Abstract
T follicular helper (Tfh) cells contribute to the establishment of humoral immunity by controlling the delivery of helper signals to activated B cells; however, Tfh development must be restrained, as aberrant accumulation of these cells is associated with positive selection of self-reactive germinal center B cells and autoimmunity in both humans and mice. Here, we show that TGF-β signaling in T cells prevented Tfh cell accumulation, self-reactive B cell activation, and autoantibody production. Using mice with either T cell–specific loss or constitutive activation of TGF-β signaling, we demonstrated that TGF-β signaling is required for the thymic maturation of CD44+CD122+Ly49+CD8+ regulatory T cells (Tregs), which induce Tfh apoptosis and thus regulate this cell population. Moreover, peripheral Tfh cells escaping TGF-β control were resistant to apoptosis, exhibited high levels of the antiapoptotic protein BCL2, and remained refractory to regulation by CD8+ Tregs. The unrestrained accumulation of Tfh cells in the absence of TGF-β was dependent on T cell receptor engagement and required B cells. Together, these data indicate that TGF-β signaling restrains Tfh cell accumulation and B cell–associated autoimmunity and thereby controls self-tolerance.
Introduction
CD4+ T lymphocytes have been known for decades to play a crucial role in helping B cells produce antibodies (1). More recently, among CD4+ T cells, T follicular helper (Tfh) cells have been described as a distinct subset with specialized helper functions. They colocalize with antigen-specific B cells within germinal centers (GCs), transient structures located within B cell follicles of secondary lymphoid tissues where somatic hypermutation of Ig variable region genes and selection of high affinity B cell clones occurs (2–4). Tfh cells are phenotypically defined by their high expression of chemokine receptor CXCR5 that promotes their migration to the B cell follicles as well as high surface levels of programmed death 1 (PD-1) (5, 6). Furthermore, Tfh cells express various receptors such as inducible T cell costimulator (ICOS), B and T lymphocyte attenuator (BTLA), and CD40L that are important for their development and/or function (2). They also produce cytokines including IL-21, which promotes B cell maturation, survival, isotype switching, and affinity maturation (7), and IL-4 or IFN-γ that can dictate isotype class switching to the appropriate Ig isotype tailored for protective immunity (8). B cell lymphoma 6 (BCL6) protein, a transcriptional repressor, plays a key role in programming Tfh cell differentiation (9–11).
Tfh cells normally differentiate from naive CD4+ T cells following immunization or infection. However, unrestrained accumulation of Tfh cells is associated with loss of B cell tolerance, development of autoantibodies, and autoimmune disorders in both humans and mice (12–15). Preventing the development of Tfh cells that normally expand in a T cell autonomous manner in the autoimmune-prone sanroque mouse model ameliorates autoantibody-related pathology (16). Collectively, these studies point to the importance of preventing unrestrained accumulation of Tfh cells.
CD4+ T cell subset differentiation is known to be highly influenced by the cytokine environment that can either enhance or repress their development. Both IL-6 and IL-21 have been described as cytokines capable of enhancing Tfh differentiation (2). However, with the recent exceptions of IL-2 and IL-10 that were shown to partially restrain Tfh cell differentiation in an infection and immunization setting, respectively (17, 18), no cytokine has been associated with controlling the spontaneous accumulation of Tfh cells observed in autoimmune diseases. CD8+ T regulatory cells (CD8+ Tregs) have been reported to prevent the unrestrained development of Tfh cells by inducing their apoptosis after interaction with Qa-1/peptide complex on the surface of Tfh cells, in a TCR-dependent manner (19, 20). Impairing the regulatory activity of CD8+ Tregs results in autoimmunity (20), while adoptive transfer of CD8+ Tregs is sufficient to reduce the number of Tfh cells and blunt the development of rheumatoid arthritis in mice (21), underlining the physiological relevance of CD8+ Treg-mediated control of Tfh cells. These regulatory cells represent 3% to 5% of peripheral CD8+ T cells, are thought to develop in the thymus (19, 22), and are characterized by the surface expression of CD44, CD122, and Ly49. In addition to CD8+ Tregs, FOXP3-expressing CD4+ T cells that have coopted a CXCR5+ phenotype have been proposed to limit the size of the Tfh cell population and GC reactions in response to immunization (23–26). These T follicular regulatory (Tfr) cells originate from thymic-derived FOXP3+ Tregs and coexpress BCL6 but at lower levels than Tfh cells (23–26). Thus, regarding the aggressive autoimmune phenotype associated with the spontaneous accumulation of Tfh cells, one challenge of broad interest is to identify factors that directly control Tfh homeostasis and/or the development of the cellular T lymphocyte populations charged with their regulation.
In this study, we reveal that TGF-β is a crucial cytokine to prevent the uncontrolled accumulation of Tfh cells. Mice deprived of TGF-β signaling in their T cells spontaneously accumulated Tfh cells in a TCR- and B cell-dependent manner leading to loss of B cell tolerance, antinuclear autoantibody production, and IgG complex deposition in the kidney. We found that not only was TGF-β signaling required for CD8+ Treg thymic development but that Tfh cells lacking TGF-β signaling had reduced sensitivity to apoptosis and were refractory to CD8+ Treg-mediated suppression. Collectively, our results demonstrate the ability of TGF-β signaling to act in a T cell intrinsic manner to restrain Tfh cell accumulation and B cell-associated autoimmunity revealing a novel mechanism in the control of self-tolerance.
Results
TGF-β signaling in T cells prevents aberrant accumulation of Tfh cells.
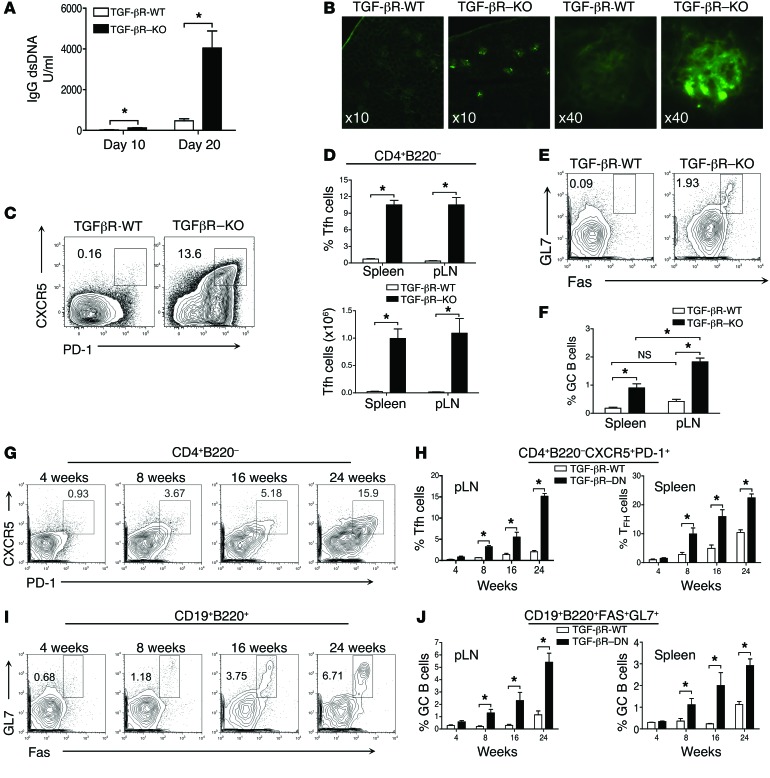
We and others have previously demonstrated that Tgf-βR2fl/fl CD4-Cre (TGF-βR–KO) mice deprived of TGF-β signaling, specifically in their αβ T cells, succumb to aggressive, early onset autoimmunity, including lymphocyte infiltration of organs and high levels of autoantibodies despite the fact that B cells are untouched by the mutation (27, 28). This phenotype, with aspects reminiscent of systemic lupus erythematosus (SLE), was also observed in mice totally deficient in TGF-β (29). In TGF-βR–KO mice, we observed high levels of circulating IgG double-stranded (ds) DNA and glomerular deposition of IgG immune complexes as compared with healthy littermate controls (Figure 1, A and B). As excessive numbers of Tfh cells have been recently associated with a loss a B cell tolerance and autoimmune disorders both in mice and humans (12–15), we analyzed for the presence of Tfh cells in TGF-βR–KO mice. In clear contrast with the few Tfh cells observed in nonimmunized WT littermate mice, both spleen and peripheral lymph nodes (pLNs) of TGF-βR–KO mice exhibited an exacerbated frequency and number of Tfh cells from their first week of age (Figure 1, C and D, and data not shown). In line with uncontrolled Tfh accumulation, GC B cells were also significantly increased in frequency and number in the spleen and pLNs of TGF-βR–KO mice (Figure 1, E and F, and data not shown). The analysis of prototypical markers on the Tfh cells that accumulate in the absence of TGF-β signaling control revealed expression of BCL6 to a similar extent as that observed in the few Tfh cells demonstrable in littermate control mice (Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/JCI76179DS1). Spontaneously accumulating Tfh cells expressed the GC-associated maker GL7, as well as high levels of ICOS, BTLA, CD40L, and IL-21, affirming their Tfh credentials (Supplemental Figure 1, B and D). However, surface expression of these B cell-activating molecules as well as the production of the B cell-activating cytokine IL-21 were clearly increased in Tfh cells from TGF-βR–KO mice compared with that from TGF-βR-WT mice as has been observed in B cell-associated autoimmunity diseases in humans (30). Moreover, in agreement with high levels of IgG2 in the blood of TGF-βR–KO mice (28), we noticed that Tfh cells that developed in an unrestrained manner in the absence of TGF-β control massively produced IFN-γ in contrast to basal Tfh cells from TGF-βR-WT mice (Supplemental Figure 1C). Furthermore, we did not detect IL-17 (data not shown), a cytokine that has been implicated in pathogenic GC formation in some circumstances (31).
Figure 1. Unrestrained accumulation of Tfh cells in the absence of TGF-β signaling in T cells.
(A) Mean (± SEM) representation of ELISA titration of dsDNA-specific IgG from serum of TGF-βR–KO and TGF-βR-WT mice at different ages from 4 independent experiments with a total of 8 mice per group. (B) Representative images of IgG staining on kidney sections from TGF-βR-WT and TGF-βR–KO mice aged 20 days from 3 independent experiments with a total of 5 animals per group. Original magnification, ×10 and ×40, as indicated in the panels. (C) Flow cytometry analysis of PD-1 and CXCR5 expression on CD4+ B220– FOXP3– T cells from pLNs of 18-day-old TGF-βR–KO and TGF-βR-WT mice. The percentage of Tfh cells are indicated on the plots. (D) Graphs illustrate the mean of frequency and total numbers of Tfh cells (± SEM) of 4 independent experiments with 12 mice per group. (E) Cytometry analysis of Fas and GL7 expression on B cells from 18-day-old mice. The percentage of GC B cells are indicated on the contour plots. (F) Graph represents mean (± SEM) of the frequency of GC B cells from 11 mice from 3 independent experiments. (G) Tfh cells in the pLNs of TGF-βR-DN mice at different ages. (H) Graphs represent mean (± SEM) of the percentage of Tfh cells from at least 3 independent experiments with 4 to 8 mice per time point. (I) Percentage of GL7+Fas+ B cells at different ages. (J) Graphs represent the mean (± SEM) of the percentage of GC B cells from 3 independent experiments with 4 to 8 animals per time point.
The ability of TGF-β signaling to contain spontaneous Tfh accumulation was confirmed in adult mice expressing a dominant-negative (DN) form of the TGF-β type II receptor specifically in their T cells (TGF-βR-DN) that attenuates TGF-β signaling leading to a progressive autoimmune syndrome that is fatal at approximately 24 weeks of age compared with 3 weeks for TGF-βR–KO mice (32). We observed a gradual accumulation of Tfh cells (Figure 1, G and H) and GC B cell development (Figure 1, I and J) in both the spleen and pLNs from TGF-βR-DN mice ruling out additional effects of TGF-β signaling on early peripheral colonization of an empty niche by T cells that could contribute to the unrestrained Tfh development observed in 2.5-week-old TGF-βR–KO mice. Thus, this first set of data reveals that TGF-β signaling regulates the spontaneous accumulation of Tfh cells and subsequent loss of B cell tolerance.
Sustained late-stage maintenance of Tfh cells following peptide immunization.
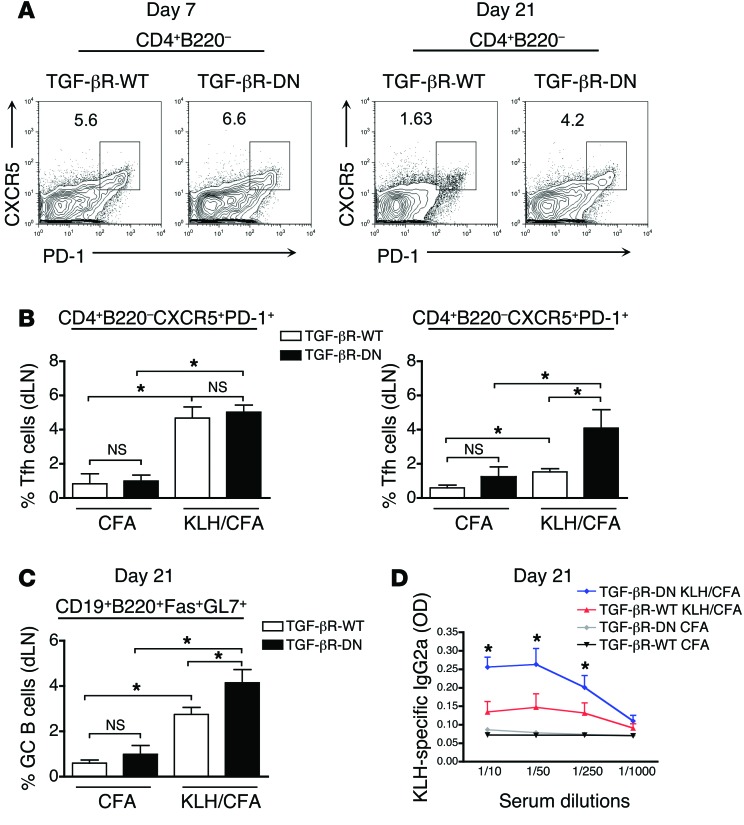
Next, we sought to investigate whether TGF-β exerts a similar repressive effect on Tfh cell homeostasis following foreign peptide immunization. As TGF-βR–KO mice succumb to an autoimmune syndrome within 3 weeks of age, we immunized TGF-βR-DN mice with KLH in CFA. Four-week-old TGF-βR-DN mice were chosen before they accumulated spontaneous Tfh cells (Figure 1G). As previously reported in mice immunized with KLH peptide in CFA and treated with TGF-β blocking antibody (33), a similar frequency of Tfh cells and GC B cells was observed at day 7 after immunization between TGF-βR-DN and TGF-βR-WT mice implying that TGF-β did not affect Tfh differentiation (Figure 2, A and B). However, 2 weeks later, while the Tfh cell pool had significantly contracted in the draining lymph nodes (dLNs) of WT mice, a significant population of Tfh cells remained detectable in the dLNs of TGF-βR-DN mice in response to KLH/CFA immunization (Figure 2, A and B). In line with this maintenance of Tfh cells, we also observed a greater induction of GC B cells (Figure 2C) and a higher IgG2a KLH-specific antibody titer in the blood of TGF-βR-DN mice as compared with TGF-βR-WT mice (Figure 2D). Thus, in addition to repressing the spontaneous accumulation of Tfh cells and preventing the development of autoreactive B cells, TGF-β signaling in T cells also regulates the size of the Tfh subset as well as the B cell response following protein immunization.
Figure 2. Longer Tfh accumulation in immunized mice with attenuated TGF-β signaling in T cells.
(A) Flow cytometry analysis of PD-1 and CXCR5 expression on CD4+B220– T cells from the inguinal dLNs of TGF-βR-DN and TGF-βR-WT mice at both day 7 and day 21 after immunization with KLH in CFA. Percentages illustrate the frequency of Tfh cells. (B) Graphs represent mean (± SEM) of the percentage of CD4+B220–PD-1+CXCR5+ Tfh cells generated on day 7 and day 21 in inguinal dLNs of mice receiving CFA alone or in combination with KLH peptide. Data consist of 2 independent experiments representing 8 mice in total. (C) The percentage of GC B cells generated in the inguinal dLNs of TGF-βR-DN and TGF-βR-WT mice on day 21 following immunization. Graphs represent the mean and SEM of 3 to 8 mice from 2 independent experiments. (D) ELISA titration of IgG2a KLH-specific antibodies in the serum of TGF-βR-DN and TGF-βR-WT mice on day 21 following immunization with either KLH in CFA or CFA alone. Graphs represent mean (± SEM) of 2 independent experiments with 8 mice.
Peripheral CD8+ Tregs decrease in the absence of T cell–specific TGF-β signaling.
There are several explanations for the spontaneous accumulation of Tfh cells in the absence of TGF-β signaling control. The absence of TGF-β signaling could affect T cell populations involved in restraining Tfh cell expansion. Alternatively, it is possible that self-reactive TGF-βR–deficient T cells preferentially differentiate into Tfh cells. Finally, T cell intrinsic mechanisms might exist that are disrupted in the absence of TGF-β signaling leading to preferential accumulation of Tfh cells.
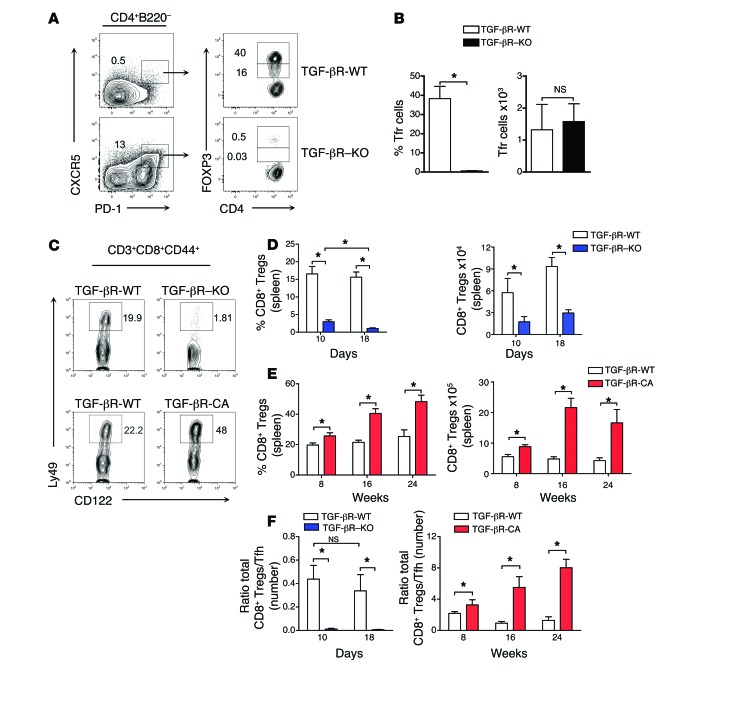
Thus, we addressed our first hypothesis by examining the development of T cell subsets implicated in repressing Tfh cells. Recently, CD4+ T cells expressing FOXP3, CXCR5, and PD-1 (Tfr cells) have been described as regulating Tfh cells in immunization-induced GCs (24–26). As TGF-β has been reported as contributing to the induction and maintenance of FOXP3 in CD4+ T cells (34, 35) and a reduced frequency of Tfr cells has been shown to aggravate lupus-like disease (36), we first analyzed the Tfr cell compartment using TGF-βR–KO mice expressing FOXP3gfp. Among CD4+ T cells that were double-positive for CXCR5 and PD-1, the frequency of Tfr cells was substantially reduced in TGF-βR–KO mice (Figure 3, A and B). However, total cell numbers were unaffected (Figure 3, A and B), suggesting that within the CXCR5+PD-1+CD4+ T cell population, TGF-β signaling selectively controls Tfh but not Tfr cell homeostasis.
Figure 3. Lack of peripheral CD8+ Tregs in the absence of TGF-β receptor signaling.
(A) CD4+B220– T cells from TGF-βR-WT or TGF-βR–KO mice were examined at 18 days, and cells expressing PD-1 and CXCR5 were separated based on GFP expression into FOXP3+ Tfr cells. Representative flow cytometric contour plots from 5 independent experiments are demonstrated and frequencies of Tfh and Tfr cells are indicated. (B) Graphs represent mean (± SEM) from 4 mice in 2 independent experiments of the frequency and total number of Tfr cells (FOXP3+ cells within the CD4+B220–CXCR5+PD-1+ gate) in the 2 axial lymph nodes of TGF-βR–KO or TGF-βR-WT mice. (C) CD8+CD3+ T cells expressing CD44hi, CD122+, and Ly49+ (CD8+ Tregs) were examined by flow cytometry in the spleens of TGF-βR–KO and TGF-βR-CA mice as well as their littermate WT controls (TGF-βR-WT). Representative contour plots illustrating the frequency of CD8+ Tregs from 4 independent experiments with between 4 and 9 mice are demonstrated. (D and E) Graphs demonstrate the frequency and total number of CD8+ Tregs in the spleens of TGF-βR–KO and TGF-βR-CA mice compared with TGF-βR-WT mice at various time points. Data is mean (± SEM) of 4 independent experiments with between 4 and 9 mice. (F) Graphs demonstrate the ratio of total numbers of CD8+ Tregs to Tfh cells in the spleens of TGF-βR–KO and TGF-βR-CA mice compared with TGF-βR-WT mice at various time points. Data are the mean (± SEM) of between 4 and 9 mice from 4 independent experiments.
Thus, we next assessed whether TGF-β signaling affects the homeostasis of the CD8+ Treg population. CD8+ Tregs are QA-1–restricted lymphocytes characterized by the surface expression of CD44hi, CD122, and Ly49 that have been shown to repress spontaneous development of Tfh cells by inducing their apoptotic death, thereby efficiently contributing to the control of autoimmune diseases in both mice and humans (20, 21, 37). While CD8+ Tregs represented, as expected, around 20% of the CD44+CD122+CD8+ T cells in the spleen from TGF-βR-WT mice, they were barely detectable in both frequency and total number in TGF-βR–KO mice (Figure 3, C and D). Moreover, we found a 1.5–2-fold increase in CD8+ Tregs in LSL-TβRICA CD4-Cre (TGF-βR-CA) mice whose T cells express a constitutive active form of the TGF-β receptor (38), as compared with TGF-βR-WT mice (Figure 3, C and E), which supports the direct contribution of TGF-β signaling in T cells on CD8+ Treg homeostasis. Consequently, the ratio of the number of CD8+ Tregs to Tfh cells in TGF-βR–KO and in TGF-βR-CA mice was more than 60-fold lower and 8-times higher, respectively, compared with control mice (Figure 3F).
TGF-β regulates thymic development of CD8+ Tregs.
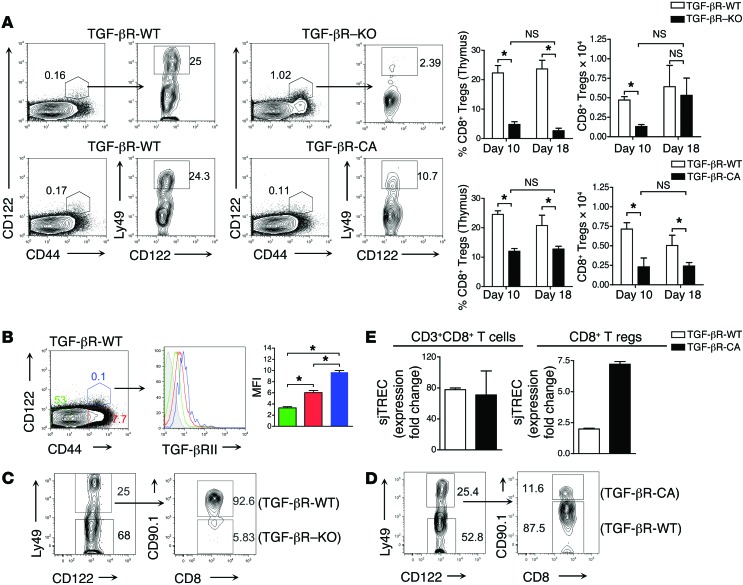
We failed to observe any difference in peripheral CD8+ Treg apoptosis using annexin/7-AAD staining and peripheral CD8+ Treg proliferation using BrdU addition to drinking water between TGF-βR-CA mice and TGF-βR-WT mice (data not shown). Therefore, we next analyzed the effects of TGF-β signaling on CD8+ Treg thymic development. In complete agreement with the lack of peripheral CD8+ Tregs in TGF-βR–KO mice, both the frequency and number of CD44hiCD122+Ly49+CD8 single-positive (CD8SP) thymocytes were considerably reduced in TGF-βR–KO mice 10 days after birth as compared with TGFR WT mice (Figure 4A). It is notable that while the numbers of CD8+ Treg thymocytes were much lower in TGF-βR–KO mice during their first 2 weeks of life, animals exhibited a similar number of CD8+ Treg thymocytes to their TGF-βR-WT littermates at 18 days of age. These data suggest a delay in the generation of CD8+ Tregs in the absence of TGF-β with CD8+CD44hiCD122+ thymocytes accumulating in the thymus rather than a block in their development. In line with a progressive dependency on TGF-β signaling for thymic maturation, we found greater expression of the TGF-βRII on WT cells as they progressed from naive CD8SP thymocytes (CD44loCD122lo) to intermediate cell populations (CD44hiCD122lo) to CD8SP Treg precursors (CD44hiCD122hi) (Figure 4B). To definitively confirm a direct effect of TGF-β signaling on CD8+ Treg thymic development, we next analyzed the origin of the CD8+ Treg thymocytes using irradiated Rag-KO mice reconstituted with equal parts BM from congenic TGF-βR-WT mice and TGF-βR–KO mice; we found that more than 90% of the CD8+ Treg thymocytes were derived from the TGF-βR-WT BM whereas CD8+ T cells were equally derived from the 2 BM origins (Figure 4C). Surprisingly, in contrast with the large amount of CD8+ Tregs at the periphery of TGF-βR-CA mice, we observed a decrease in the CD8+ Treg proportion and number in the thymus of TGF-βR-CA mice compared with TGF-βR-WT mice (Figure 4, A and D). However, this discrepancy can be explained by analysis of the frequency of signal joint TCR excision circles (sjTRECs) in the peripheral cell population implying an exacerbated thymic export of mature CD8+ Tregs in TGF-βR-CA mice compared with TGF-βR-WT mice, whereas sjTREC content in total CD8+ T cells was unchanged (Figure 4E). Taken together, these data indicate TGF-β as a crucial cytokine for the development of CD8+ Tregs facilitating their rapid thymic differentiation to increase the peripheral number of these cells to restrain the Tfh cell population.
Figure 4. CD8+ Treg thymic development is defective in the absence of TGF-βR signaling.
(A) Flow cytometry analysis illustrating the frequency of thymic CD8+ Tregs identified in TGF-βR–KO or TGF-βR-CA mice aged 18 days and their WT controls by gating on CD8SP thymocytes followed by CD44hiCD122+ T cells that express Ly49. Graphs show mean (± SEM) of frequency and total number of thymic CD8+ Tregs from 3 independent experiments with 6 to 8 mice. (B) TGF-βR-II expression on thymic CD8SP subsets in 18-day-old TGF-βR-WT mice. CD44–CD122– (green), CD44+CD122– (red), and CD44+CD122+ (blue) subsets are demonstrated on a representative histogram. Graph represents mean (± SEM) of mean fluorescence intensity (MFI) of TGF-βRII from 3 independent experiments with 3 mice. (C and D) BM from congenic WT mice was combined with BM from either TGF-βR–KO mice (C) or TGF-βR-CA mice (D) and grafted into irradiated recipient Rag-KO mice. The thymus was examined 5 weeks after reconstitution by flow cytometry for the presence of CD8+ Tregs in CD44+CD122+ cells, and the percentage of CD8+ Tregs from each BM are shown. Data are representative of 2 independent experiments with 5 mice. (E) Signal joint TCR excision circles (sjTRECs) were measured by real-time qPCR from genomic DNA of sorted CD8+ Tregs or total CD8+ T cells. Data are presented as mean (± SEM) of expression fold changes, normalized with CD45 expression obtained from triplicate wells. Data are representative of 2 independent experiments with 8 pooled spleens.
Aberrantly accumulating Tfh cells lacking TGF-βRII are refractory to CD8+ Treg control.
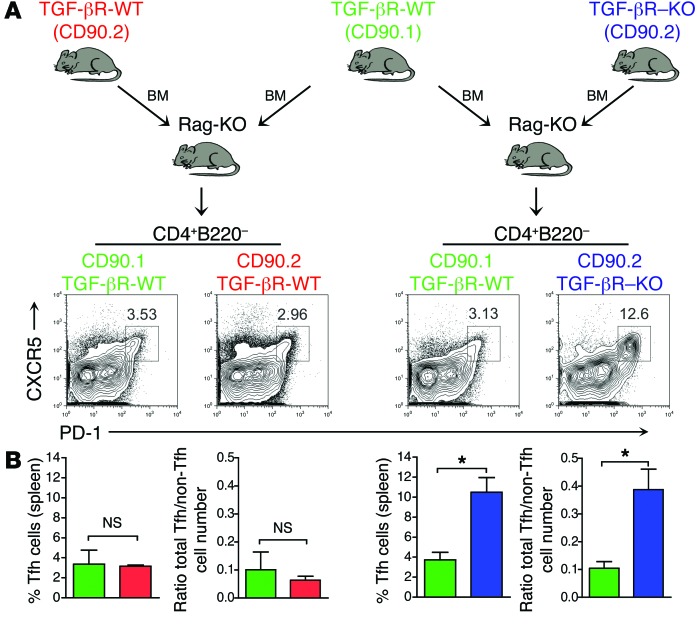
In the absence of TGF-β signaling control, there is a clear delay in the constitution of the CD8+ Treg pool. Thus, we next investigated whether the large number of Tfh cells observed in the absence of TGF-β signaling in T cells was due to the defect in CD8+ Treg development. To address this hypothesis, we generated mixed BM chimera mice by reconstituting irradiated Rag-KO mice with BM (1:1) from both TGF-βR–KO mice and congenic TGF-βR-WT mice and analyzed them for the presence of Tfh cells and their BM origins. The CD8+ Treg compartment was fully reconstituted mainly from TGF-βR-WT BM cells as illustrated in Figure 4C. Strikingly, the CD4+ T cell compartment derived from the BM of TGF-βR–KO mice was highly enriched in Tfh cells in the presence of TGF-βR-WT BM–derived cells (Figure 5, A and B), strongly suggesting that aberrantly accumulating Tfh cells lacking TGF-βRII were refractory to TGF-βR-WT CD8+ Treg control and implicating a T cell intrinsic mechanism permitting spontaneous Tfh cell accumulation in the absence of TGF-β control. Furthermore, the ratio of the total number of Tfh cells to non-CD4+ Tfh cells was 5 times higher in the T cells derived from TGF-βR–KO BM compared with that from TGF-βR-WT BM (Figure 5B) pointing out a selective accumulation of Tfh cells among CD4+ T cells in the absence of TGF-β signaling and not a result of the global CD4+ T cell expansion. It is also noteworthy that no aberrant accumulation of TGF-βR-WT Tfh cells was observed in the BM chimera (Figure 5, A and B), ruling out any cytokine bystander effect from the well-known inflammatory environment provided by the TGF-βR–KO T cells (27, 28). Similarly, TGF-βR-DN Tfh cells were highly represented 3 weeks after immunization of irradiated Rag-KO mice previously reconstituted with mixed BM from TGF-βR-WT and TGF-βR-DN mice (Supplemental Figure 2, A and B). These data firmly establish an intrinsic effect of TGF-β signaling in repressing uncontrolled Tfh cell accumulation and shows that Tfh cells escaping TGF-β control are refractory to regulatory cells.
Figure 5. Uncontrolled Tfh cell accumulation was not controlled by CD8+ Tregs.
(A and B) Mixed BM chimera in irradiated Rag-KO mice reconstituted with BM from TGF-βR–KO mice or littermate control (TGF-βR-WT) mice (both CD90.2+) with BM from congenic CD90.1+ TGF-βR-WT mice were analyzed 5 weeks later. Flow cytometry analysis of the expression of PD-1 and CXCR5 on CD4+B220–FOXP3– T cells is illustrated, and the percentage of Tfh cells is shown on the contour plots. Graphs demonstrate mean (± SEM) of the percentage of Tfh cells or the ratio of the total number of Tfh cells to non-Tfh cells in the spleens of reconstituted mice contributed by each BM from 2 independent experiments with 4 to 5 mice.
Spontaneous Tfh cell accumulation occurs in a TCR- and B-cell dependent manner.
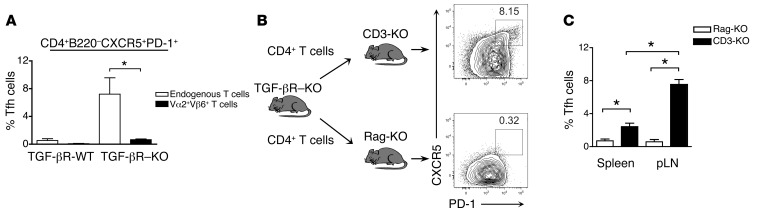
We next investigated the mechanisms that could be involved in the uncontrolled Tfh cell accumulation and autoreactive B cell activation in the absence of TGF-β signaling. We first analyzed whether self-reactive TGF-βR–deficient T cells might preferentially become Tfh cells either by spontaneous activation due to tonic TCR signaling irrespective of TCR specificity or alternatively from preferential activation of self-reactive CD4+ T cells. To discriminate between these two scenarios, we bred TGF-βR–KO mice onto TEa TCR transgenic mice (39). In the latter mice, a transgene encoding a Vα2+Vβ6+ TCR specific for the foreign Ea52–68 peptide bound to I-Ab was expressed by more than 90% of peripheral T cells with the remaining T cells expressing endogenously rearranged TCR genes. Analysis for the presence of Tfh cells within the TCR transgenic (Vα2+, Vβ6+) and endogenous TCR (Vα2–, Vβ6–) CD4+ T cells revealed that they were all derived from the endogenous TCR-expressing CD4+ T cells (Figure 6A), strongly supporting a TCR-dependent differentiation of TGF-βR–deficient CD4+ T cells into Tfh cells that likely occurs upon self-antigen recognition.
Figure 6. Spontaneous Tfh cell accumulation was dependent on TCR and B cell signals.
(A) TEa mice were bred with TGF-βR–KO mice resulting in a mixed cell population of TCR-restricted Vα2+Vβ6+ TEa cells and a small population of endogenous T cells with mixed TCRs. Vα2+Vβ6+CD4+ TEa cells and the endogenous Vα2–Vβ6–CD4+ T cells were differentiated by flow cytometry in mice 4 to 6 weeks after birth, and the development of CD4+B220–PD-1+CXCR5+ Tfh cells in each cell population was examined. WT littermates were used as controls. Graph represents mean (± SEM) from 2 independent experiments with 5 mice. (B) CD4+ T cells were sorter purified from TGF-βR–KO mice and injected into either Rag-KO or CD3-KO mice and examined 6 weeks later. The percentages of CD4+B220–PD-1+CXCR5+FOXP3– Tfh cells in the pLNs are demonstrated in representative contour plots. (C) Graph is mean (± SEM) of the percentage of Tfh cells in the pLNs and in the spleen of either Rag-KO or CD3-KO mice representing 4 mice in 2 independent experiments.
B cells play a key role in Tfh cell development (40). As B cells have been implicated in infection and immunization models as important mediators of signals required to complete Tfh cell maturation and development in addition to their role as antigen-presenting cells (41, 42), we next investigated whether the contribution of B cells was similarly required for the aberrant accumulation of Tfh cells in the absence of TGF-β signaling in T cells. Interestingly, Rag-KO mice adoptively transferred with CD4+ T cells failed to spontaneously generate TGF-βR–KO Tfh cells, whereas a large cell population of TGF-βR–KO Tfh cells was observed in CD3-KO mice (Figure 6, B and C) implying that though Tfh cell accumulation in the absence of TGF-β control is dependent upon TCR specificity, B cells are still required.
Aberrantly accumulating Tfh cells are more resistant to apoptosis.
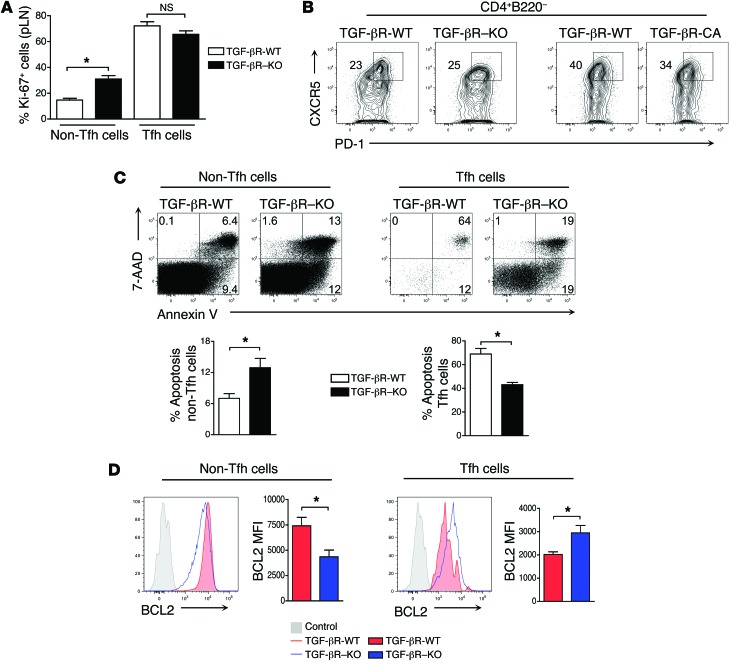
Because the BM transfer experiments shown in Figure 5 suggested that in the absence of TGF-β control, Tfh cells escape regulatory activity provided by the WT BM–derived cells, we investigated intrinsic mechanisms that could explain the aberrant accumulation of the Tfh cell population. As exacerbated accumulation of Tfh cells can be a consequence of either excessive proliferation or greater survival, we addressed each of these possibilities. In line with the reported higher basal cycling capability of Tfh cells (43), Tfh cells in TGF-βR-WT and TGF-βR–KO mice were highly divided compared with non-Tfh (CD4+B220–PD-1negCXCR5neg) cells (Figure 7A). However, the percentage of cells undergoing proliferation was similar in Tfh cells from TGF-βR–KO and control mice suggesting an increase of differentiation or survival rather than excessive expansion of existing Tfh cells in the absence of TGF-β control (Figure 7A). To assess whether naive T cells lacking TGF-β signaling differentiated into Tfh cells to a greater extent than WT T cells, we cultured non-Tfh naive CD4+ T cells over-responding to or lacking TGF-β signaling under Tfh polarizing conditions adapted from published work (33), that routinely gave between 20% to 50% Tfh cells after 4 days of culture. Neither over-activation of TGF-β signaling nor its deprivation in T cells affected the rate of differentiation into CD4+CXCR5hiPD-1hi Tfh cells from naïve CD4+ T cells, excluding a role for TGF-β signaling in the repression of initiation of differentiation of Tfh cells (Figure 7B).
Figure 7. Tfh cells lacking TGF-βR signaling upregulate BCL2 and are resistant to apoptosis.
(A) Graph is mean (± SEM) of the percentage of Ki-67+ cells in Tfh and non-Tfh subpopulations in TGF-βR–KO and TGF-βR-WT mice aged 18 days. Graph represents 4 to 5 mice in 2 independent experiments. (B) CD4+ cells from TGF-βR-WT, TGF-βR–KO, and TGF-βR-CA mice were depleted of CD44hi, PD-1, CXCR5, and FOXP3+ cells by cell sorting, cultured in Tfh polarizing conditions with CD90.1+ feeder cells, and examined by flow cytometry at day 4 for the induction of Tfh cells double-positive for PD-1 and CXCR5. The percentages of differentiated Tfh cells are illustrated on the contour plots and are representative of 2 independent experiments. (C) Tfh and non-Tfh (CD4+B220–CXCR5–PD-1–FOXP3–) subpopulations in TGF-βR–KO and TGF-βR-WT mice were examined by flow cytometry for apoptosis by costaining for annexin V and 7-AAD. Tfh data from TGF-βR-WT mice were derived by concatenating 3 independent samples to have sufficient events. The percentage of apoptotic cells are illustrated on the contour plots. Graphs represent the mean and SEM of 2 independent experiments with 4 to 5 mice. (D) Histograms represent intracellular BCL2 expression in non-Tfh and Tfh cells from both TGF-βR–KO and TGF-βR-WT mice aged 16 to 20 days after flow cytometry analysis by concatenating 3 independent samples. Graphs illustrate mean (± SEM) of mean fluorescence intensity (MFI) for BCL2 representing 2 independent experiments with a total of 5 mice.
Because Tfh cells from TGF-βR–KO mice cannot be kept in check by the regulatory effect of WT CD8+ Tregs known to regulate Tfh cells by inducing their apoptosis, we finally investigated the survival ability of Tfh cells responding or not to TGF-β signaling. We looked at apoptosis in Tfh cells using annexin V and 7-AAD costaining. In line with a previous report of increased sensitivity to apoptosis in TGF-βR–KO mice (44), we demonstrated that peripheral CD4+ non-Tfh cells from TGF-βR–KO mice were more susceptible to apoptosis compared with WT controls (Figure 7C). However, in clear contrast, TGF-βR–KO Tfh cells were significantly less inclined to apoptosis than Tfh cells from TGF-βR-WT mice (Figure 7C). In addition to specificity for the Tfh subset, this resistance to apoptosis in the absence of TGF-β signaling seemed to involve intrinsic mechanisms, since we observed that TGF-βR-WT Tfh cells remained more sensitive to apoptosis than TGF-βR–KO Tfh cells in mixed BM chimera mice (data not shown). Consistent with their apoptosis resistance, we found high levels of the antiapoptosis protein BCL2 in TGF-βR–KO Tfh cells as compared with those from TGF-βR-WT mice (Figure 7D) and no difference in the levels of the proapoptotic molecules, Bim or Fas (data not shown). Collectively, these data demonstrate that the Tfh cells escaping TGF-β control have clearly reduced sensitivity to apoptosis, implying their resistance to apoptosis as a T cell intrinsic mechanism promoting their aberrant accumulation and the associated rupture in B cell tolerance.
Discussion
New B cell receptor specificities, including those with autoreactivity, arise continuously during the GC reaction (45–47). Aberrant accumulation of Tfh cells has been associated with positive selection of self-reactive GC B cells and autoimmunity in both mice and humans (30, 48). Elucidating the mechanisms that prevent development of high-affinity antibodies to dsDNA and self-proteins is central to understanding autoimmune diseases such as lupus erythematosus, rheumatoid arthritis, and myasthenia gravis and hence, to developing specific therapeutic approaches. We showed here that in the absence of TGF-βR signaling in T cells, Tfh cells spontaneously accumulated in an uncontrolled manner accompanied by B cell activation, autoantibody production, and immune complex deposition in the kidney. Mechanistically, Tfh cell accumulation in the absence of TGF-β signaling was T cell intrinsic, dependent on TCR engagement, required B cells, and was associated with improved survival, increased levels of antiapoptotic BCL2, and resistance to CD8+ Treg regulation. We also demonstrated an essential role for TGF-β signaling in the thymic development of CD8+ Tregs. Thus, this study reveals the importance of T cell control by TGF-β to prevent the aberrant accumulation of Tfh cells and development of B cell autoimmunity.
Our findings, in both TGF-βR–KO and TGF-βR-DN mice, indicated that T cells, either escaping or modulating TGF-β control, respectively, could accumulate spontaneously as Tfh cells. It is notable that these 2 mouse models have been associated with high levels of inflammatory cytokines such as IFN-γ (28, 49), which is known to lead to BCL6 overexpression and excessive development of Tfh cells (43). However, our work rules out any bystander cytokine action on Tfh cell population control in these mouse models, and thus underlines a direct effect of TGF-β on T cells to control Tfh cell homeostasis. Although we have not directly assessed the TCR repertoire and specificity of the aberrantly expanded Tfh cells in TGF-βR–KO mice, our finding that T cells lacking TGF-β signaling were unable to develop into Tfh cells when their TCR was substituted for a transgenic TCR specific for a defined foreign antigen strongly argues for T cell autoreactivity to self-peptides as was suggested for the global CD8+ and CD4+ T cell populations (50). Germ-free mice have been found to have a lower frequency of Tfh cells and reduced autoantibody titers in an autoimmune arthritis model (51); however, we excluded reactivity against the microbiota since TGF-βR–KO mice placed under a large spectrum of antibiotics, before and after birth, spontaneously develop large numbers of Tfh cells (data not shown). However, as Tfh cells tend to be derived from T cells binding peptide/MHC with a high affinity (52), consistent with the observation that prolonged TCR engagement during viral persistence predisposes activated T cells towards Tfh development (53), it is possible that TGF-β signaling might play an intrinsic role in preventing unwanted Tfh cell accumulation by heightening the antigen affinity required for TCR activation. In line with this hypothesis, we have found the ability of TGF-β to restrain T cell reactivity towards low-affinity self-antigen is the result of altered TCR signaling thresholds (unpublished observations, Marie and Hennino).
Our work suggests that TGF-β limits the size of the already established Tfh cell population. Thus, we propose that TGF-β prevents the aberrant accumulation of Tfh cells that spontaneously accumulate during life in response to self-antigen. It is known that some Tfh cells are spontaneously generated in nonimmunized mice, likely in response to environmental antigens or self-antigens. Compared with other CD4+ T cells, we and others have found that these cells are endowed with a higher proliferative ability (43), but we also show here that they are more susceptible to apoptosis. These cells accumulate with age in humans (54) and could be involved in autoantibody production. Supporting this idea, autoantibodies are known to accumulate in mice and humans with age (55, 56). Though the role of spontaneously developed Tfh cells remains to be clearly identified, it seems clear that aberrant accumulation of Tfh cells has been associated with loss of B cell tolerance, massive development of autoantibodies, and autoimmune disorders in both humans and mice (15, 48). We propose that control of the size of these spontaneously developed Tfh cells relies on a tight equilibrium between cell cycling and apoptosis maintaining a low number of spontaneously developed Tfh cells to avoid loss of B cell tolerance and that TGF-β is at the core of this equilibrium. We revealed that in the absence of TGF-β control, a large part of the spontaneously developed cells escape apoptosis and noxiously accumulate in the organism. Interestingly, contraction of CD8+ effector T cells, an apoptotic phenomenon crucial to protect the organism from tissue damage due to exacerbated immune responses, behaves similarly, employing a TGF-β–mediated mechanism to repress BCL2 expression (57). Thus, one role for TGF-β in maintaining organism integrity could be controlling, by apoptosis, the size of the T lymphocyte populations whose uncontrolled expansions are toxic to the organism. In addition to apoptosis resistance, it is possible that other factors such as the high levels of ICOS observed in TGF-βR–KO T cells could contribute to the massive Tfh cell development regarding the role ICOS signaling plays in the development of Tfh cells (42). However, this contribution should be minor since we failed to find aberrant accumulation of TGF-βR-WT Tfh in TGF-βR–KO:TGF-βR-WT mixed BM chimera mice.
The origin of TGF-β controlling Tfh cell homeostasis remains an open question, regarding the ubiquitous expression of this cytokine within the organism. However, it is possible that Tfh cells exhibit a difference in sensitivity to TGF-β during their life. As BCL6 has been shown to be a repressor of TGF-β signaling (58), recently differentiated Tfh cells might survive the proapoptotic effect of TGF-β and later, when their BCL6 levels are reduced (59), they might become susceptible to TGF-β–induced apoptosis. Indeed, a small population of Tfh cells remains long after the immune response and maintains BCL6 at low levels (59). In response to immunization or infection, as suggested for CD8+ T cells, a large wave of TGF-β produced by the organism and observed after day 8–10 could increase TGF-β signaling and thus Tfh sensitivity to apoptosis (57). Interestingly, both Tfr cells and CD8+ Tregs demonstrated surface staining for latency-associated peptide that noncovalently binds to mature TGF-β and controls its activity (data not shown). Both cell subsets would represent attractive candidates as the GC source of TGF-β.
In addition to affecting the sensitivity of Tfh cells to apoptosis, our work revealed that TGF-β signaling in T cells is also required for the thymic development of CD8+ Tregs, known to regulate the size of Tfh cells by inducing their apoptosis (19, 21). Interestingly, other regulatory cell populations that also recognize self-peptides such as FOXP3+ Tregs, CD8αα+ intraepithelial lymphocytes, and invariant NKT (iNKT) cells (60–62), differentiate in the thymus in a TGF-β–dependent manner, emphasizing a fundamental role for TGF-β in the differentiation of self-reactive regulatory cells. Though the mechanisms by which TGF-β controls the development of all of these regulatory subsets are not completely understood, some similarities can be pointed out. Similar to iNKT cells, it is likely that the development of CD8+ Tregs undergoes different development stages based on CD44 and CD122 expression that involves both IL-15 and TGF-β (60, 63). Previously, CD8+ Treg development has been shown to be dependent on IL-15 with the CD44+CD122+ cell population being significantly depleted in IL-15–KO mice with a consequent absence of fully differentiated CD44+CD122+Ly49+CD8+ Tregs, though the acquisition of Ly49 is known to be independent of IL-15 (64). Our data suggests that the development of CD8+ Tregs is blocked at the CD44+CD122+ stage in the absence of TGF-β signaling where precursors massively accumulate and fail to rapidly acquire Ly49 implying that the optimal acquisition of Ly49 is under TGF-β control. Thus, TGF-β would accelerate the differentiation/maturation process of CD8+ Tregs. In TGF-βR–KO mice, we observed a delay in CD8+ Treg development. Very few CD8+ Tregs were produced in the thymus during the first 2 weeks of the animals, whereas similar numbers to that observed in TGF-βR-WT mice were present after 2 weeks of age. These data suggest that either other factors could gradually take over in the absence of TGF-β signaling control or that TGF-β signaling contributes only to the early development of CD8+ Tregs. Interestingly, such a delay in the thymic development of FOXP3+ regulatory cells in the absence of TGF-β control has also been reported but identification of contributing factors remains elusive (62). Thus, we propose that TGF-β enhances the generation of regulatory cell populations in the thymus to allow their rapid presence at the periphery and allow early control of the homeostasis of putative autoreactive lymphocytes. However, it is important to note that in addition to their resistance to apoptosis, Tfh cells escaping TGF-β control could also be refractory to regulatory mechanisms mediated by FOXP3+ CD4+ Tfr cells or other regulatory cell populations similar to total CD4+ T cells lacking the TGF-β receptor that have been described as being refractory to FOXP3+ CD4+ T cells (28). Whether TGF-β production by these regulatory cell populations could also contribute to controlling autoreactive Tfh cells, and thus B cell autoreactivity, should be the focus of future investigations.
In sum, the data reported here demonstrate that TGF-β signaling in T cells is crucial to restrain the size of the Tfh cell population, which, when left unchecked, leads to a break in B cell tolerance. By increasing their sensitivity to apoptosis, TGF-β exerts an intrinsic cellular mechanism preventing the damaging accumulation of Tfh cells. Given that an increased frequency of Tfh cells has been shown to positively correlate with autoantibody production in patients suffering from SLE (13), and TGF-β production is deficient in SLE patients (65) and lupus-prone (New Zealand Black and White) F1 mice (66), the modulation of Tfh cells by TGF-β might represent a promising therapeutic intervention in diseases associated with B cell autoreactivity. Taken together, these results highlight a new role for TGF-β in CD4+ T cell subset development with important implications for understanding the loss of B cell tolerance mechanisms.
Methods
Mice.
Tgf-βR2fl/fl CD4-Cre (TGF-βR–KO) mice were previously reported (28). Cre-negative littermates were used as WT controls. DN-TGF-βRII (TGF-βR-DN) mice were provided by Ronald Gross (National Cancer Institute, NIH, Bethesda, Maryland, USA) (32). FOXP3gfp and TEa mice were provided by Alexander Y. Rudensky (Sloan-Kettering Institute, New York, New York, USA). LSL-TβRICA mice were generated in house as previously described (38). CD3-KO, C57BL/6 (B6), Thy1.1 congenic B6, and Rag2–/– (Rag-KO) mice were purchased from Charles River Laboratories. Mice were housed and bred in a specific pathogen-free animal facility, AniCan, at the Cancer Research Center Lyon.
Cell isolation and adoptive cell transfer.
Single-cell suspensions were prepared from the thymus, spleen, or peripheral lymph nodes in the case of autoimmunity (pLN; pool of inguinal, brachial, axillary, and cervical lymph nodes) or draining lymph nodes (dLN; inguinal) in the case of immunization by manual disruption using glass slides. For cell sorting, cells were first purified with Miltenyi Biotec beads followed by sorting on a FACSAria (BD Biosciences). For adoptive cell transfer, CD4+ T cells were prepurified by CD4 negative selection (Miltenyi Biotec) followed by sorting to high purity before 5 × 105 CD4+ T cells were injected into recipient mice. For in vitro Tfh differentiation, CD4+B220–PD-1–CXCR5–CD44loCD62LhiFOXP3– T cells were isolated by a combination of CD4+ Miltenyi beads and cell sorting before culture. For real time qPCR, CD8+ Tregs were purified by CD8 negative selection followed by cell sorting to high purity.
Mouse immunization.
Mice were injected subcutaneously in the tail base with 2 × 50 μl of keyhole limpet hemoctyanin (KLH; 0.5 mg/ml; Calbiochem) emulsified in an equal volume of complete Freund adjuvant (CFA; 1 mg/ml; Sigma-Aldrich). Control mice were injected with CFA alone. Mice were sacrificed, and the draining inguinal lymph nodes were examined on day 7 or 21 following immunization.
Antibodies and flow cytometry.
Cells were preincubated with anti-CD16/32 and stained for 30 minutes at 4°C with the following antibodies. CD4 (RM 4-5), PD-1 (RPMI-30), B220 (RA3-6B2), ICOS (7E.17G9), BTLA (8F4), GL-7 (GL-7), CD8 (53-6.7), Ly49C/I/F/H (14B11), CD122 (TM-b1), CD40L (MR1), FOXP3 (FJK-16s), CD90.1 (Thy1.1), Vα2 (B20.1), Vβ6 (RR4-7), IL-21 (FFA21), and IFN-γ (XMG1.2) were purchased from eBioscience; CXCR5 (2G8), CD44 (1M7), CD3 (145-2C11), CD19 (1D3), CD95 (J02), BCL6 (K112-91), BCL2 (3F11), and Ki-67 (B56) were purchased from BD Biosciences. Biotinylated mouse TGF-βRII was purchased from R&D Systems. Streptavidin PeCy7, FITC, and APC (BD Biosciences) were also used. Flow cytometric analysis was carried out using a CyAn (Beckman Coulter) or BD Fortessa (BD Biosciences), and analysis of cells was performed using FlowJo software (Tree star Inc.). Fluorescence minus one (FMO) controls were used to set positive staining gates. For apoptosis staining, cells were incubated at 37°C for 3 hours in RPMI media without fetal calf serum prior to staining with annexin V and 7-AAD (BD Biosciences) in appropriate binding buffer (BD Biosciences) according to the manufacturer’s instructions. For intracellular cytokine staining, cells were stimulated with 500 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) for 3 hours in the presence of Golgiplug (BD Pharmingen). Cells were fixed and permeabilized using the BD cytofix/cytoperm kit (BD Pharmingen). For intranuclear staining, the FOXP3 staining kit was used (eBioscience).
Immunofluorescence histology.
Kidney tissues were embedded in Tissue-Tek OCT compound (Sakura Finetek) and snap frozen over liquid nitrogen. Sections (6 μm) were cut with a Leica X cryostat onto Poly-Prep slides (Sigma-Aldrich). Slides were fixed in cold acetone/ethanol (3:1) and blocked with 1% BSA (Sigma-Aldrich). Goat anti-mouse IgG Alexa Fluor 488 (Invitrogen) was used to detect IgG immune complex deposition. Slides were mounted with fluoromount (Sigma-Aldrich) and analyzed with a Zeiss 780 Confocal microscope.
BM chimeras.
BM was isolated and depleted of CD3+ T cells using Miltenyi Biotec anti-CD3 beads. Donor BM–derived cells (1 × 106) were injected intravenously into Rag-KO recipient mice that had been irradiated (8 Gy). Mice were analyzed 5 to 6 weeks after reconstitution.
ELISA.
IgG-specific dsDNA was detected using a commercially available ELISA kit according to the manufacturer’s instructions and read at a wavelength of 450 nm (Alpha Diagnostic International). Values (U/ml) were automatically generated from a standard curve. For the antigen-specific ELISA, 96-well plates (Nunc) were coated overnight with 2 μg of KLH peptide, blocked with 1% BSA/PBST, and incubated with serial serum dilutions. Bound Ig was detected using anti-IgG2a (Invitrogen) directly labeled with streptavidin-HRP conjugate. Color was visualized by the addition of o–phenylenediamine dihydrochloride (OPD; Sigma-Aldrich). Absorbance at wavelength 490 nm was read using a microplate reader (Tecan), and OD values were compared.
Real-time PCR analysis.
Signal joint TREC analysis to detect recent thymic emigrant status of peripheral T cells was performed as described (67). Genomic DNA was isolated from FACS-sorted CD8+ Tregs using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s instructions. SjTREC and CD45 primers and probes with a FAM reporter dye and TAMRA quencher dye were used as published (68, 69). A CD45 reference gene was used to correct for input genomic DNA of purified lymphocytes. A ROX internal reference dye (Invitrogen) was added to the mixture. The reaction was run on an ABI Prism 7000 Sequence Detection System (Applied Biosystems), and fold-change was calculated using the 2–ΔΔCt method.
In vitro Tfh differentiation.
CD4+PD-1–CXCR5–CD44loCD62Lhi FOXP3– T cells were isolated by cell sorting and cultured with irradiated (1,500 rad) splenocytes from congenic WT mice plus soluble CD3 (1 μg/ml) in the presence of polarizing reagents for 4 or 7 days. CD4+ T cells (5 × 105) were cultured in complete RPMI media (RPMI, 10% FCS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin) with 1 × 106 splenocytes in 96-well flat bottom plates. Polarizing reagents for the generation of Tfh cells were 45 ng/ml IL-21 and 50 ng/ml IL-6. Cells were supplemented with 45 ng/ml IL-21 on day 2 of culture and IL-2 (200 U) on day 3 and day 6. Recombinant cytokines were purchased from R & D systems.
Statistics.
Mean values and SEM were calculated. An unpaired, 2-tailed Student’s t test was used as appropriate to calculate significance between 2 groups. P ≤ 0.05 was considered significant and illustrated with an asterisk (*). All statistical analysis was carried out with GraphPad Prism Version 4. Nonsignificant comparisons are noted in the figures.
Study approval.
Experiments were performed in accordance with the animal care guidelines of the European Union and French laws and were validated by the local Animal Ethic Evaluation Committee, Comité d’Ethique en Expérimentation Animale (CECCAPP; Lyon, France).
Supplementary Material
Acknowledgments
We thank the staff of the core facilities at the Cancer Research Center Lyon and Julie Noiret for their technical assistance. We also thank Colin Havenar-Daughton and Valerie Dardalhon for critically reading the manuscript as well as all the Marie lab members for their helpful discussions. We would also like to thank Isabelle Durand for expert cell sorting. This work was supported by grants from Atip-Avenir program (to J.C. Marie), FRM INE20091217951 (to J.C. Marie), ANR investissement d’avenir ANR-10-LABX-61 (to J.C. Marie), and the Fondation Schueller-Bettencourt. M.J. McCarron was supported by a grant from the Foundation for Medical Research (Fondation pour la Recherche Médicale; SPF20110421356). J.C. Marie is a Helmholtz Association investigator.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(10):4375–4386. doi:10.1172/JCI76179.
References
- 1.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 4.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237(1):72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 5.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106(6):1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 6.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179(8):5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 7.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207(2):353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10(4):385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson N, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 14.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435(7041):452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein JS, Hernandez SG, Craft J. T cells that promote B-Cell maturation in systemic autoimmunity. Immunol Rev. 2012;247(1):160–171. doi: 10.1111/j.1600-065X.2012.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206(3):561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballesteros-Tato A, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai G, et al. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. J Immunol. 2012;189(3):1294–1302. doi: 10.4049/jimmunol.1102948. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011;23(6):446–452. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leavenworth JW, Tang X, Kim HJ, Wang X, Cantor H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J Clin Invest. 2013;123(3):1382–1389. doi: 10.1172/JCI66938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan BA, Kraj P, Weber DA, Ignatowicz L, Jensen PE. Positive selection of a Qa-1-restricted T cell receptor with specificity for insulin. Immunity. 2002;17(1):95–105. doi: 10.1016/S1074-7613(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 23.Alexander CM, Tygrett LT, Boyden AW, Wolniak KL, Legge KL, Waldschmidt TJ. T regulatory cells participate in the control of germinal centre reactions. Immunology. 2011;133(4):452–468. doi: 10.1111/j.1365-2567.2011.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187(9):4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 27.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25(3):455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25(3):441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Dang H, et al. SLE-like autoantibodies and Sjogren’s syndrome-like lymphoproliferation in TGF-β knockout mice. J Immunol. 1995;155(6):3205–3212. [PubMed] [Google Scholar]
- 30.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly — TFH cells in human health and disease. Nat Rev Immunol. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y, et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol. 2013;191(4):1614–1624. doi: 10.4049/jimmunol.1300479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191(7):1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaeth M, et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med. 2014;211(3):545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195(1–2):121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Bartholin L, et al. Generation of mice with conditionally activated transforming growth factor β signaling through the TβRI/ALK5 receptor. Genesis. 2008;46(12):724–731. doi: 10.1002/dvg.20425. [DOI] [PubMed] [Google Scholar]
- 39.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7(2):197–208. doi: 10.1016/S1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 40.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209(7):1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannons JL, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32(2):253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SK, et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37(5):880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32(5):642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207(10):2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A. 1996;93(5):2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9(12):845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 49.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–181. doi: 10.1016/S1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 50.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7(6):443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 51.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10(4):375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208(5):987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havenith SH, et al. CXCR5+CD4+ follicular helper T cells accumulate in resting human lymph nodes have superior B cell helper activity. Int Immunol. 2014;26(3):183–192. doi: 10.1093/intimm/dxt058. [DOI] [PubMed] [Google Scholar]
- 55.Manoussakis MN, Tzioufas AG, Silis MP, Pange PJ, Goudevenos J, Moutsopoulos HM. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin Exp Immunol. 1987;69(3):557–565. [PMC free article] [PubMed] [Google Scholar]
- 56.Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013;8(4):e60726. doi: 10.1371/journal.pone.0060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31(1):131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Long J, Dai F, Liang M, Feng XH, Lin X. BCL6 represses Smad signaling in transforming growth factor-β resistance. Cancer Res. 2008;68(3):783–789. doi: 10.1158/0008-5472.CAN-07-0008. [DOI] [PubMed] [Google Scholar]
- 59.Kitano M, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34(6):961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Doisne JM, et al. iNKT cell development is orchestrated by different branches of TGF-β signaling. J Exp Med. 2009;206(6):1365–1378. doi: 10.1084/jem.20090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konkel JE, et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol. 2011;12(4):312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9(6):632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 63.Castillo EF, Acero LF, Stonier SW, Zhou D, Schluns KS. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 2010;116(14):2494–2503. doi: 10.1182/blood-2010-03-277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci U S A. 2011;108(5):2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. Decreased production of TGF-β by lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1998;160(5):2539–2545. [PubMed] [Google Scholar]
- 66.Saxena V, et al. Dual roles of immunoregulatory cytokine TGF-β in the pathogenesis of autoimmunity-mediated organ damage. J Immunol. 2008;180(3):1903–1912. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sempowski GD Rhein ME. Measurement of mouse T cell receptor excision circles. Curr Protoc Immunol. 2004;Chapter 10:Unit 10.31. doi: 10.1002/0471142735.im1031s63. [DOI] [PubMed] [Google Scholar]
- 68.Broers AE, et al. Quantification of newly developed T cells in mice by real-time quantitative PCR of T-cell receptor rearrangement excision circles. Exp Hematol. 2002;30(7):745–750. doi: 10.1016/S0301-472X(02)00825-1. [DOI] [PubMed] [Google Scholar]
- 69.den Braber I, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36(2):288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.