Abstract
Poultry meat and its products are widely consumed by humans globally, however, Aeromonas infections in poultry have been reported in different parts of the world with devastating effects. This study was carried out to assess the antibiogram and biofilm forming potential of Aeromonas isolated from chicken fecal samples. Aeromonas isolates were screened for antibiotic susceptibility using antibiotics disk and biofilm producing potentials on abiotic surfaces. Nineteen isolates recovered from chicken feces were 100% sensitive to ciprofloxacin, gentamicin and the tetracyclines. About 53% of Aeromonas isolates were resistant to erythromycin and 47% resistant to streptomycin. Eight isolates (42.1%) were found to be moderate producers of biofilm, 31.6% (6/19) were weak producers of biofilm, 10.5% (2/19) were non biofilm producers while 15.8% (3/19) were strong producers. The present investigation shows a prevalence of potentially pathogenic Aeromonas strains in chicken feces, suggesting potential group at risk for Aeromonas infection which could be dissemination to other animals or humans with close contact and the wider community.
Keywords: Aeromonas strains, Diseases, Infections, Pathogenic, Public health
1. Introduction
Poultry meat and its products are among the most widely consumed food all over the world. Chicken meat is delicious, nutritious and a good source of protein characterized by good flavor and easily digested. Aeromonas infections in poultry have been reported in different parts of the world with devastating effects (Dashe et al., 2013). A higher occurrence of Aeromonas from chicken source (Smita and Brahmabhatt, 2011) suggests that chicken could be a potential host for the spread of Aeromonas infection and present a possible threat to public health. Considering the high frequency of Aeromonas in poultry stool samples (Jindal et al., 1993), poultry carcasses and poultry plant processing water (Barnhart et al., 1989; Zanella et al., 2012), there is need to investigate the presence of Aeromonas in chicken samples.
Aeromonas species has the ability of colonizing several ecological niches. Aeromonas intestinal colonization is as a result of several virulence factors. Virulence in Aeromonas is multifactorial and not yet understood. Microbial colonization of mucosal surfaces is a complex process which results in infection, however, for most microbial infections it is thought to involve biofilm formation. A biofilm is an accretion of organisms entrenched in a polysaccharide matrix of their own making and adhering to a surface. Bacteria in biofilms are free-floating planktonic cells and show more resistance to antimicrobial agents and host defenses. They may also express more virulent phenotypes as a result of gene activation through bacterial communication (“quorum sensing”) or gene transfer (Greenberg, 1999; Costerton et al., 1999; Kirov et al., 2002). An important component in biofilm formation is the ability to move over and colonize surfaces after the initial attachment.
Aeromonas species have been described as an emerging food borne pathogen involved in human gastroenteritis ranging from mild diarrheal to cholera-like illness (Vila et al., 2003; Igbinosa et al., 2012). Aeromonas has also been implicated in meningitis, cellulites, otitis, endocarditis, osteomyelitis, peritonitis, bacteremia, and septicemia, among others diseases (Albert et al., 2000; Zanella et al., 2012). Aeromonas spp. have emerged as important human pathogens associated with food borne disease outbreaks (Fukushema et al., 2007; Awaad et al., 2011; Dashe et al., 2013). Biochemically, Aeromonas are Gram negative rods, oxidase and catalase positive, facultative anaerobe, motile, glucose fermenting. The ubiquitous nature of Aeromonas in aquatic, clinical and environmental sources has made it possible for forms of life such as human and domestic animals to have close contact with and become infected with Aeromonas species. Aeromonas have been implicated in water and food borne disease outbreak in different parts of the world especially in developing countries where hygiene and access to quality water supply is a challenge (Odeyemi and Ahmad, 2013).
The use of antibiotics has been vital in the treatment of infectious diseases caused by bacteria which has contributed to the rise in average life expectancy in the Twentieth century. However, bacteria that cause disease have become resistant to antimicrobial chemotherapy and are an increasing public health challenge (Sharma et al., 2010). The antibiotic susceptibility of an isolate is usually required for effective clinical control.
Microbial resistance to antibiotics is partially as a result of bacterial dynamism in adapting to its environment as well as increasing use, and misuse, of existing antibiotics in agriculture, human and veterinary medicine. Antimicrobial resistance among enteric pathogens is a part of major problem in developing countries where there is a high occurrence of gastroenteric illnesses and many antibiotics fall routinely into inadequate use. Antibiotic resistance is mostly pertinent in pathogenic Aeromonas species due to the frequent occurrence of multiple antibiotic resistances besides the classical resistance to beta-lactamic antibiotics (Kampfer et al., 1999; Vila et al., 2002). Also, these bacteria could possess integron (Igbinosa et al., 2013) which enables them to receive and transfer antibiotic resistance genes, giving rise to the risk from resistant bacterial infections (Marchandin et al., 2003; Zanella et al., 2012). There is a need for periodic surveillance of drug resistance of these organisms in different geographical areas and different sources for appropriate guidance for choice of antimicrobial agent for empiric therapy.
2. Materials and methods
2.1. Isolation procedure
The survey area of the study was Fort Cox agricultural farm in the Eastern Cape province of South Africa. Chicken fecal samples were collected at random in the months of September and December 2010 from the poultry segment of the farm and transported to the laboratory in cold chain. About 10 g of samples was inoculated in buffered peptone water (pH 7.2) for enrichment and incubated at 36 °C for 18–24 h. Enriched culture media were spread onto GSP agar plates for Aeromonas isolation. After 24 h incubation, phenotypic yellow colonies were picked and purified (Igbinosa et al., 2013). Pure colonies were transferred unto nutrient agar plates and slants. Isolates were identified based on biochemical characteristics using API 20NE profiling kit. The strips were then read, and final identification was made using API lab plus software (bioMerieux, Marcy l’Etoile, France).
2.2. Antibiotic phenotyping of isolates
Antibiotic susceptibilities of isolates were carried out using the following antibiotics: amoxycillin (30 μg), ampicillin–sulbactam (20 μg), aztreonam (30 μg), cefotaxime (30 μg), cephalothin (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), erythromycin (15 μg), gentamicin (10 μg), kanamycin (30 μg), nalidixic acid (30 μg), neomycin (30 μg), nitrofurantoin (300 μg), norfloxacin (10 μg), oxytetracycline (30 μg), penicillin G (10 μg), streptomycin (10 μg), tetracycline (10 μg), tobramycin (10 μg), trimethoprim–sulfamethoxazole (25 μg). Antibiotics were selected on the basis of antibiotics used as food additive in agriculture and those used for the treatment of Aeromonas associated infections. Pure isolates were grown on nutrient agar plates for 18 h afterward 4–6 colonies were suspended in normal physiological saline and adjusted to turbidity of 0.5-M McFarland standard. Subsequently, the isolate suspension was spread onto Muller Hinton agar (biolab) plates. Plates were allowed to dry and impregnated with the appropriate antibiotic disks. Plates were incubated at 36 °C for 24 h after which zones of inhibition were measured and recorded (Igbinosa et al., 2013). The inhibition and zone margins were selected as the areas showing no visible growth. The sizes of the zones were interpreted using published standards of the Clinical Laboratory Standard Institute Guidelines (CLSI, 2006) and the isolates reported as susceptible, intermediate or resistant against the antimicrobial agents tested.
2.3. Biofilm formation assay
Aeromonas isolates were grown for 18 h in trypticase soy broth at 36 °C and centrifuged for 2 min at 12,000 rpm. Cell pellets were washed and re-suspended in phosphate-buffered saline (pH 7.2) turbidity equivalent of 0.5 McFarland standard (Basson et al., 2007). Wells of sterile 96-well U-bottomed polystyrene microtiter plates was inoculated with 180 μl trypticase soy broth and 20 μl of standardized cell suspensions in order to determine bacteria adherence to abiotic material (Jacobs and Chenia, 2011; Igbinosa et al., 2013). Aeromonas hydrophila ATCC 7966 was used as positive control while wells containing only broth were used as negative control. Microtitre plates were incubated at 36 °C for 24 h. The absorbance reading of each well was obtained at 570 nm using an automated microtiter-plate reader (Synergy mx BiotekR USA). Assays were done in triplicate and the results averaged (Jacobs and Chenia, 2011; Igbinosa et al., 2013). Biofilm formation was classified as non-adherent, weakly, moderately or strongly-adherent. The cut-off OD (ODc) for the microtiter plate test was defined as three standard deviations above the mean OD of the negative control. Isolates were classified as follows: ODODC = non-adherent, ODC < OD(2 × ODC) = weakly adherent; (2 × ODC) < OD ⩽ (4 × ODC) = moderately adherent and (4 × ODC) < OD = strongly adherent (Jacobs and Chenia, 2011; Igbinosa et al., 2013).
3. Results and discussion
3.1. Occurrence of Aeromonas species
Biochemical identification of isolates was carried out using API 20NE kit. Based on biochemical identification of isolates, six Aeromonas species isolates were recovered from fecal samples collected in September 2010 while thirteen Aeromonas isolates were recovered from fecal samples collected in December 2010 resulting in a total of nineteen (19) isolates. Although, other microorganisms might have been present in the samples, however, Aeromonas was the interest microorganism of study. The isolation media used differentially and selectively narrowed the possibility of other microorganisms. Aeromonas have the capability of adapting to different ecological niche (Mateos et al., 1993; Arora et al., 2006) and possess astonishing properties which permit their survival and ability to survive and flourish in diverse condition (Agarwal, 1997; Arora et al., 2006), thereby allowing their cosmopolitan occurrence in nature. Aeromonas recognition as an emerging food borne pathogen is on the increase.
3.2. Antibiotic phenotyping of isolates
Most of the Aeromonas isolates were resistant to erythromycin (macrolides) but were sensitive to tetracycline, chloramphenicol, nitrofurantoin, quinolone, fluoroquinolones and aminoglycosides as shown in Table 1. The tetracyclines showed absolute sensitivity against all Aeromonas isolates. A similar observation of Aeromonas susceptibility to tetracycline has been reported in different geographical regions (Zanella et al., 2012; Awan et al., 2009; Mahmoud and Tanios, 2008). Although tetracycline has been used as growth stimulant in poultry feed for many years, the low resistance Aeromonas observed against tetracycline indicate minimal repercussion of tetracycline as antibiotics of choice in poultry farming. The aminoglycosides (gentamicin and tobramycin) showed brilliant activity against Aeromonas isolates with gentamicin showing absolute activity (Table 1). In a study carried out by Awan et al. (2009), Aeromonas isolated from food samples including fresh and frozen chicken demonstrated absolute sensitivity to gentamicin. This also corroborates with the report of (Dallal et al., 2012) who found excellent gentamicin activity against Aeromonas isolated from minced meat and chicken samples. The cephalosporins (cefotaxime) showed very high potency against the isolates compared to (cephalothin) which showed approximately average activity, which signify that Aeromonas species have variable susceptibility to cephalosporins. Aeromonas resistance to first generation cephalosporins, is expected given that motile Aeromonas demonstrate beta-lactamase activity and frequently the presence of metallo-beta-lactamases of expanded effect (Morita et al., 1994).
Table 1.
Patterns of Antibiotic phenotype of Aeromonas isolates from chicken feces.
| Antimicrobial agents | Susceptible |
Intermediate |
Resistance |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Amoxycillin (30 μg) | 0 | 0 | 0 | 0 | 19 | 100 |
| Ampicillin–sulbactam (20 μg) | 2 | 10.5 | 0 | 0 | 17 | 89.5 |
| Aztreonam (30 μg) | 16 | 84.2 | 0 | 0 | 3 | 15.8 |
| Cefotaxime (30 μg) | 16 | 84.2 | 2 | 10.5 | 1 | 5.3 |
| Cephalothin (30 μg) | 11 | 57.9 | 3 | 15.8 | 5 | 26.3 |
| Chloramphenicol (30 μg) | 14 | 73.7 | 1 | 5.3 | 4 | 21.1 |
| Ciprofloxacin (5 μg) | 19 | 100 | 0 | 0 | 0 | 0 |
| Erythromycin (15 μg) | 6 | 31.6 | 3 | 15.8 | 10 | 52.6 |
| Gentamicin (10 μg) | 19 | 100 | 0 | 0 | 0 | 0 |
| Kanamycin (30 μg) | 12 | 63.2 | 0 | 0 | 7 | 36.8 |
| Nalidixic acid (30 μg) | 15 | 78.9 | 1 | 5.3 | 3 | 15.8 |
| Neomycin (30 μg) | 12 | 63.2 | 4 | 21.1 | 3 | 15.8 |
| Nitrofurantoin (300 μg) | 15 | 78.9 | 0 | 0 | 4 | 20.1 |
| Norfloxacin (10 μg) | 13 | 68.4 | 1 | 5.3 | 5 | 26.3 |
| Oxytetracycline (30 μg) | 19 | 100 | 0 | 0 | 0 | 0 |
| Penicillin G (10 μg) | 0 | 0 | 0 | 0 | 19 | 100 |
| Streptomycin (10 μg) | 8 | 42.1 | 2 | 10.5 | 9 | 47.3 |
| Tetracycline (10 μg) | 19 | 100 | 0 | 0 | 0 | 0 |
| Tobramycin (10 μg) | 17 | 89.5 | 0 | 0 | 2 | 10.5 |
| Trimethoprim-sulfamethoxazole (25 μg) | 10 | 52.6 | 2 | 10.5 | 7 | 36.8 |
Trimethoprim-sulfamethoxazole complex showed appreciable susceptibility against Aeromonas isolates and resistance rate of 36.8%. This observation is similar to reports of (Zanella et al., 2012) and corroborates the finding of Ghenghesh et al. (2013), in which all Aeromonas isolates from chicken samples showed absolute sensitivity to Trimethoprim-sulfamethoxazole. Although, some literature argue the effectiveness of sulfamethoxazole–trimethoprim (SXT) complex against Aeromonas as a result of reports of other authors showing Aeromonas resistance (von Graevenitz and Altwegg, 1991). However, a study carried out on sulfamethoxazole alone against Aeromonas isolates showed poor activity but as a complex SXT, its efficacy against the isolates improved significantly (Awan et al., 2009), which is in conformity with the result obtained in the present study.
The penicillins (amoxycillin and penicillin) were absolutely inactive against all isolates while a slight variability of Ampicillin–sulbactam was insignificant. Several researchers have documented Aeromonas resistance to penicillins (Kaskhedikar and Chhabra, 2009; Zanella et al., 2012; Ghenghesh et al., 2013). In general, Most Aeromonas isolates are intrinsic or chromosomally mediated resistance against ampicillin (Rall et al., 1998). The resistance is as a result of at least four β-lactamases (von Graevenitz and Altwegg, 1991; Awan et al., 2009). Apart of the antimicrobial agent that showed absolute activity, the following antibiotics showed excellent activity (>70% strains were sensitive) against all the isolates tested. These include aztreonam, cefotaxime, chloramphenicol, nalidixic acid, nitrofurantoin, and tobramycin. A similar observation of Aeromonas sensitivity to cefotaxime, chloramphenicol, nitrofurantoin and tobramycin has been documented (Awan et al., 2009) while Aeromonas sensitivity to aztreonam, nalidixic acid among others has been reported (Zanella et al., 2012).
Aeromonas has been reported to show excellent sensitivity against chloramphenicol nitrofurantoin and tetracycline (von Graevenitz and Altwegg, 1991; Pasquale et al., 1994; Vivekanandhan et al., 2002). A similar observation has been documented (Awan et al., 2009) which is in accordance with the result obtained in the present study.
3.3. Biofilm formation assay
Biofilm formation is considered a vital virulence factor, aiding bacterial colonization by cell adhesion to epithelial cells and intestinal villi, reducing bacterial sensitivity to antimicrobial agents, and the reducing recognition of the bacteria by the immunologic system (Davey and O’Toole, 2000; Zanella et al., 2012). A quantitative assessment of biofilm producing potential of Aeromonas isolates showed significant variation among the isolates. Eight isolates (42.1%) were found to be moderate producers of biofilm while 6 (31.6%) of the isolates were weak producers of biofilm. In general, 2 (10.5%) isolates were non producers of biofilm whereas 3 (15.8%) were strong producers as shown in Fig. 1. The existence of biofilm forming Aeromonas from poultry and poultry workers has been documented (Zanella et al., 2012), which corroborates the result of this study. Aeromonas biofilm forming ability was also reported by Kirov et al. (2002), and was considered a potential virulence factor.
Figure 1.
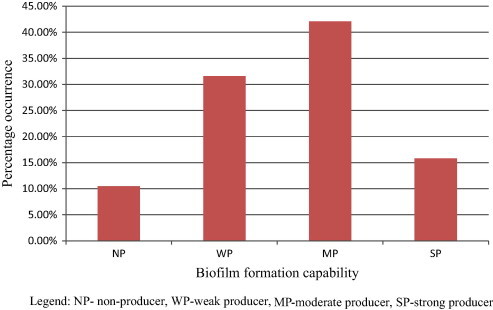
Biofilm producing potential of Aeromonas isolates isolated from chicken feces.
In conclusion, the present investigations show a high prevalence of potentially pathogenic Aeromonas strains in chicken feces. This suggests a potential group at risk for Aeromonas gastroenteric diseases and the dissemination of Aeromonas to other animals or humans with close contact and the wider community. Periodical screening of poultry birds across different geographical location is essential.
Acknowledgement
The author is grateful to University of Fort Hare for financial assistance.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal, R.K., 1997. Characterization of virulence factors of aeromonads isolated from foods of animal origin (Ph.D. thesis), Deemed University, IVRI, Izatnagar, India.
- Albert M.J., Ansaruzzaman M., Talukder K.A., Chopra A.K., Kuhn I., Rahman M., Faruque A.S.G., Islam M.S., Sack R.B., Mollby R. Prevalence of enterotoxin genes in Aeromonas spp. isolated from chicken and diarrhea, healthy controls, and the environment. J. Clin. Microbiol. 2000;38:3785–3790. doi: 10.1128/jcm.38.10.3785-3790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Agarwal R.K., Bist B. Comparison of ELISA and PCR vis-a‘-vis cultural methods for detecting Aeromonas spp. in foods of animal origin. Int. J. Food Microbiol. 2006;106:177–183. doi: 10.1016/j.ijfoodmicro.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Awaad M.H., Hatem M.E., Wafaa A., Asia E., Fathi A. Certain epidemiological aspects of Aeromonas hydrophila infection in chickens. J. Am. Sci. 2011;7:761–770. [Google Scholar]
- Awan M.B., Maqbool A., Bari A., Krovacek K. Antibiotic susceptibility profile of Aeromonas spp. isolates from food in Abu Dhabi, United Arab Emirates. New Microbiol. 2009;32:17–23. [PubMed] [Google Scholar]
- Barnhart H.M., Pancorbo O.C., Dreesen D.W., Shotts E.B. Recovery of Aeromonas hydrophila from carcasses and processing water in a broiler processing operation. J. Food Prot. 1989;52:646–649. doi: 10.4315/0362-028X-52.9.646. [DOI] [PubMed] [Google Scholar]
- Basson A., Flemming L.A., Chenia H.Y. Evaluation of adherence, hydrophobicity, aggregation characteristics and biofilm development of Flavobacterium johnsoniae-like isolates from South African aquaculture systems. Microb. Ecol. 2007;55:1–14. doi: 10.1007/s00248-007-9245-y. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) 7th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2006. Methods for Dilution of Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard M7–A7. [Google Scholar]
- Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Dallal M.M.S., Yazdi M.K.S., Avadisians S. Study of prevalence and antibiotic resistance in Aeromonas species isolated from minced meat and chicken samples in Iran. Afr. J. Microbiol. Res. 2012;6:460–464. [Google Scholar]
- Dashe Y.G., Raji M.A., Abdu P.A., Oladele B.S. Aeromonas hydrophila infections in chickens affected by fowl cholera in Jos Metropolis, Nigeria. Int. J. Microbiol. Immunol. Res. 2013;1:032–036. [Google Scholar]
- Davey M.E., O’Toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushema H., Katsube K., Hata Y., Kishi R., Fujiwara S. Rapid separation and concentration of food-borne pathogens in food samples prior to quantification by viable-cell counting and real-time PCR. Appl. Environ. Microbiol. 2007;73:92–100. doi: 10.1128/AEM.01772-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghenghesh K.S., El-Mohammady H., Levin S.Y., Zorgani A. Antimicrobial resistance profile of Aeromonas species isolated from Libya. Libyan J. Med. 2013;8:21320–21321. doi: 10.3402/ljm.v8i0.21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E.P. Quorum sensing in gram-negative bacteria. ASM News. 1999;63:371–377. [Google Scholar]
- Igbinosa I.H., Igumbor E.U., Aghdasi F., Tom M., Okoh A.I. Emerging Aeromonas species infections and their significance in public health. Sci. World J. 2012;2012:13. doi: 10.1100/2012/625023. Article ID 625023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbinosa I.H., Chigor V.N., Igbinosa E.O., Obi L.C., Okoh A.I. Antibiogram, adhesive characteristics, and incidence of class 1 integron in Aeromonas species isolated from two South African rivers. Biomed. Res. Int. 2013;2013:8. doi: 10.1155/2013/127570. Article ID 127570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A., Chenia H.C. Biofilm formation and adherence characteristics of an Elizabethkingia meningoseptica isolate from Oreochromis mossambicus. Ann. Clin. Microbiol. Antimicrob. 2011;10:16. doi: 10.1186/1476-0711-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal N., Garg S.R., Kumar A. Comparison of Aeromonas spp. isolated from human, livestock and poultry faeces. Israel J. Vet. Med. 1993;48:80–82. [Google Scholar]
- Kampfer P., Christmann C., Swing J., Huys G. In vitro susceptibilities of Aeromonas genomic species to 69 antimicrobial agents. Syst. Appl. Microbiol. 1999;22:662–669. doi: 10.1016/S0723-2020(99)80019-8. [DOI] [PubMed] [Google Scholar]
- Kaskhedikar M., Chhabra D. Multiple drug resistance of Aeromonas hydrophila isolates from chicken samples collected from Mhow and Indore city of Madhyapradesh. Vet. World. 2009;2:31–32. [Google Scholar]
- Kirov S.M., Tassell B.C., Semmler A.B.T., O’Donovan L.A., Rabaan A.A., Shaw J.G. Lateral flagella and swarming motility in Aeromonas species. J. Bacteriol. 2002;184:547–555. doi: 10.1128/JB.184.2.547-555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A.M., Tanios A.I. Pathogenicity of Aeromonas hydrophila in chickens. Egypt J. Comp. Pathol. Clin. Pathol. 2008;21:88–110. [Google Scholar]
- Marchandin H., Godreuil S., Darbas H., Jean-Pierre H. Extended-spectrum β-lactamase TEM-24 in an Aeromonas clinical strain: acquisition from the prevalent Enterobacter aerogenes clone in France. Antimicrob. Agents Chemother. 2003;47:3994–3995. doi: 10.1128/AAC.47.12.3994-3995.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos D., Anguita J., Naharro G., Paniagua C.J. Influence of growth temperature on the production of extracellular virulence factors and pathogenicity of environmental and human strains of Aeromonas hydrophila. J. Appl. Bacteriol. 1993;74:111–118. doi: 10.1111/j.1365-2672.1993.tb03003.x. [DOI] [PubMed] [Google Scholar]
- Morita K., Watanabe N., Kurata S., Kanamori M. β-Lactam resistance of motile Aeromonas isolates from clinical and environmental sources. Antimicrob. Agents Chemother. 1994;38:353–355. doi: 10.1128/aac.38.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeyemi O.A., Ahmad A. Anti-biogram and resistogram profiling of Aeromonas species isolated from Malaysian aquatic sources. J. Coastal Life Med. 2013;1:108–112. [Google Scholar]
- Pasquale V., Baloda S.B., Dumontet S., Krovacek K. An outbreak of Aeromonas hydrophila infection in turtles (Pseudemis scripta) Appl. Environ. Microbiol. 1994;60:1678–1680. doi: 10.1128/aem.60.5.1678-1680.1994. We. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall V.L.M., Iaria S.T., Heidtman S., Pimenta F.C., Gamba R.C., Pedroso D.M.M. Aeromonas species isolated from Pintado fish (Pseudoplatystoma sp.): virulence factors and drug susceptibility. Rev. Microbiol. 1998;29:222–227. [Google Scholar]
- Sharma I., Kumar A., Pramanik A.K. Antibiotic sensitivity test of Aeromonads isolated from foods of animal origin including fish. Assam Univ. J. Sci. Technol. 2010;5:43–47. [Google Scholar]
- Smita, Brahmabhatt M.N. Prevalence of Aeromonas species in chicken samples collected from retail shops of Anand (Gujarat) J. Vet. Pub. Health. 2011;9:115–117. [Google Scholar]
- Vila J., Marco F., Soler M., Chacón M., Figueras M.J. In vitro antimicrobial susceptibility of clinical isolates of Aeromonas caviae, Aeromonas hydrophila, and Aeromonas veronii biotype sobria. J. Antimicrob. Chemother. 2002;49:701–702. doi: 10.1093/jac/49.4.701. [DOI] [PubMed] [Google Scholar]
- Vila J., Ruiz J., Gallardo J., Vargas M., Soler L., Figueras M.J., Gascon J. Aeromonas spp. and traveler’s diarrhea: clinical features and antimicrobial resistance. Emerg. Infect. Dis. J. 2003;9:552–555. doi: 10.3201/eid0905.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanandhan G., Savithamani K., Hatha A.A., Lakshamanaperumalsamy P. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int. J. Food Microbiol. 2002;76:165–168. doi: 10.1016/s0168-1605(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Von Graevenitz A., Altwegg M. Manual of Clinical. Microbiology. 5th ed. American Society for Microbiology; Washington, D.C., USA: 1991. Aeromonas and Plesiomonas. [Google Scholar]
- Zanella J.D.P., da Luz R.B., Fadanelli R., Figueiró M.P., Delamare A.P.L., da Costa S.O.P., Echeverrigaray S. High Prevalence of Aeromonas spp. in poultry farmers from a rural community of South Brazil. Asian Pac. J. Mol. Biol. Biotechnol. 2012;20:93–98. [Google Scholar]


