condition dependence in several ejaculate traits [16–18], to explore the effects of n-3 LC-PUFA dietary manipulation on competitive fertilization success. We used controlled heterospermic artificial inseminations to show that males fed diets enriched with n-3 LC-PUFA achieved significantly higher paternity success than their counterparts fed nutritionally impaired diets, thus confirming that ejaculate ‘fitness’ in this species is highly condition dependent and functionally dependent on resource acquisition.
2. Material and methods
(a). Study population and dietary treatments
The experimental male guppies (n = 60), aged three months at the start of the trials, were assigned haphazardly to one of two experimental diet treatments (n = 30 per treatment) that differed in n-3 LC-PUFA levels (hereafter ‘n-3LC-enriched’ and ‘n-3LC-reduced’). Both diets were compositionally similar, comprising identical quantities of basal ingredients, with the only exception being the type of added lipid sources. Both diets contained similar levels of saturated fatty acids and n-6 PUFA, but differed in their n-3 LC-PUFA content (measured as 12.9% in the n-3LC-enriched diet and 1.8% in the n-3LC-reduced diet; see the electronic supplementary material, table S1). The variation in n-3 LC-PUFA content was offset by the proportional variation of monounsaturated fatty acids (primarily oleic acid). Once assigned to their treatments, males were reared individually in separate 2 l aquaria for three months and fed ad libitum the crumbled diet once daily (6 days per week) until they were tested at six months old. Male standard length (SL: distance in millimetres from the snout to tip of caudal peduncle) was measured after the three month feeding trials and did not differ significantly between treatments (mean ± s.e.: n-3LC-enriched = 16.87 ± 0.2; n-3LC-reduced = 16.69 ± 0.18; t-test, t = 0.70; p = 0.49).
(b). Artificial insemination
Each replicate comprised a pair of rival males (one from each diet treatment; n = 30 pairs) and three virgin females (n = 90). We used artificial insemination [19] to inseminate equal numbers of sperm from the two males into three (unrelated) females. Immediately after insemination, each female was placed in a 2 l plastic aquarium and fed live Artemia nauplii until parturition. Tissue samples from the caudal fin of males and females, and the whole bodies of newborn fish, were collected and stored in pure ethanol for paternity analyses (see below). Only offspring arising from the female producing the largest brood in each replicate were used for subsequent paternity analyses (i.e. if more than one of the three females from a given replicate produced offspring, we only considered the largest brood for our analysis).
(c). Paternity analyses
We used up to four microsatellite markers to assign paternity to each brood, including TTA, Pr46, KonD15 and KonD21 (Genbank accession numbers: AF164205, AF127242, AF368429, AF368430, respectively). Genomic DNA was extracted from offspring using the EDNA HISPEX extraction kit (Fisher Biotec, Subiaco, Western Australia). PCR products were analysed on an ABI3730 Sequencer and visualized using GeneMarker v. 1.91 (http://www.softgenetics.com); paternity was assigned using CERVUS v. 3.0 (http://www.fieldgenetics.com). Only broods comprising three or more offspring were included in our subsequent analysis (final sample size: n = 26 independent broods).
(d). Statistical analysis
We used a generalized linear model (GLM) to analyse the effect of diet treatment on the relative paternity share of competing males. For each family, we selected at random one focal male, such that in 50% of cases the focal male was assigned to the n-3LC-enriched treatment and 50% to the n-3LC-reduced treatment. Our model included the proportion of offspring sired by the focal male as the response variable and treatment (diet) as a fixed effect. The model was weighted by family size (total number of offspring) and specified a quasi-binomial error distribution to account for overdispersion. All analyses were conducted using the ‘glm’ function in R v. 3.1.0 [20].
3. Results
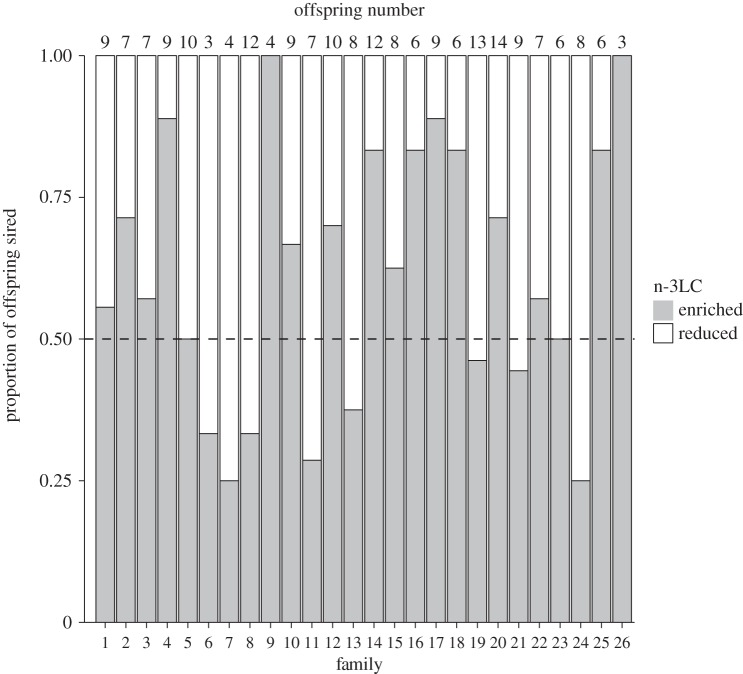
The mean number of offspring per family was 7.92 ± 0.4 s.e. (range = 3–14; total number of offspring = 206; see figure 1 for relative paternity share in each brood). The GLM revealed that dietary n-3 LC-PUFA levels had a significant effect on paternity success, confirming that on average n-3LC-reduced males sired a significantly lower proportion of offspring (mean ± s.e.: 0.39 ± 4.5) than their n-3LC-enriched rivals (0.61 ± 4.5; t = −2.42; p = 0.02; Cohen's d = 0.96; figure 1).
Figure 1.
Proportion of offspring sired by n-3LC-enriched and n-3LC-reduced males in each of the (n = 26) families.
4. Discussion
Our study reveals a clear link between diet quality and reproductive performance in male guppies, thus corroborating previous evidence that PUFA play a critical role in regulating sperm and semen quality in several species [11,12,21–23], including guppies [24]. Our finding that males fed diets enriched with n-3 LC-PUFA sired significantly more offspring than their counterparts fed reduced n-3 LC-PUFA levels complements recent evidence from crickets that males fed diets enriched with vitamin E and β-carotene produce competitive superior ejaculates [5]. In both cases, nutritional stress played a role in modulating the competitive performance of ejaculates, a finding that is likely to have important implications for postcopulatory sexual selection and the evolution of female multiple mating (polyandry).
One important implication of our findings is that variation in the availability of n-3 LC-PUFA, and/or differences among individual males in patterns of resource acquisition and allocation, will generate considerable phenotypic variance in ejaculates (e.g. [25]). Such environmental effects may explain, at least in part, the emerging evidence that ejaculates are inherently variable (see §1), despite evidence for directional and/or stabilizing selection on these traits (e.g. [26,27]). To the extent that condition itself has a genetic basis, polyandry may enhance female fitness by biasing paternity in favour of genetically superior males (reviewed in [28]). Thus, as with traits subject to precopulatory sexual selection, ejaculates may serve as ‘honest’ signals of male condition and genetic quality, and therefore serve as targets for viability selection.
Finally, our results have broader implications in the context of linking n-3 LC-PUFA availability to fertility and reproductive fitness in a broad range of species. There is already some speculation that global declines in the production of n-3 LC-PUFA by marine phytoplankton (the primary source of all dietary n-3 LC-PUFA available for both the marine and the terrestrial environments) may be linked to climate change [29]. Any such decline in the n-3 LC-PUFA content of marine products may further compound the trend towards reduced n-3 LC-PUFA levels in modern animal (including human) diets [11], with concomitant impacts on health and fertility. Our results suggest that patterns of sexual selection may be similarly impacted by these changes, which in turn has the potential to influence population and community dynamics in affected groups (e.g. [30]). We therefore require further investigation to determine the generality of these effects in other species, and the possible implications for patterns of sexual selection in affected populations.
Supplementary Material
Acknowledgements
We thank Cameron Duggin, Fernando Norambuena, Maxine Lovegrove and Sherralee Lukehurst for assistance, three anonymous reviewers for helpful feedback and Alastair Wilson for statistical advice.
Ethics statement
This work was approved by UWA's Animal Ethics Committee (RA/3/100/513).
Funding statement
We thank the Australian Research Council and the University of Western Australia for funding.
References
- 1.Malo AF, Gomendio M, Garde J, Lang-Lenton B, Soler AJ, Roldan ERS. 2006. Sperm design and sperm function. Biol. Lett. 2, 246–249. ( 10.1098/rsbl.2006.0449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleven O, Laskemoen T, Fossoy F, Robertson RJ, Lifjeld JT. 2008. Intraspecific variation in sperm length is negatively related to sperm competition in passerine birds. Evolution 62, 494–499. ( 10.1111/j.1558-5646.2007.00287.x) [DOI] [PubMed] [Google Scholar]
- 3.Hettyey A, Roberts JD. 2006. Sperm traits of the quacking frog, Crinia georgiana: intra- and interpopulation variation in a species with a high risk of sperm competition. Behav. Ecol. Sociobiol. 59, 389–396. ( 10.1007/s00265-005-0062-3) [DOI] [Google Scholar]
- 4.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. ( 10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 5.Almbro M, Dowling DK, Simmons LW. 2011. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol. Lett. 14, 891–895. ( 10.1111/j.1461-0248.2011.01653.x) [DOI] [PubMed] [Google Scholar]
- 6.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. 2012. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 27, 1466–1474. ( 10.1093/humrep/des065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciereszko A, Dabrowski K. 2000. Effect of ascorbic acid supplement in vitro on rainbow trout sperm viability. Aquacult. Int. 8, 1–8. ( 10.1023/A:1009253330451) [DOI] [Google Scholar]
- 8.Fricke C, Bretman A, Chapman T. 2008. Adult male nutrition and reproductive success in Drosophila melanogaster. Evolution 62, 3170–3177. ( 10.1111/j.1558-5646.2008.00515.x) [DOI] [PubMed] [Google Scholar]
- 9.Gage MJG, Cook PA. 1994. Sperm size or numbers? Effects of nutritional stress upon eupyrene and apyrene sperm production strategies in the moth Plodia interpunctella (Lepidoptera: Pyralidea). Funct. Ecol. 8, 594–599. ( 10.2307/2389920) [DOI] [Google Scholar]
- 10.Santos JEP, Bilby TR, Thatcher WW, Staples CR, Silvestre FT. 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim. 43, 23–30. ( 10.1111/j.1439-0531.2008.01139.x) [DOI] [PubMed] [Google Scholar]
- 11.Wathes DC, Abayasekara DRE, Aitken RJ. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77, 190–201. ( 10.1095/biolreprod.107.060558) [DOI] [PubMed] [Google Scholar]
- 12.Abayasekara DRE, Wathes DC. 1999. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostag. Leukotr. Ess. 61, 275–287. ( 10.1054/plef.1999.0101) [DOI] [PubMed] [Google Scholar]
- 13.Stubbs CD, Smith AD. 1984. The modification of mammalian polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 779, 89–137. ( 10.1016/0304-4157(84)90005-4) [DOI] [PubMed] [Google Scholar]
- 14.Connor WE, Lin DS, Wolf DP, Alexander M. 1998. Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm. J. Lipid Res. 39, 1404–1411. [PubMed] [Google Scholar]
- 15.delBarco-Trillo J, Roldan ERS. 2014. Effects of metabolic rate and sperm competition on the fatty-acid composition of mammalian sperm. J. Evol. Biol. 27, 55–62. ( 10.1111/jeb.12275) [DOI] [PubMed] [Google Scholar]
- 16.Devigili A, Kelley JL, Pilastro A, Evans JP. 2013. Expression of pre- and postcopulatory traits under different dietary conditions in guppies. Behav. Ecol. 24, 740–749. ( 10.1093/beheco/ars204) [DOI] [Google Scholar]
- 17.Gasparini C, Devigili A, Dosselli R, Pilastro A. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3, 4940–4953. ( 10.1002/ece3.870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman MM, Kelley JL, Evans JP. 2013. Condition-dependent expression of pre- and postcopulatory sexual traits in guppies. Ecol. Evol. 3, 2197–2213. ( 10.1002/ece3.632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JP, Zane L, Francescato S, Pilastro A. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363. ( 10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team. 2014. R: a language and environment for statistical computing, version 3.1.0. Vienna, Austria: R Foundation for Statistical Computing; (http://cran.r-project.org/) [Google Scholar]
- 21.Rooke JA, Shao CC, Speake BK. 2001. Effects of feeding tuna oil on the lipid composition of pig spermatozoa and in vitro characteristics of semen. Reproduction 121, 315–322. ( 10.1530/rep.0.1210315) [DOI] [PubMed] [Google Scholar]
- 22.Al-Daraji HJ, Al-Mashadani HA, Al-Hayani WK, Al-Hassani AS, Mirza HA. 2010. Effect of n-3 and n-6 fatty acid supplemented diets on semen quality in Japanese quail (Coturnix coturnix japonica). Int. J. Poult. Sci. 9, 656–663. ( 10.3923/ijps.2010.656.663) [DOI] [Google Scholar]
- 23.Robbins WA, Xun L, FitzGerald LZ, Esguerra S, Henning SM, Carpenter CL. 2012. Walnuts improve semen quality in men consuming a western-style diet: randomized control dietary intervention trial. Biol. Reprod. 87, 1–8. ( 10.1095/biolreprod.112.101634) [DOI] [PubMed] [Google Scholar]
- 24.Rahman MM, Turchini GM, Gasparini C, Norambuena F, Evans JP. 2014. The expression of pre- and postcopulatory sexually selected traits reflects levels of dietary stress in guppies. PLoS ONE 9, e105856 ( 10.1371/journal.pone.0105856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson MR, Beckerman AP. 2013. Quantifying multivariate plasticity: genetic variation in resource acquisition drives plasticity in resource allocation to components of life history. Ecol. Lett. 16, 281–290. ( 10.1111/ele.12047) [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 27.Johnson DW, Monro K, Marshall DJ. 2013. The maintenance of sperm variability: context-dependent selection on sperm morphology in a broadcast spawning invertebrate. Evolution 67, 1383–1395. ( 10.1111/evo.12022) [DOI] [PubMed] [Google Scholar]
- 28.Evans JP, Simmons LW. 2008. The genetic basis of traits regulating sperm competition and polyandry: can selection favour the evolution of good- and sexy-sperm? Genetica 134, 5–19. ( 10.1007/s10709-007-9162-5) [DOI] [PubMed] [Google Scholar]
- 29.Kang JX. 2011. Omega-3: a link between global climate change and human health. Biotechnol. Adv. 29, 388–390. ( 10.1016/j.biotechadv.2011.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saino N, Szép T, Ambrosini R, Romano M, Moller AP. 2004. Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proc. R. Soc. Lond. B 271, 681–686. ( 10.1098/rspb.2003.2656) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.