Abstract
Millions of patients worldwide are affected by craniofacial deformations caused by congenital defects or trauma. Current surgical interventions have limited therapeutic outcomes; therefore, methods that would allow cartilage restoration are of great interest. A number of studies on embryonic limb development have shown that chondrogenesis is initiated by cellular condensation, during which mesenchymal progenitors aggregate and form 3D structures. Here, we demonstrated efficient regeneration of avascular elastic cartilage from in vitro–grown mesenchymal condensation, which recapitulated the early stages of chondrogenesis, including transient vascularization. After transplantation of vascularized condensed progenitors into immunodeficient mice, we used an intravital imaging approach to follow cartilage maturation. We determined that endothelial cells are present inside rudimentary cartilage (mesenchymal condensation) prior to cartilage maturation. Recreation of endothelial interactions in culture enabled a recently identified population of adult elastic cartilage progenitors to generate mesenchymal condensation in a self-driven manner, without requiring the support of exogenous inductive factors or scaffold materials. Moreover, the culture-grown 3D condensed adult–derived progenitors were amenable to storage via simple freezing methods and efficiently reconstructed 3D elastic cartilage upon transplantation. Together, our results indicate that transplantation of endothelialized and condensed progenitors represents a promising approach to realizing a regenerative medicine treatment for craniofacial deformations.
Introduction
It is becoming clear that manipulating dynamic intercellular interactions with proper spatiotemporal control is essential for developing 3D organs from stem cells (1). For example, pioneering studies using animal models have suggested that endothelial cells not only form passive conduits to deliver nutrients and oxygen but also support proper organogenesis and regeneration through the elaboration of paracrine trophogens and/or direct cell-cell contact, especially in well-vascularized organs such as the liver, pancreas, and lungs (2–6). In parallel with these improved understandings of basic developmental biology, early human organogenesis is now being recapitulated in a dish to grow 3D rudimentary organs (organ buds) from stem cells by reconstituting organogenetic cellular interactions (7). However, the promise of rudimentary organ self-organization for generating relatively simple and “avascular” tissues has barely been explored.
The earliest events during cartilage development, specifically that of hyaline cartilage, have been extensively studied in the context of limb development. In this process, the progenitor cells condense to form an immature tissue aggregate, called a mesenchymal condensation, and they develop into fully mature cartilage with significant amounts of extracellular matrix (ECM) postnatally. However, little is known about the earliest cellular processes in ear (elastic) cartilage development, which also require self-organization. Here, we identified the presence of endothelial cells during the formation of rudimentary ear cartilage from mesenchymal progenitors. By reconstituting intercellular interactions in culture, endothelial cells stimulate the condensation of recently identified human ear–derived cartilage progenitor cells (CPCs) (8, 9) in a self-driven manner, without any exogenous support. These 3D condensed progenitors can be stored until future transplant use by a simple slow-freezing method. Importantly, we found that the transplantation of progenitor-derived mesenchymal condensations with endothelial cells much more efficiently reconstructed 3D cartilage compared with conventional methods. Overall, these results suggest the importance of rudimentary cartilage (mesenchymal condensation) transplants that include supporting cell types for growing a relatively simple and avascular tissue, which could be useful for realizing next-generation regenerative medicine therapies.
Results
Early vascularization of murine CPCs prior to terminal differentiation.
Adult cartilage is a unique avascular, aneural, and alymphatic tissue. Due to the simplicity of this tissue, tissue engineering is considered to be the most promising and relevant method for treating chondrogenic defects (10). Although previous molecular studies have shown that mesenchymal progenitors or dedifferentiated chondrocytes can be stimulated to adopt a chondrogenic fate by extrinsic factors, such as TGFs or FGFs (11), little is known about the cellular dynamics that allow progenitor cells to form a 3D mass of cartilage. To establish a method for stimulating chondrocyte differentiation of ear-derived CPCs with high efficiency, we initially sought to analyze the ontogenetic multicellular dynamics during chondrogenesis. Specifically, we focused on the earliest process of chondrogenesis, during which mesenchymal condensation is established, to identify the critical cellular types to recapitulate in culture.
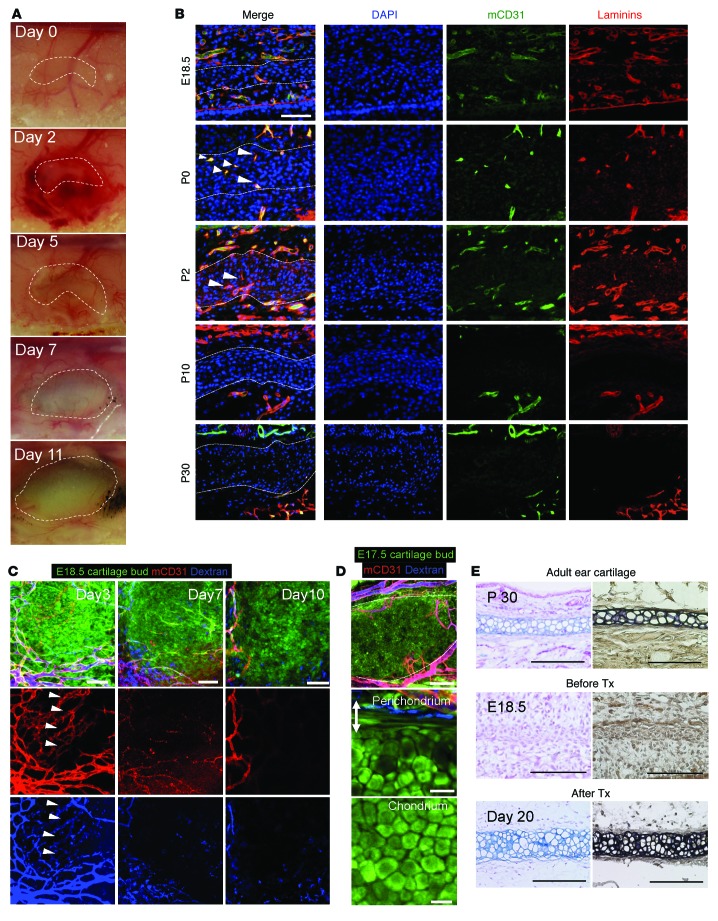
To gain insight into the dynamic cellular processes that occur during early chondrogenesis, we established a live imaging platform to track the fate of CPCs during ear-derived elastic cartilage development (8, 9). An explanted immature ear cartilage sample was introduced under a transparent cranial window, because the easy optical access allowed for repeated, long-term, and noninvasive imaging (12, 13). Interestingly, we found that the immature cartilage was vascularized within 3 days after transplantation (Figure 1A). To eliminate the possibility of this result being an artifact related to the ectopic transplant model, we performed histological analysis of native murine ear cartilage at multiple developmental stages. The presence of endothelial cells supported by basement membrane proteins was confirmed inside the mesenchymal condensation from P0 to P2, as visualized by coimmunostaining for mCD31 and laminins (Figure 1B). To further analyze the cartilage formation process, we performed repeated intravital confocal microscopy, and the results showed the formation of a clearly patent endothelial network inside of and/or adjacent to the immature cartilage (Figure 1C, arrowheads), as visualized using fluorophore-conjugated dextran and an Alexa Fluor 647–conjugated mouse-specific CD31 (mCD31) antibody infused via tail-vein injection.
Figure 1. Rudimentary ear cartilage (mesenchymal condensation) is transiently vascularized in vivo.
(A) Gross observations of E18.5 CAG-EGFP murine immature cartilage transplants at multiple time points. (B) Immunohistological analyses of mCD31 and laminins in ear cartilage from P0 to P30. Dotted line indicates the border between the chondral and perichondral layers of the mouse ear cartilage. Scale bar: 50 μm. (C) Intravital confocal images of the same field of view at 3, 7, and 10 days after transplantation. Arrowhead indicates the vascularized area of the transplants. Endothelial cells and blood perfusion were visualized intravitally using Alexa Fluor 647–conjugated mCD31 antibody and fluorophore-conjugated dextran injected via the tail vein. Scale bars: 75 μm. (D) Formation of mature cartilage 20 days after transplantation. The developed chondral layer was composed of homogenous polygonal mature chondrocytes, whereas the perichondrium (double-headed arrow, outer fibrous layer) was composed of spindle-shaped putative progenitor cells that were proximate to blood vessels, similar to normal ear cartilage. Scale bars: 200 μm (top and middle panels) and 25 μm (bottom panel). (E) Terminal differentiation from murine progenitor cells into mature chondrocytes after transplantation into a cranial window. Alcian blue and EVG staining confirmed the formation of mature elastic cartilage from E18.5 immature cartilage transplants after 20 days compared with E18.5 and P30 cartilage sections. Tx; transplantation. Scale bars: 100 μm.
Interestingly, the mass and volume of the immature cartilage dramatically increased soon after vascularization, suggesting an extensive amplification of the progenitor cell population around days 2–7 (Figure 1, A and C). Afterward, the round CPCs adopted a polygonal chondrocyte–like morphology (Figure 1C and D, bottom), as seen in the histological analysis around days 10–20 (Figure 1E), and formed fully developed 3D elastic cartilage without any residual endothelial cells (Figure 1, C–E). Blood vessels were maintained in the perichondral layer after 20 days in both the transplant and adult cartilage (Figure 1, B and D, middle), and this tissue layer was previously shown to harbor CPCs with high chondrogenic potential in humans and mice (8, 9). The formation of mature elastic cartilage in this ectopic model was further confirmed by both Alcian blue and elastica van Gieson (EVG) staining (Figure 1E). Although the classic literature suggests an orchestration between vascular regression and skeletogenesis in the chick limb (14), few studies have reported the role of endothelial cells in embryonic ear cartilage development. Thus, it is important to note that the above results imply the importance of endothelial cells in the early stages of chondrogenesis in mammalian ear cartilage development.
To analyze whether transient vascularization occurs during cartilage regeneration in humans, human CPCs (hCPCs) were stimulated to undergo chondrogenic differentiation using a previously reported protocol (9) and introduced into a cranial window. Intravital imaging analysis showed that Kusabira-Orange–labeled (KOFP-labeled) cells were transiently vascularized until day 30, as demonstrated by the infusion of fluorophore-conjugated dextran. At day 60, the transplants had differentiated into mature chondrocytes and formed avascular cartilage (Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/JCI76443DS1). Similarly, transplants derived from pellet culture, a standard regenerative method, were also invaded by functional vessels prior to cartilage formation (Supplemental Figure 1C). Future studies are required to determine whether perichondral circulation alone, not whole parts of developing elastic cartilage, is sufficient. Thus, we observed the processes of initial vascularization and subsequent vessel regression not only during the course of natural cartilage development but also during cartilage regeneration.
Transient amplification of CPCs during the early-vascularized stage.
Macroscopic and histological analyses of developing ear cartilage showed a dramatic increase in size after the early-vascularized stage (Supplemental Figure 2A), suggesting extensive amplification of the progenitors and subsequent matrix deposition. Quantitative analysis of mCD31 and laminins costaining at multiple developmental stages showed that from P0 to P30, the average distance from the CPCs to the endothelial cells was shortest (17.8 ± 8.7 μm) at P2, indicating that P2 was the most highly vascularized stage in the immature cartilage (Supplemental Figure 2B). To examine progenitor cell proliferation during this early-vascularized stage, we performed immunohistological analysis for the cell-cycle marker Ki67 (8, 9). The presence of proliferating CPCs was demonstrated by immunostaining against the primary cell surface receptor for hyaluronan (CD44) (ref. 15 and Supplemental Figure 2C, upper panel). In addition, we quantified the number of Ki67-positive cells in the chondral layer, and the results suggested that the ubiquitous appearance of transient proliferating cells significantly peaked at P0 to approximately P2 (Supplemental Figure 2, C and D), coinciding with the well-vascularized stage (Figure 1B and Supplemental Figure 2B). These results suggest that one possible effect of early vascularization is to promote the initial expansion of CPCs.
To assess the effect of endothelial cells on cell proliferation, hCPCs were cocultured with human endothelial cells in a Transwell system. Human CPCs were isolated from the ear perichondrium, as previously described (9). We plated the primary hCPCs at a low density (1,000 cells/cm2) and then cocultured them with HUVECs, or with other cell types in control experiments, in an insert. On day 12 of the coculture, we quantified the total number of hCPCs using whole-well imaging based on nuclear staining, and the results showed extensive proliferation of CPCs in the presence of endothelial cells (Supplemental Figure 2E). Interestingly, other cell types, such as mesenchymal stem cells, dermal fibroblasts, and chondrocytes, did not stimulate this proliferation. These results support the hypothesis that the endothelial cell–specific amplification of CPCs may at least be responsible for the initial growth of immature cartilage.
Self-condensation of human ear–derived CPCs by recapitulating early endothelial cell interactions.
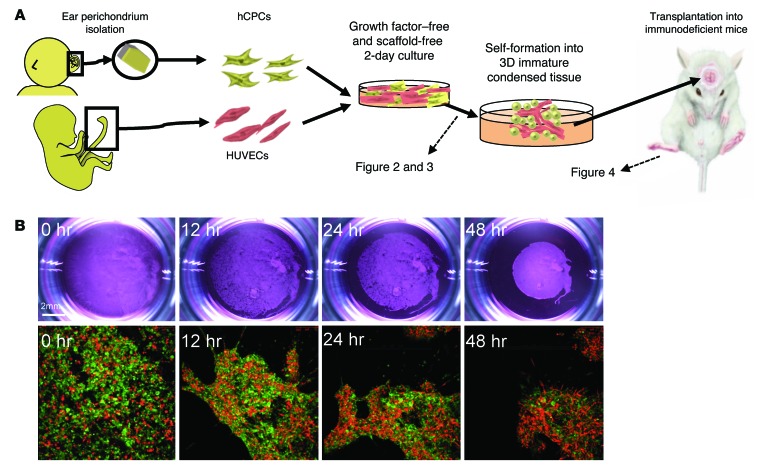
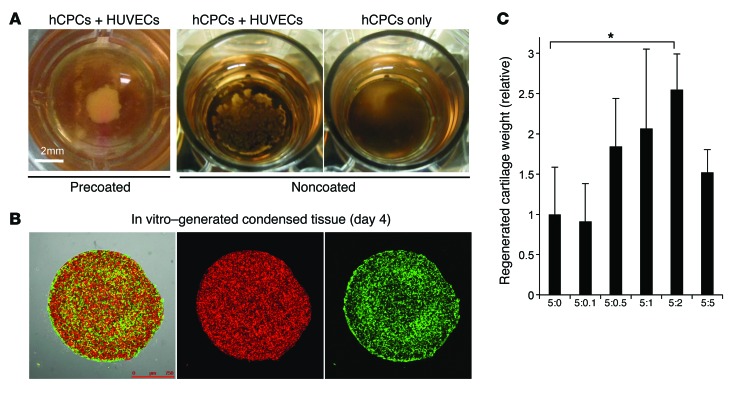
The above results led us to examine the potential contribution of early endothelial interactions in inducing progenitors to regenerate human elastic cartilage. Hence, we established a culture platform to recapitulate endothelial cell interactions in culture by modifying our published protocols (Figure 2A). After several refinements of the culture protocols, 2 × 106 hCPCs and 8 × 105 HUVECs were resuspended and plated on Matrigel that had been presolidified on a 24-well plate (7). Strikingly, without the aid of conventional scaffold materials, the mixed cells autonomously condensed to form a transplantable 3D tissue mass 24 hours after being plated onto the precoated Matrigel (Figure 2B and Supplemental Video 1). Endothelial cell–derived secreted factors provided with a Transwell were not sufficient to induce mechanically stable and condensed tissue formation. The autonomous cell condensation process required both Matrigel presolidification and endothelial cell coculture, suggesting that endothelial cells help strengthen the contractile force of the CPCs to achieve self-condensation under the defined conditions (Figure 3A). Confocal microscopic analysis showed rigorous endothelial sprouting inside the in vitro–derived condensed tissues after 4 days of culture (Figure 3B).
Figure 2. Self-driven condensation of hCPCs by endothelial cell coculture on a soft substrate.
(A) Schematic diagram of our culturing method. (B) Time-dependent changes in macroscopic and fluorescence microscopic images in vitro. hCPCs self-assembled into condensed 3D immature cartilage in vitro, without the aid of scaffolds or soluble factors. Green indicates HUVECs; red indicates human cartilage from CPCs. Bottom row original magnification: ×10.
Figure 3. Protocol optimization for growing prevascularized condensation from human progenitor cells.
(A) Comparison between Matrigel-precoated and normal cell culture plates on which hCPC single cultures or hCPC and endothelial cell cocultures were plated, showing that Matrigel presolidification was essential to induce the 3D condensation of hCPCs and HUVECs. Scale bar: 2 mm. (B) Confocal imaging of in vitro–generated condensed tissue at day 4. Green indicates HUVECs; red indicates hCPCs. Scale bar: 750 μm. (C) Protocol optimization of the hCPC and HUVEC mixing ratio for the regeneration of human cartilage. See also Supplemental Figure 3. *P < 0.01 by Mann-Whitney U test with Bonferroni’s correction.
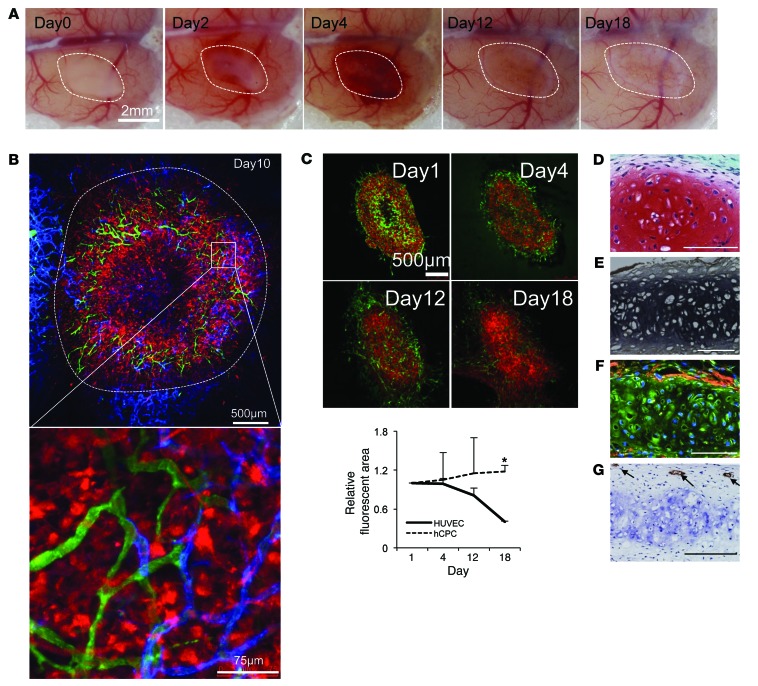
The 3D tissues, or vascularized condensed progenitors, that were generated were physically stable and could be manipulated with a spatula in vitro. Hence, we directly transplanted the tissues into immunodeficient mice to assess their terminal differentiation in vivo. Optimal ratios of hCPCs and HUVECs in the coculture were determined by subrenal capsular transplantation analysis, and the results indicated that a 5:2 hCPC/HUVEC ratio was the most efficient for cartilage regeneration in vivo (Supplemental Figure 3 and Figure 3C). Under the optimized conditions, we then transplanted the tissues into a cranial window to track the fate of the endothelial cells in vivo. Macroscopic examination revealed that the initial vascularization was recapitulated in the human vascularized transplants at day 3, similar to what was observed in murine E18.5 ear cartilage transplants (Figure 4A). At day 10, perichondral localization of FITC single-positive functional human vessels at the surrounding chondrium was demonstrated intravitally using an infusion of FITC-conjugated dextran and an Alexa Fluor 647–conjugated mCD31 antibody (Figure 4B). The quantification of transplanted fluorescent HUVECs over time showed that the majority of cells in the transplants regressed over time until 18 days after transplantation except for surrounding perichondral layers (Figure 4C). At day 30, the generated tissues were harvested, and histological analysis demonstrated that the cells in the vascularized transplants had differentiated into mature chondrocytes (Supplemental Figure 4) and formed an elastic cartilage that was rich in proteoglycans and elastic fibers (Figure 4, D and E). Furthermore, immunohistochemistry revealed that the regenerated cartilage contained a collagen I–positive perichondrium enveloping an aggrecan-positive chondral layer (Figure 4F). After cartilage maturation, the human endothelial cells remained inside the regenerated perichondral layer, similar to that observed in native cartilage (Figure 4G).
Figure 4. Recapitulation of transient vascularization prior to human cartilage progenitor maturation.
(A) Macroscopic observation of transplanted day-2 self-condensed hCPCs with HUVECs at multiple time points, showing the initial vascularization and subsequent vessel regression. Scale bar: 2 mm. (B) Intravital visualization of the vasculature inside the transplanted tissues along with the surrounding recipient vessels. Green, dextran; red, CPCs; blue, mouse-specific Alexa Fluor 647–conjugated CD31. Scale bars: 500 μm and 75 μm (inset). (C) Time-dependent changes in the number of transplanted HUVECs over CPCs. Upper panels are representative images for the subsequent quantification analysis. Data represent the mean ± SD (n = 3, *P < 0.01). Green, HUVECs; red, CPCs. (D–G) Safranin O (D) and EVG (E) staining of vascularized tissue transplants at day 30 indicated a significant number of cartilage ECM proteins characteristic of elastic cartilage. Aggrecan (green) and collagen I (red) coimmunostaining (F) showed that the reconstructed cartilage developed both perichondral and chondral layers. Human CD31 immunostaining (G) showed that human endothelial cells remained in the perichondral layer, similar to that observed in normal ear cartilage. Scale bars: 100 μm.
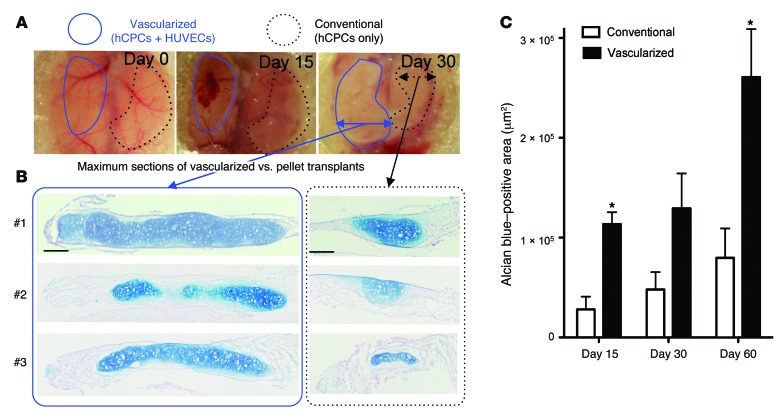
Evaluation of cartilage formation efficiency compared with a conventional pellet culture transplant.
To compare the cartilage formation efficiency of this method with that of a conventional method, CPCs were subjected to pellet culture, which is a well-characterized method for cartilage differentiation (16), and transplanted into the contralateral portion of the vascularized transplant inside the cranial window (Figure 5A). In this series of experiments, the initial seeding cell number (2 × 106) in each method was completely identical so as to make a fair comparison. Alcian blue staining at day 15 showed that the CPCs began producing proteoglycans earlier than did the pellet culture transplants (Supplemental Figure 5A, upper panel), suggesting that they were efficiently induced to undergo chondrogenic differentiation. At day 30, the vascularized transplants were strongly positive for proteoglycans (Supplemental Figure 5A, middle panel). At day 60, terminally differentiated mature chondrocytes were distributed homogenously in the majority of the transplants in which progenitors were transplanted along with endothelial cells (Figure 5B, left). In contrast, a much smaller proportion of the pellet transplants, which lacked endothelial cells, were positive for proteoglycans at days 15, 30, and 60 (Figure 5B, right). Quantification of the Alcian blue–positive area in sections taken from the widest point of the specimens showed that the average area of the vascularized transplants was 3.27-fold larger than that of the conventional pellet–cultured transplants (Figure 5C and Supplemental Figure 5B). Previous studies have reported a beneficial effect of sFlt1 expression on in vivo articular cartilage regeneration by muscle-derived stem cells, indicating that constitutive inhibition and reduction of VEGF signaling or vasculogenesis/angiogenesis is beneficial for cartilage regeneration (17, 18). Although our results at least superficially contradict these previous observations, we assume that this mechanism is potentially relevant in the later phase of our system, i.e., the vessel regression phase of the cartilage regenerative process. These results suggest that early endothelialization at the condensed stage and subsequent regression ultimately promote rapid and efficient elastic cartilage formation from human progenitors in vivo.
Figure 5. In vitro–grown condensed progenitor cells efficiently reconstruct human elastic cartilage in vivo.
(A) Gross observation of the transplants at days 3, 15, and 30. Conventional pellet culture transplants (dotted black outlines) were placed into the contralateral portion of the vascularized transplants (blue outlines). (B) Alcian blue–stained sections of the vascularized transplants and pellet transplants at day 60 (n = 3 independent transplantation experiments). Scale bars: 200 μm. (C) Quantification of Alcian blue–positive areas for the widest points of the sections. Data represent the mean ± SEM (n = 3, *P < 0.05).
The establishment of functional blood vessels is essential for successful cartilage formation from progenitors in vivo.
To elucidate whether host vessel anastomosis and functional blood perfusion are essential, we established a model in which the transplants were completely separated from the host circulation by a nanomesh (pore size = 0.45 μm). This approach allowed only the entry of diffusive factors from the preexisting host vessels and did not allow blood flow (Supplemental Figure 6, A and B). Interestingly, once functional vessel formation was inhibited, the CPCs failed to form a mature cartilaginous tissue (Supplemental Figure 6C). Immunohistological analysis showed that perfusion-deficient transplants were negative for collagen II (COL2) but were positive for caspase 3 antigen at day 15, suggesting that the apoptotic cell death pathway was activated (Supplemental Figure 6D). Thus, in addition to intercellular interactions with endothelial cells, blood perfusion into the transplants seems to be essential for the successful maturation of progenitors into chondrocytes.
En bloc cryopreservation of self-condensed progenitors.
Establishing a method to preserve pieces of vascularized condensed progenitors until future transplantation is important for facilitating the broader use of this regenerative therapy worldwide. For this reason, we sought to evaluate a potential en bloc cryopreservation method that would allow us to remove restrictions regarding the timing and volume of the transplants. Vascularized condensed progenitors grown from hCPCs were removed with a spatula on day 4 of culture and subjected to cryopreservation en bloc under 2 conditions: a slow-freezing method and a reported vitrification method (Supplemental Figure 7A and ref. 19). We examined cell viability just after thawing based on trypan blue staining, and the results showed that the slow-freezing method using TC-Protector was quite effective for tissue cryopreservation in terms of cellular survival (88% cells were viable with the slow-freezing method, whereas only 74% were viable with the vitrification method). Based on this optimization, human tissues cryopreserved for 30 days were thawed and directly transplanted into the subcutaneous space of immunodeficient mice. One month after transplantation, the hCPCs had successfully reconstructed human cartilage in vivo (Supplemental Figure 7, B and C). These results suggested that the generated 3D and vascularized condensed progenitors could be stored and ready to use for transplantation using a conventional slow-freezing technique without losing their tissue-regenerative capacity.
Discussion
The spatiotemporal control of dynamic cellular interactions is essential for controlling stem cell behavior and ultimately contributes to the growth of 3D and complex organs from stem cells (20). Although the importance of supporting cell types in well-vascularized organ regeneration is being intensely studied, little is known about avascular organs such as cartilage. To the best of our knowledge, we are the first to report the supportive role of endothelial cells in establishing avascular tissues from human tissue–specific progenitors. More specifically, early interactions with endothelial cells trigger the initial expansion of CPCs and promote the self-aggregation of a 3D condensation of progenitors without any scaffold materials and in the absence of exogenous factors. Although the molecular mechanism of this significant effect on promoting avascular tissue regeneration remains to be determined, our results highlight the unique role of endothelial cells in elastic cartilage regeneration from progenitors. This knowledge presents a possible new paradigm in regenerative biology, i.e., the importance of intercellular communication, that could be applied to the regeneration of simple and avascular tissues as well as complex organ systems.
Due to its avascular nature, a number of antiangiogenetic proteins have been purified from extracts of adult cartilage (21). Most studies have specifically used fully developed mature cartilage, which inhibits angiogenesis from surrounding vessels. From this perspective, our live imaging system provides a new tool for analyzing the chronological process of the exclusion of preexisting vessels, rather than the protection in the mesenchymal condensation that naturally occurs during the early stages of chondrogenesis. Further studies on time-dependent molecular and cellular changes related to vascular regression will possibly aid in the identification of a new antiangiogenic agent for targeting tumor neovascularization.
Millions of reconstructive surgeries for craniofacial deformities or traumas are performed annually worldwide (22). Current surgical treatments generally rely on the use of autologous tissue grafts, such as rib cartilage. However, these approaches place a significant burden on donor sites and are associated with poor long-term tissue maintenance, highlighting the need for novel therapeutic strategies. To overcome these limitations, we have recently identified a promising progenitor cell population in the adult ear perichondrium, both in humans and mice, which has been shown to regenerate both chondrium and perichondrium (8, 9). The maintenance of self-renewing progenitors in the perichondrium is expected to maintain the regenerated tissue over the long term.
Current regenerative strategies for 3D cartilage reconstruction follow the basic principles of tissue engineering and use 3 components: cells, scaffolds, and growth factors (23). However, ensuring the efficient regeneration of a cartilage structure clearly remains a major challenge (24). More specifically, only a small fraction (10%~20%) of mesenchymal progenitors have been shown to successfully undergo mature chondrocyte differentiation in vivo (25), highlighting the need for strategies to stimulate efficient cartilage formation from human progenitor cells. We succeeded here in establishing a simple and highly efficient regenerative technique by transplanting rudimentary cartilage (mesenchymal condensation) self-assembled from cocultures of human progenitor cells and endothelial cells. Upon transplantation, these tissues efficiently regenerated elastic cartilage by mimicking transient vascularization.
Notably, we also demonstrated that the generated 3D tissues could be stored using a conventional slow-freezing technique. Preserved vascularized condensed progenitors were ready to use for transplantation and maintained their tissue-reconstructive capacity in vivo. This potential for cryopreservation will dramatically accelerate the clinical application of this method worldwide, because limitations associated with the location and timing of culture could be overcome in the near future. Taken together, when combined with HLA-matched endothelial cells and our recently identified adult progenitor cells, the transplantation of allogeneic vascularized condensed progenitors represents a promising new approach for the realization of next-generation regenerative medicine therapies for patients with craniofacial defects.
Methods
Isolation and cultivation of hCPCs.
Elastic cartilage samples were obtained from microtia patients. We stripped off the adipose tissue and microscopically separated the perichondral layer. The dissected tissues were then cut into small pieces and digested for 2 hours at 37°C with shaking in PBS containing 0.2% type II collagenase (Worthington Biochemical Corp.). The cells were passed through a 100-μm nylon mesh (BD Biosciences) and then washed 3 times with PBS. The cell suspensions were cultured in DMEM/F12 medium (Sigma-Aldrich) supplemented with 10% FBS (lot 7219F; ICN Biochemicals Inc.) and 1% antibiotic-antimycotic solution (Sigma-Aldrich) in 5% CO2 at 37°C.
Retroviral transduction.
For live imaging, the cells were infected with retroviruses expressing EGFP or Kusabira-Orange (KOFP), as previously described (13). Briefly, the retroviral vector pGCDNsam IRES-EGFP or KOFP was used to transfect 293GP and 293GPG packaging cells (provided by Masafumi Onodera, National Research Institute for Child Health and Development, Tokyo, Japan), in which viral particle production was triggered using a tetracycline-inducible system (26). Supernatants collected from retrovirus-infected cultures were passed through a 0.45-μm filter (Whatman; GE Healthcare) and used immediately for infection. KOFP displays a major absorption wavelength maximum at 548 nm, with a slight shoulder at 515 nm, and a bright orange fluorescence, with an emission peak at 561 nm.
Transplantation in vivo.
For the mouse explanted immature cartilage transplantation experiments, pregnant GFP mice (C57BL/6-Tg [CAG-EGFP]) were obtained from Japan SLC Inc. After carefully removing the surrounding connective tissue, immature ear–derived cartilage was dissected from E18.5 CAG-EGFP mice and transplanted into preformed cranial windows in NOD/SCID mice (Sankyo). For the human cartilage reconstruction experiments, in vitro–generated vascularized condensed progenitor cells were collected by hand with a microspatula and directly transplanted into the cranial windows or subcapsular sites of NOD/SCID mice (13). The transplantation procedures were described in our previous article (27). Subcutaneous transplantation was also used in a part of this study aimed at future clinical use, since the primary indication for elastic cartilage regeneration will be craniofacial deformities, as noted in our previous article (9). As controls, CPCs from the same donor were also subjected to conventional pellet culture (28). Approximately 2 × 106 cells were placed in a 15-ml polypropylene tube and centrifuged at 500 g for 5 minutes at 4°C. After 2 days of culture in chondrogenic induction medium (9), the pelleted cells were introduced into the contralateral portion of the cranial window. The in vivo fate of the transplanted cells was monitored by intravital imaging using a Leica TCS SP5 confocal microscope (Leica Microsystems).
Visualization of functional blood vessels in vivo.
Tail-vein injections of 1% tetramethylrhodamine-conjugated dextran (MW 2 × 106) and FITC-conjugated dextran (MW 2 × 106) (all from Invitrogen) were used to identify vessel lumens. To visualize the murine vasculature, Alexa Fluor 647–conjugated mouse-specific CD31 antibody (BD Biosciences — Pharmingen) was injected intravenously (27).
Tissue processing and immunostaining.
Tissues were fixed overnight at 4°C in 4% PFA, processed, and embedded in paraffin. Transverse sections (4 μm) were placed on MAS-coated slides (Matsunami Glass Ind. Ltd.) for either immunostaining or standard histological staining with H&E, Alcian blue, and EVG. Immunostaining was preceded by antigen retrieval in citrate buffer (pH 6.0) at 70°C. The primary antibodies used were anti-human CD31 (Dako), anti-laminins (Dako), anti-mouse CD31 (BioLegend), anti-human type II collagen polyclonal antibodies (Chemicon), and anti-Ki67 (Abcam). Tissue sections were incubated with Alexa Fluor–conjugated secondary antibodies (Life Technologies) for 1 hour at room temperature, followed by DAPI (Sigma-Aldrich) for nuclear staining. The images were acquired using a Zeiss LSM510 laser scanning microscope.
Quantification of Alcian blue–positive areas.
The widest point of the sections from each vascularized or conventional transplant were stained with Alcian blue and imaged for further quantification. Image J software (NIH) was used to quantify the Alcian blue–positive area, as described previously. Briefly, the blue color was defined by selecting 1 blue area and then clicking the Image/Adjust/Color threshold tool and the Sample button. Then, the image was converted into an 8-bit binary image, and the Alcian blue–positive area was calculated using the Analyze/Measure tool.
Endothelial cell Transwell coculture experiments.
HUVECs from passages 3–5 were used for the coculture experiments. Before being cocultured with the CPCs, the endothelial cells were plated into 6-well Transwell membrane inserts (BD Biosciences) at 3 × 104 cells/Transwell in DMEM/F12 with 10% FBS. As controls, CPCs, mesenchymal stem cells, dermal fibroblasts, and chondrocytes were plated in Transwell inserts at the same density and grown in the same manner as the endothelial cells. The Transwell inserts were placed above CPCs that had been plated at a density of 1 × 103 cells/cm2 1 day before beginning the coculture, and the cultures were fed DMEM/F12 with 10% FBS every 3 days. The cells were cultured for 12 days, fixed, and then stained with DAPI (Sigma-Aldrich) to visualize the cell nuclei. The number of cells in each well was determined using IN Cell Investigator software (GE Healthcare) based on nuclear staining.
Self-condensation of human progenitor cells by endothelial cell coculture.
Human CPCs were isolated and cultured from ear perichondrium according to a previously reported protocol (9). HUVECs (Lonza) were maintained in endothelial growth medium (EGM) (Lonza) at 37°C in a humidified 5% CO2 incubator. To generate human vascularized condensed progenitors in vitro, 1 well in a 24-well plate was coated and incubated for 10 minutes at 37°C using 300 μl of Matrigel (BD Biosciences) diluted in an equal volume of EGM. Then, 2 × 106 hCPCs and 8 × 105 HUVECs were resuspended in a mixture of EGM and DMEM/F12 medium (Sigma-Aldrich) supplemented with 10% FBS and 1% antibiotic-antimycotic solution (Sigma-Aldrich) and plated onto the Matrigel presolidified well. After 4 to 6 days of culture, 3D tissues were autonomously formed and collected by hand with a microspatula for transplantation into preformed cranial windows in NOD/SCID mice. To analyze the optimal ratio of mixing for cartilage regeneration, 2 × 106 cells composed of a mixture of hCPCs and HUVECs at ratios of 5:5, 5:2, 5:1, 5:0.5, 5:0.1, and 5:0 (hCPC/HUVEC) were cocultured and transplanted into subrenal capsular sites to compare their cartilage-forming efficiency at day 30 after transplantation.
Cryopreservation of vascularized condensed progenitors en bloc.
In vitro–generated 3D tissues were immersed in TC-Protector cell-freezing medium (BUF050; AbD Serotec, Bio-Rad) for time periods ranging from several hours to overnight and then frozen at –80°C. The tissues were stored at –80°C in a freezer for up to 30 days. To perform the transplantation experiments, the cryotubes containing the tissues were placed at room temperature to thaw the cryoprotectant solution, and the tissues were removed and briefly soaked in culture medium to remove the cryoprotectants. Then, the tissues were quickly transplanted under the skin of immunodeficient mice.
Statistics.
The data are expressed as the mean ± SD or SEM of at least 4 independent experiments. The number of each experiment is shown in the figure legends. Comparisons between 3 or 4 groups were analyzed using the Kruskal-Wallis test by rank, and post-hoc comparisons were made using the Mann-Whitney U test with Bonferroni’s correction. Two-tailed P values of less than 0.05 were considered statistically significant.
Study approval.
The mice were bred and maintained according to our institutional guidelines for the care and use of laboratory animals. All animal studies were approved by the IACUC of Yokohama City University (approval nos. 11-63 and 11-68). Elastic cartilage samples were obtained from microtia patients following the approved guidelines established by the ethics committee of Yokohama City University (approval no. 03-074).
Supplementary Material
Acknowledgments
We thank M. Enomura, T. Ogaeri, and N. Sasaki for their technical support. We are especially grateful to M. Yamato, K. Sekine, and Y.-W. Zheng for their critical evaluation of the manuscript. This work was supported by Grants-in-Aid (24106510, 24689052, and 24659785) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and by a Health and Labor Sciences Research grant (12103252). This work was also supported by Research and Development Project III grant no. 22 from Yokohama City University, Japan.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(10):4325–4334. doi:10.1172/JCI76443.
References
- 1.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120(1):59–64. doi: 10.1016/S0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294(5542):559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 6.Takebe T, et al. Self-organization of human hepatic organoid by recapitulating organogenesis in vitro. Transplant Proc. 2012;44(4):1018–1020. doi: 10.1016/j.transproceed.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Takebe T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, et al. Presence of cartilage stem/progenitor cells in adult mice auricular perichondrium. PLoS One. 2011;6(10):e26393. doi: 10.1371/journal.pone.0026393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi S, et al. Reconstruction of human elastic cartilage by a CD44+ CD90+ stem cell in the ear perichondrium. Proc Natl Acad Sci U S A. 2011;108(35):14479–14484. doi: 10.1073/pnas.1109767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28(11):1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 12.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428(6979):138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 13.Takebe T, et al. Generation of functional human vascular network. Transplant Proc. 2012;44(4):1130–1133. doi: 10.1016/j.transproceed.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Pacifici M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev Dyn. 2001;222(3):522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- 15.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26(34):7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Kubo S, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60(1):155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano T, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Takebe T, Taniguchi H. Human iPSC-derived miniature organs: a tool for drug studies. Clin Pharmacol Ther. 2014;96(3):310–313. doi: 10.1038/clpt.2014.110. [DOI] [PubMed] [Google Scholar]
- 21.Langer R, Brem H, Falterman K, Klein M, Folkman J. Isolations of a cartilage factor that inhibits tumor neovascularization. Science. 1976;193(4247):70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- 22.Goessler UR, Stern-Straeter J, Riedel K, Bran GM, Hormann K, Riedel F. Tissue engineering in head and neck reconstructive surgery: what type of tissue do we need? Eur Arch Otorhinolaryngol. 2007;264(11):1343–1356. doi: 10.1007/s00405-007-0369-y. [DOI] [PubMed] [Google Scholar]
- 23.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 24.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23(3):403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79–89. [PubMed] [Google Scholar]
- 26.Suzuki A, et al. Feasibility of ex vivo gene therapy for neurological disorders using the new retroviral vector GCDNsap packaged in the vesicular stomatitis virus G protein. J Neurochem. 2002;82(4):953–960. doi: 10.1046/j.1471-4159.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 27.Takebe T, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 28.Sakai S, et al. Rotating three-dimensional dynamic culture of adult human bone marrow-derived cells for tissue engineering of hyaline cartilage. J Orthop Res. 2009;27(4):517–521. doi: 10.1002/jor.20566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.