Abstract
Nucleotomy is a common surgical procedure to treat disc herniations. The potential occurrence of segmental instability after surgery, however, is suspected to necessitate re-operation and fusion. Although in vitro studies support the theory of destabilization after nucleotomy, a prior, in-house animal study contrarily revealed an increase in stability after surgery. To identify which structural compartment of the motion segment is decisive for increased stability after nucleotomy in vivo, the flexibilities of ovine motion segments were measured after different stepwise reductions at the anterior and posterior spinal column. Different test groups were used in which nucleotomy had been performed during surgery in vivo and under isolated in vitro conditions, respectively. In accordance with expectations, in vitro nucleotomy on ovine motion segments significantly increased flexibility. By contrast, nucleotomy significantly decreased flexibility 12 weeks after surgery. After removal of the posterior structures, however, the differences in flexibility diminished. The present results thus suggest that it might not exclusively be the trauma to the intervertebral disc during surgery which is decisive for post-operative stability, but rather adaptive mechanisms in the posterior structures. Therefore, care should be taken to minimize the damage to the posterior structures in the course of the surgical approach, which more likely compromises stability.
Keywords: intervertebral disc, nucleotomy, stability, animal study, in vivo, in vitro
1. Introduction
Nucleotomy is a common surgical procedure used to treat disc herniations combined with nerve root compressions or constrictions to the spinal cord in the middle-aged population [1,2]. Annual costs for decompressive surgery, including nucleotomy and laminectomy, amount to approximately 4 billion dollars, which is one-quarter of the total direct costs spent on the surgical treatment of back pain in the USA [3].
To decompress neural structures and to minimize the risk of reherniations, a sufficient amount of nucleus tissue needs to be removed. Experimentally, it is generally agreed that the structural damage due to nucleotomy significantly destabilizes the motion segment [4]. In vivo, however, there is a lack of consensus whether, and to which extent, segmental stability is affected and might thus contribute to failed back surgery syndrome, reoperations and, finally, the need for fusion [5–9].
Given the absence of a congruent definition for in vivo lumbar spine instability, the identification of a potential mechanical impairment of the motion segment after nucleotomy poses diagnostic problems [8,10,11]. Additionally, diagnostic tools are limited to imaging and clinical examinations. However, both have been reported to occasionally deviate from each other and, moreover, they fail to provide unambiguous biomechanical evidence for instability. Medical imaging, for example, was shown to be unreliably indicative for biomechanical instability [12]. False assessment of radiographs might be due to poor image quality and individual spinal profiles of the patient [13].
Owing to the vague knowledge of the biomechanical situation regarding segmental stability after nucleotomy in vivo, a previously published, in-house animal study offered an interesting contribution to the controversy [14]. The study revealed significantly decreased ex vivo flexibilities in ovine lumbar motion segments 12 weeks after nucleotomy when compared with the intact controls (INT). On the basis of preliminary tests and despite assuming the existence of endogenous compensatory mechanisms after nucleotomy in vivo, the authors hypothesized precisely the opposite prior to the study.
The aim of the present biomechanical study on sheep, therefore, was to closely investigate the mechanisms behind the contradictory segmental flexibilities after in vitro and in vivo nucleotomy. To illuminate to which compartment of the motion segment the discrepancy between in vitro and in vivo can be attributed, a test design was developed in which repeating flexibility tests were conducted after stepwise reductions in ovine motion segments.
2. Material and methods
Two general experimental approaches were pursued. In the in vitro part of the study, several defects in ovine motion segments, including nucleotomy, were created under laboratory conditions, each immediately followed by a flexibility measurement. For the ex vivo part, in contrast, flexibility testing was performed using ovine specimens in which nucleotomy was created during surgery 12 weeks before.
2.1. Experimental design
A total of 18 lumbar motion segments from female merino sheep aged between 2 and 4.5 years were divided into the following three test groups: in vitro 1, in vitro 2, ex vivo.
2.1.1. In vitro 1 and in vitro 2 (n = 6 each)
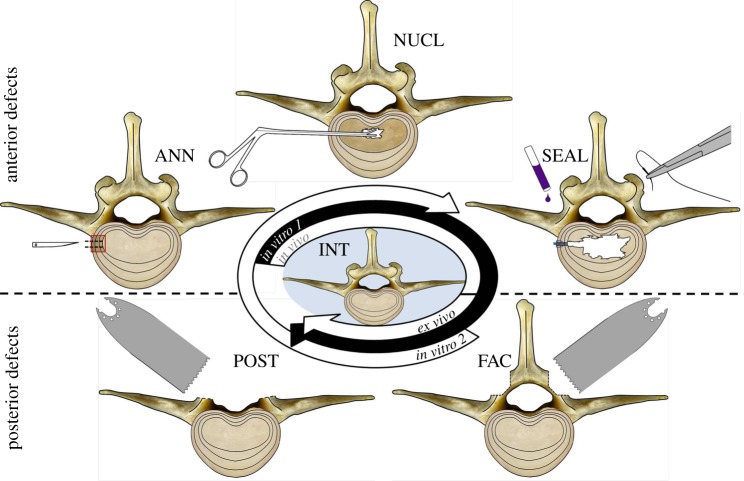
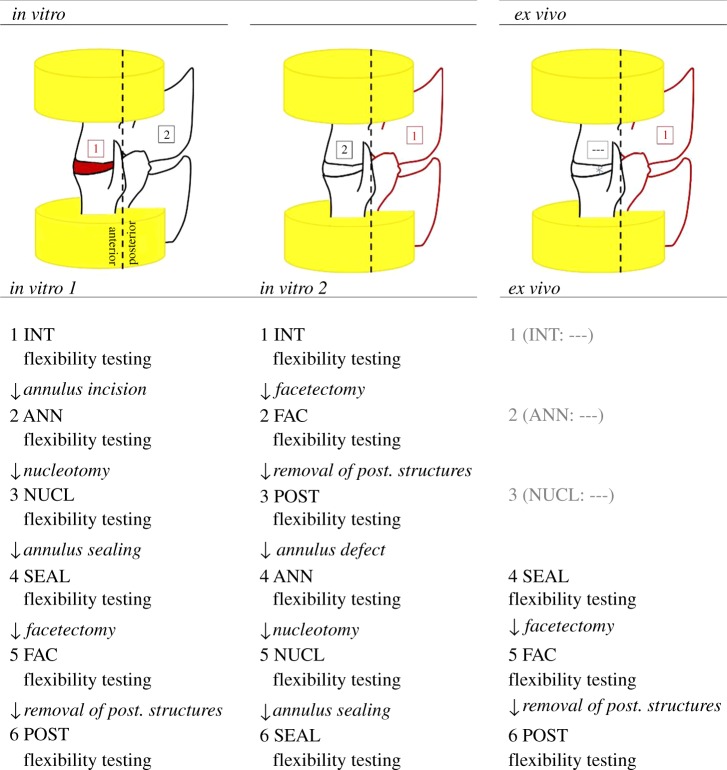
Intact lumbar motion segments (INT) served as controls for the ex vivo investigations (4 × L1/2, 4 × L2/3, 2 × L3/4, and 2 × L5/6). The mechanical influence of the following defects to the anterior and posterior spinal column was subsequently investigated (figure 1). Anterior column: annulus incision (ANN), nucleotomy (NUCL) and annulus sealant (SEAL). Posterior column: facetectomy (FAC) and total removal of posterior structures (POST). In both in vitro groups, defects to the anterior as well as the posterior column were created under laboratory conditions. For both in vitro groups and also for the ex vivo group described below, all defects were carried out essentially in the same way. ANN was created by a precise oblique cut of about 2.5 mm on the left side of the intervertebral disc using a microsurgical scalpel blade (sterile micro blades no. 367; Aesculap AG, Tuttlingen, Germany). For NUCL, 0.20 g (0.17–0.23 g) of nucleus tissue was removed in test group in vitro 1 and 0.20 g (0.17–0.21 g) in test group in vitro 2 using the annulus incision to reach the nucleus cavity. SEAL represented the state in which the former annulus incision was closed by suture and glue (Dermabond; Ethicon Products, Norderstedt, Germany), and additional superficial coverage with a collagen sponge (Lyostypt; Aesculap AG). Stepwise removal of the facet joints (FAC) and, finally, the remaining posterior structures (POST) was conducted using an oscillating saw and rongeur forceps. In POST, only the anterior spinal column with preservation of the anterior and posterior longitudinal ligaments remained. All bony and ligamentous structures posterior to the origins of the pedicles were removed. The only difference between both in vitro groups was the reversed order of defects. For in vitro 1, anterior column defects were set before the posterior and vice versa for in vitro 2. Flexibility tests were performed after each defect state. The resulting test sequences are depicted in figure 2. The differing sequences in in vitro 1 and in vitro 2 were performed to gain adequate combinations between anterior and posterior column defects for comparison with the ex vivo segments.
Figure 1.
Transverse view of ovine motion segments for illustration of the different anterior (upper row) and posterior (lower row) defects. Note that each single test step exclusively depicts the manipulation performed without inclusion of the former defect state. The test sequences of in vitro 1 and in vitro 2 started with flexibility measurements of the intact state (centre). Ex vivo, only posterior defects were measured. Anterior defects had been previously created in vivo. INT, intact; ANN, annulus incision; NUCL, nucleotomy; SEAL, annulus sealant; FAC, facetectomy; POST, posterior structures removed. (Online version in colour.)
Figure 2.
Schematic test design of subsequent defect states of the two in vitro groups and the ex vivo group. (Online version in colour.)
2.1.2. Ex vivo (n = 6)
The segments for this group were extracted from six sheep 12 weeks after surgery. The motion segments had already been used before as controls in the context of a former animal study investigating the potential of newly developed hydrogels for disc regeneration. In each sheep, the surgical procedure included the combination of ANN, NUCL and SEAL. Only the defects to the posterior spinal column were created in the laboratory. The defects were set in strict accordance with the procedure described for the in vitro groups above. For NUCL, 0.20 g (0.16–0.23 g) of nucleus tissue was removed from the intervertebral disc. Surgical access was achieved using a retroperitoneal approach from the left lateral side. Across the animals, the operated lumbar disc levels were uniformly distributed as 2 × L1/2, 2 × L2/3, 1 × L3/4 and 1 × L5/6. This was the same distribution as in vitro. No damage was caused to the posterior structures during surgery. The total study design was described in detail previously [14]. As ANN, NUCL and SEAL were created during surgery, only SEAL (immediate post-operative state) as well as FAC and POST could be measured in stepwise flexibility tests after sacrifice of the animals (ex vivo).
2.2. Preparation
All 18 motion segments of the three test groups were carefully cleaned of soft tissue while keeping the biomechanically relevant structures, including the intervertebral disc, facet joints and peri-discal ligaments, intact. To mount the specimens in the universal spine tester, the proximal and the distal end of the upper and the lower vertebra of the motion segments were embedded in polymethylmethacrylate (PMMA; Technovit 3040, Heraeus Kulzer, Wehrheim, Germany). Care was taken to adjust the transverse midplane of the intervertebral disc space horizontally between the PMMA blocks to minimize the influence of shear on flexibility measurements [15,16]. Afterwards, specimens were sealed in double plastic bags and stored at −20°C in a freezer. Prior to flexibility testing, specimens were thawed overnight at +4°C in a refrigerator.
2.3. Test protocols
Flexibility tests were performed in a custom-made spinal flexibility testing machine [17]. The range of motion (RoM) and the neutral zone (NZ) were investigated by plotting the motion data, recorded with a motion tracking system with six cameras (Vicon MX13; Vicon, Oxford, UK), against the load applied to the specimen. The RoM is the maximal deflection a specimen reaches in the main motion direction when a defined load is applied. The NZ describes the deflection that is reached with minimal resistance of the specimen. It is a good indicator of the laxity and thus of the instability of a specimen [18]. Rotation of specimens around an axis was done at a rate of 1° s−1 for flexion/extension (FE) and lateral bending (LB) right/left and at a rate of 0.5° s−1 for axial rotation (AR) left/right until a pure moment of ±3.75 Nm was reached. A total of 3.5 cycles were conducted, of which the first two cycles were used for preconditioning of the segments and the third for data analysis. During the tests, the motion segments were kept moist by spraying with physiological 0.9% saline solution.
2.4. Statistics
A Levene test showed that the data were non-normally distributed. To evaluate differences between one defect state of all three test groups, significance was tested with the non-parametric, multiple comparison Kruskal–Wallis test. Differences between one defect state of only two comparative groups were identified using the non-parametric, two-sample Mann–Whitney U-test. The unpaired, two-sample Wilcoxon signed-rank test was used when differences between two successive defect states within one experimental group were determined. Statistics calculations were performed using spss software (v. 19; SPSS Inc., Chicago, IL, USA). The level of significance was set at α = 0.05.
3. Results
3.1. In vitro 1 versus in vitro 2
RoM and NZ of the intact state did not significantly differ across all motion directions between both in vitro groups (FE: p = 0.522; LB: p = 1; AR: p = 0.631; tables 1–4). Likewise, RoM and NZ of the final defect states of in vitro 1 (POST) and in vitro 2 (SEAL) did not reveal significant differences, even though the order of defects between both groups had been reversed (FE: p = 0.631; LB: p = 0.200; AR: p = 0.749).
Table 1.
RoM of the different defect states of the test groups in vitro 1 and ex vivo. FE, flexion/extension; LB, lateral bending; AR, axial rotation; INT, intact; ANN, annulus incision; NUCL, nucleotomy; SEAL, annulus sealant; FAC, facetectomy; POST, posterior structures removed; n.s., not significantly different; n.i., no increase. p-value related to the previous defect state.
|
in vitro 1 |
ex vivo |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RoM (°) | range | increase (+%) | p-value | RoM (°) | range | increase (+%) | p-value | ||
| FE | INT | 7.5 | 6.3–8.6 | — | — | — | — | — | — |
| ANN | 7.7 | 6.3–8.7 | 3 | n.s. | — | — | — | — | |
| NUCL | 8.9 | 7.5–10.0 | 16 | 0.016 | — | — | — | — | |
| SEAL | 9.1 | 7.5–10.2 | 2 | n.s. | 4.6 | 3.4–6.8 | — | — | |
| FAC | 10.9 | 9.9–16.5 | 20 | 0.031 | 7.4 | 5.5–9.9 | 61 | 0.016 | |
| POST | 13.5 | 10.9–17.8 | 24 | 0.031 | 12.8 | 12.1–13.5 | 73 | 0.016 | |
| LB | INT | 8.4 | 5.8–9.4 | — | — | — | — | — | — |
| ANN | 8.4 | 5.8–9.7 | n.i. | n.s. | — | — | — | — | |
| NUCL | 9.8 | 7.3–10.9 | 18 | 0.031 | — | — | — | — | |
| SEAL | 10.0 | 7.5–11.0 | 2 | n.s. | 6.3 | 4.3–8.0 | — | — | |
| FAC | 11.2 | 10.4–12.7 | 12 | 0.016 | 10.3 | 9.6–11.6 | 64 | 0.016 | |
| POST | 11.3 | 10.3–13.2 | 1 | n.s. | 11.0 | 10.0–14.2 | 7 | 0.016 | |
| AR | INT | 1.2 | 0.7–1.6 | — | — | — | — | — | — |
| ANN | 1.2 | 0.7–1.7 | n.i. | n.s. | — | — | — | — | |
| NUCL | 1.7 | 1.1–2.2 | 42 | 0.016 | — | — | — | — | |
| SEAL | 1.7 | 1.1–2.2 | n.i. | n.s. | 1.0 | 0.6–1.6 | — | — | |
| FAC | 5.6 | 4.5–6.3 | 230 | 0.016 | 5.4 | 4.4–6.5 | 440 | 0.016 | |
| POST | 5.7 | 4.5–6.3 | 2 | n.s. | 5.5 | 5.0–8.6 | 2 | 0.016 | |
Table 2.
RoM of the different defect states of the test group in vitro 2. For abbreviations see table 1.
|
in vitro 2 |
|||||
|---|---|---|---|---|---|
| RoM (°) | range | increase (+%) | p-value | ||
| FE | INT | 7.3 | 4.7–9.5 | — | — |
| FAC | 10.7 | 7.6–12.0 | 47 | 0.016 | |
| POST | 13.1 | 9.8–18.4 | 22 | 0.016 | |
| ANN | 13.3 | 10.3–20.4 | 2 | n.s. | |
| NUCL | 13.8 | 11.9–23.0 | 4 | 0.016 | |
| SEAL | 14.0 | 12.0–23.1 | 1 | n.s. | |
| LB | INT | 8.3 | 6.6–11.3 | — | — |
| FAC | 9.7 | 7.9–13.3 | 17 | 0.016 | |
| POST | 10.4 | 8.0–14.9 | 7 | 0.016 | |
| ANN | 10.7 | 8.5–15.7 | 3 | 0.016 | |
| NUCL | 12.5 | 10.7–18.3 | 17 | 0.016 | |
| SEAL | 12.5 | 10.6–18.4 | n.i. | n.s. | |
| AR | INT | 1.3 | 0.7–1.7 | — | — |
| FAC | 3.4 | 2.5–6.0 | 162 | 0.016 | |
| POST | 3.7 | 2.7–7.5 | 9 | 0.016 | |
| ANN | 3.8 | 2.9–8.7 | 3 | 0.016 | |
| NUCL | 5.7 | 5.2–11.6 | 50 | 0.016 | |
| SEAL | 5.7 | 5.0–12.0 | n.i. | n.s. | |
Table 3.
NZ of the different defect states of the test groups in vitro 1 and ex vivo. For abbreviations see table 1.
|
in vitro 1 |
ex vivo |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| NZ (°) | range | increase (+%) | p-value | NZ (°) | range | increase (+%) | p-value | ||
| FE | INT | 1.6 | 0.6–1.7 | — | — | — | — | — | — |
| ANN | 1.4 | 0.9–1.7 | n.i. | n.s. | — | — | — | — | |
| NUCL | 2.9 | 2.3–4.1 | 107 | 0.028 | — | — | — | — | |
| SEAL | 3.0 | 2.5–4.2 | 3 | n.s. | 1.8 | 0.7–2.4 | — | — | |
| FAC | 3.6 | 2.7–7.3 | 20 | 0.043 | 3.5 | 2.4–3.7 | 94 | 0.028 | |
| POST | 5.8 | 3.5–7.3 | 61 | n.s. | 6.3 | 3.5–8.4 | 80 | 0.028 | |
| LB | INT | 1.7 | 0.8–2.9 | — | — | — | — | — | — |
| ANN | 2.0 | 0.7–2.6 | 18 | n.s. | — | — | — | — | |
| NUCL | 4.5 | 2.7–5.4 | 125 | 0.043 | — | — | — | — | |
| SEAL | 4.6 | 2.7–5.9 | 2 | n.s. | 2.8 | 1.3–3.7 | — | — | |
| FAC | 5.2 | 3.3–7.2 | 13 | 0.028 | 4.9 | 4.4–7.1 | 75 | 0.028 | |
| POST | 5.7 | 3.7–7.6 | 10 | n.s. | 5.9 | 4.2–8.5 | 20 | n.s. | |
| AR | INT | <0.1 | 0.0–0.2 | — | — | — | — | — | — |
| ANN | <0.1 | 0.0–0.3 | n.i. | n.s. | — | — | — | — | |
| NUCL | 0.3 | 0.1–0.5 | 42 | 0.028 | — | — | — | — | |
| SEAL | 0.2 | 0.1–0.4 | n.i. | n.s. | 0.2 | 0.1–0.6 | — | — | |
| FAC | 0.6 | 0.5–1.2 | 300 | 0.028 | 1.6 | 0.8–2.4 | 800 | 0.028 | |
| POST | 0.8 | 0.5–1.1 | 33 | n.s. | 1.8 | 1.0–2.8 | 13 | n.s. | |
Table 4.
NZ of the different defect states of the test group in vitro 2. For abbreviations see table 1.
|
in vitro 2 |
|||||
|---|---|---|---|---|---|
| NZ (°) | range | increase (+%) | p-value | ||
| FE | INT | 1.7 | 1.0–4.0 | — | — |
| FAC | 2.4 | 1.3–4.4 | 41 | 0.046 | |
| POST | 3.1 | 1.6–9.5 | 29 | 0.028 | |
| ANN | 3.0 | 1.7–12.3 | n.i. | n.s. | |
| NUCL | 5.9 | 4.5–15.2 | 97 | 0.028 | |
| SEAL | 5.8 | 4.5–15.3 | n.i. | n.s. | |
| LB | INT | 2.3 | 1.2–4.5 | — | — |
| FAC | 2.5 | 1.5–5.2 | 9 | 0.046 | |
| POST | 2.6 | 1.6–6.8 | 4 | n.s. | |
| ANN | 3.2 | 2.1–7.6 | 23 | 0.028 | |
| NUCL | 6.5 | 5.7–12.9 | 103 | 0.028 | |
| SEAL | 6.2 | 5.8–12.4 | n.i. | n.s. | |
| AR | INT | 0.2 | 0.0–0.4 | — | — |
| FAC | 0.5 | 0.1–0.8 | 150 | 0.028 | |
| POST | 0.5 | 0.1–0.9 | n.i. | 0.046 | |
| ANN | 0.4 | 0.1–1.9 | n.i. | n.s. | |
| NUCL | 1.1 | 0.6–4.3 | 175 | 0.028 | |
| SEAL | 0.9 | 0.8–3.6 | n.i. | n.s. | |
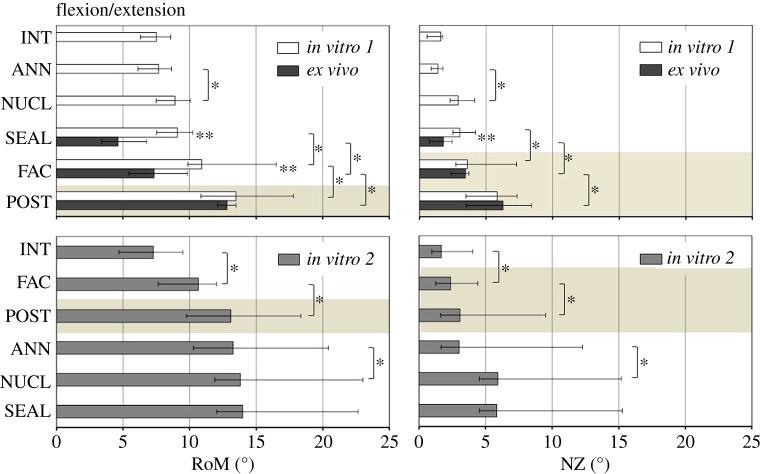
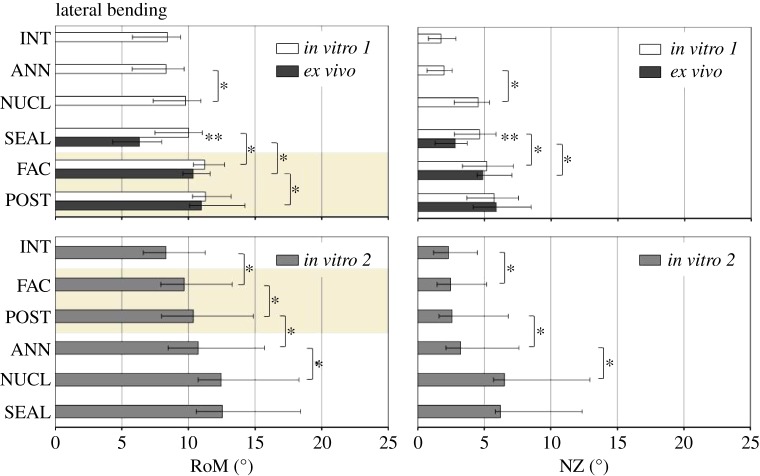
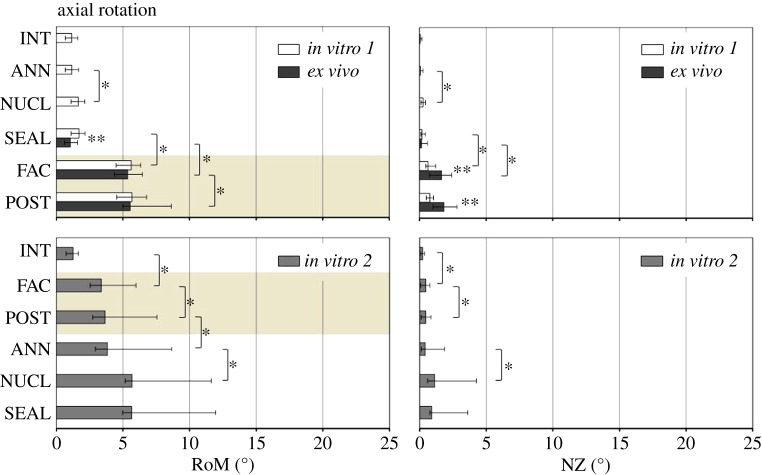
Within in vitro 1 and in vitro 2, respectively, NUCL and FAC caused significant increases in RoM and NZ compared with the prior defect state, independent of the motion direction (figures 3–5). The impact of ANN and POST on RoM and NZ, in contrast, revealed both a dependency of the motion direction as well as the test group, and hence of the combination of anterior and posterior column defects. Regarding RoM and NZ in in vitro 1, no significant influence of the annulus incision was found with intact posterior structures. By contrast, ANN in in vitro 2, with posterior structures removed, significantly increased RoM and NZ in LB, and only the RoM in AR. Similar correlations between anterior and posterior column defects were found for POST. While in in vitro 1 significant increases after removal of the posterior structures were only found for the RoM in FE (p = 0.031), in in vitro 2 significant increases in POST were found in all of the three motion directions (FE: p = 0.016; LB: p = 0.016; AR: p = 0.016). The NZ was not significantly influenced by POST in in vitro 1. By contrast, POST significantly increased NZ in in vitro 2 in FE and AR.
Figure 3.
RoM and NZ for the three test groups in subsequent defect states in FE. Asterisks reveal significant differences (p < 0.05) between (**) and within (*) the groups. Highlighted with a light brown background are the defect states in which all three test groups were comparable. INT, intact; ANN, annulus incision; NUCL, nucleotomy; SEAL, sealant; FAC, facetectomy; POST, posterior structures removed. (Online version in colour.)
Figure 4.
RoM and NZ for the three test groups in subsequent defect states in LB. Highlighted with a light brown background are the defect states in which all three test groups were comparable. Asterisks reveal significant differences (p < 0.05) between (**) and within (*) the groups. For abbreviations, see figure 3. (Online version in colour.)
Figure 5.
RoM and NZ for the three test groups in subsequent defect states in AR. Highlighted with a light brown background are the defect states in which all three test groups were comparable. Asterisks reveal significant differences (p < 0.05) between (**) and within (*) the groups. For abbreviations, see figure 3. (Online version in colour.)
3.2. In vitro versus ex vivo
Removed nucleus volumes did not differ significantly among the test groups, neither between the two in vitro groups nor between the in vitro groups and the ex vivo group (p = 0.585).
NUCL significantly increased RoM and NZ under in vitro conditions compared with INT. By contrast, by 12 weeks after surgery for ANN, NUCL and SEAL, ex vivo segments revealed a significantly decreased RoM in FE and LB compared with both intact in vitro controls (p = 0.028). The ex vivo NZ of SEAL showed no difference from the intact in vitro controls in all motion directions (p ≥ 0.075). When the ex vivo RoM of SEAL was compared with the same defect state in vitro (SEAL-in vitro 1), ex vivo segments were significantly less flexible in FE (p = 0.004), LB (p = 0.006) and AR (p = 0.037). The ex vivo NZ of SEAL was significantly smaller in FE (p = 0.002) and LB (p = 0.015) than in the in vitro equivalent. In AR, no significant difference was evident between both groups.
Stepwise removal of the facet joints and posterior structures significantly increased RoM of the ex vivo segments, independent of the motion direction (p = 0.016). Consequently, the differences between both in vitro 1 and in vitro 2, as well as ex vivo, diminished with successive removal of the posterior structures. In LB and AR, the differences had already disappeared after isolated removal of the facets. In FE, in contrast, significant differences remained after facetectomy (p = 0.017). In FE, the entire removal of the posterior structures was necessary to compensate for the differences in RoM between in vitro and ex vivo. Remarkably, after stepwise removal of the posterior structures, RoM of in vitro 2 also did not differ significantly from in vitro 1 and ex vivo, although FAC and POST in in vitro 2 were measured in combination with an intact anterior spinal column. Comparable results to RoM were found for the NZ in FE. In FE, NZ did not reveal significant differences between all three test groups in FAC and POST. In LB, the differences also diminished after facetectomy, but only between in vitro 1 and ex vivo. The NZs of the nucleotomized segments of in vitro 1 and ex vivo, however, remained significantly higher in FAC and POST than in in vitro 2 with an intact anterior column. Finally, contrasting results were found in AR compared with RoM. In FAC and POST, there were no significant differences in NZ between in vitro 1 and in vitro 2. By contrast, ex vivo NZ was significantly higher than the in vitro counterparts in both defect states.
4. Discussion
This study, using an ovine nucleotomy model, sought potential mechanisms by which the different structural compartments of the motion segment might contribute to spinal (in)stability after surgical impairment of the intervertebral disc.
The results of this study on sheep revealed that in vitro nucleotomy significantly increases the flexibility and laxity of ovine motion segments. By 12 weeks post-surgery, contrasting effects were found when comparing ex vivo with the in vitro equivalent. In FE and LB, the differences between in vitro and ex vivo, both for the RoM as well as for the NZ, diminished after removal of the posterior structures. In AR, the RoM also closely correlated with the in vitro results after removal of the posterior structures, while the NZ significantly increased. Similar RoMs and NZs in the intact state of both in vitro groups suggest a fairly good comparability between the specimens of in vitro 1 and in vitro 2. Furthermore, similar RoMs and NZs for the last measurements of both groups (POST-in vitro 1 and SEAL-in vitro 2) suggest that the reversed order of anterior and posterior defects did not have a significant influence on results.
An isolated annulus defect (ANN), combined with intact posterior structures of the motion segment (in vitro 1), did not result in a significant increase in segmental RoM and NZ, independent of the motion direction. Contrastingly, in in vitro 2, with posterior structures removed, ANN significantly increased RoM in LB and AR and also NZ in LB. In particular, the facet joints as part of the posterior structures of the spine are known to be principally involved in limiting AR [19]. In sheep, the articulating surfaces of the facet joints are cone-shaped with the lower articular processes surrounding the upper ones. This anatomy suggests a biomechanical relevance in limiting the extent of AR and LB and might, therefore, explain why ANN only affects segmental mechanics after removal of the posterior structures in in vitro 2.
The annulus sealant (SEAL), in contrast to ANN, did not have significant effects on flexibility or laxity, independent of whether the posterior structures had been previously removed or not. Given the stepwise test design, however, the relevance of this result cannot finally be clarified. In this study, significant increases in RoM due to nucleotomy might have overlapped potentially existing stabilizing effects of SEAL. Significantly higher RoM after NUCL is assumed to be caused by the structural damage to the intervertebral disc after removal of the natural nucleus tissue. This finding is in accordance with expectations prior to the study and common literature [20,21].
Although approximately equal nucleus volumes were removed in vitro and ex vivo, lower flexibility for ex vivo NUCL was found. This did not meet expectations. Intra-operative measurements on pigs suggested that defects to the anterior column of the spine result in instability of the motion segment [22]. Over a period of 12 weeks in this study, restrictions in movement might be the result of a persistent muscle activation leading to shortenings of muscles and ligaments and finally contractures.
In vivo, nucleotomy was found to provoke degenerative signs in the ovine disc within 12 weeks [23]. However, no significant annulus healing was found to occur within 12 weeks of the injury [24]. A potential stabilization based solely on scar formation at the disc, which might have compensated for the segmental instability after disruption of the natural tissue compound, can therefore be excluded. Accordingly, other structural compartments of the motion segment must be the main reason for the observed motion restrictions.
In contrast to this study, flexibility tests using canine and caprine motion segments revealed higher RoM of degenerated discs 1 and 12 weeks after enzyme injection, respectively [25,26]. As degeneration progresses slowly, the observation period of one week in the canine study is very short. Motion segments, therefore, have presumably reached an early and potentially unstable phase. In the caprine study, the decrease in disc height index, an important indicator for disc degeneration, was specified to be 6%. Despite differences in the calculation method, in our prior in vivo study, loss of disc height index 12 weeks after NUCL was found to be approximately 40% [14]. This might indicate a more severe degree of disc degeneration in this study, and hence presumably a more advanced state of adaptation to pathology.
While RoM of nucleotomized segments of in vitro 1 and ex vivo significantly differed when the posterior structures were preserved, stepwise removal of the posterior structures balanced out these differences. Furthermore, even RoM in in vitro 2 was similar when posterior structures had been removed, although the intervertebral disc was kept intact. NZ results proved to be comparable with regard to FE and LB. Significant differences between SEAL in in vitro 1 and ex vivo were equalized by removal of posterior structures. In FE, the NZ of in vitro 2 was also comparable to in vitro 1 and ex vivo in FAC and POST. These findings strongly suggest that the significant differences between in vitro and ex vivo do not arise from the intervertebral disc, but rather from the remaining posterior parts of the spine.
Contrasting effects as for the RoM were found in AR for the NZ between in vitro and ex vivo. While no significant differences could be found between SEAL in vitro 1 and SEAL ex vivo, the removal of the facet joints and the remaining posterior structures gave rise to significantly increased laxities of the ex vivo segments. In contrast to FE and LB, this result suggests that exposure to the in vivo environment might have initiated structural changes of biomechanical relevance at the intervertebral disc 12 weeks after surgery. Significantly increased disc degeneration scores and significantly decreased disc height indices compared with intact, which were already proven in our former related study [14], strengthen this assumption. Degenerative changes might have weakened the annulus fibrosus. This might explain the biomechanical instability after facetectomy and, in consequence, indicate the relevance of the annulus for segmental stability in AR, while in FE and LB the role of the annulus is negligible. In FE and LB, it rather seems to be the posterior structures which are responsible for segmental stiffness. Further study is required to investigate whether this is a characteristic of sheep only or is similar in humans.
Although an extensive nucleotomy was performed in this study, no biomechanically verifiable instability of the ex vivo segments resulted. During surgery, defects were added exclusively to the intervertebral disc using a retroperitoneal approach. By contrast, to adequately approach and remove herniated disc material in the clinical scenario (partial), resection of the posterior structures is required, including interlaminar framing in about 73%, hemilaminectomy in 12% and laminectomy in about 5% of patients [27]. According to Adams & Hutton [28], the combination of facet joint capsules, supra- and interspinous ligaments as well as the ligamentum flavum account for about two-thirds of the stability of the motion segment. The higher ex vivo bending stiffnesses might, therefore, give a hint to the importance of the preservation of the stabilizing posterior structures during surgical access.
5. Conclusion
This sheep study is the first to indicate higher bending stiffnesses of the motion segment after nucleotomy in vivo. This is in contrast to the in vitro situation and contrary to expectations prior to the study. In this test design, the defects which were set during surgery in vivo were strictly limited to the anterior column of the ovine motion segment. After removal of the posterior structures, however, similar flexibilities between ex vivo and in vitro strongly suggest that the former differences in flexibility are not based on structural adaptations of the intervertebral disc after surgical intervention. The underlying cause for the motion restrictions might rather be explained by an adaptive stiffening of the posterior structures. Depending on the motion direction, the present results might, therefore, indicate that after decompressive surgery trauma to the intervertebral disc is not exclusively decisive for post-operative stability of the motion segment. Findings imply that it is damage to the posterior, passive spine-supporting structures of the motion segment that must be avoided, because it mainly endangers the motion segment for potential instability after surgery. Further investigations are recommended to state this result more precisely and to gain knowledge about potentially similar effects in humans.
Acknowledgement
Technical assistance by Simone Schädler and Martin Braekau is gratefully acknowledged.
Funding statement
This work was funded by the German Research Foundation (WI 1352/17-1).
References
- 1.Saruhashi Y, Mori K, Katsuura A, Takahashi S, Matsusue Y, Hukuda S. 2004. Evaluation of standard nucleotomy for lumbar disc herniation using the Love method: results of follow-up studies after more than 10 years. Eur. Spine J. 13, 626–630. ( 10.1007/s00586-004-0690-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. 2001. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine 26, 652–657. ( 10.1097/00007632-200103150-00019) [DOI] [PubMed] [Google Scholar]
- 3.Katz JN. 2006. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J. Bone Joint Surg. Am. 88(Suppl. 2), 21–24. ( 10.2106/JBJS.E.01273) [DOI] [PubMed] [Google Scholar]
- 4.Zollner J, Heine J, Eysel P. 2000. Effect of enucleation on the biomechanical behavior of the lumbar motion segment. Zentralbl. Neurochir. 61, 138–142. [DOI] [PubMed] [Google Scholar]
- 5.Halldin K, Zoega B, Lind B, Cederlund CG. 2005. Clinical application of a new three-dimensional radiological classification of lumbar disc herniations. Ups. J. Med. Sci. 110, 159–165. [PubMed] [Google Scholar]
- 6.Halldin K, Zoega B, Nyberg P, Karrholm J, Lind BI. 2005. The effect of standard lumbar discectomy on segmental motion: 5-year follow-up using radiostereometry. Int. Orthop. 29, 83–87. ( 10.1007/s00264-005-0636-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotilainen E, Valtonen S. 1993. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir. 125, 120–126. ( 10.1007/BF01401838) [DOI] [PubMed] [Google Scholar]
- 8.Nachemson A. 1985. Lumbar spine instability. A critical update and symposium summary. Spine 10, 290–291. ( 10.1097/00007632-198504000-00019) [DOI] [PubMed] [Google Scholar]
- 9.Schaller B. 2004. Failed back surgery syndrome: the role of symptomatic segmental single-level instability after lumbar microdiscectomy. Eur. Spine J. 13, 193–198. ( 10.1007/s00586-003-0632-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friberg O. 1987. Lumbar instability: a dynamic approach by traction-compression radiography. Spine 12, 119–129. ( 10.1097/00007632-198703000-00007) [DOI] [PubMed] [Google Scholar]
- 11.Iguchi T, Kanemura A, Kasahara K, Sato K, Kurihara A, Yoshiya S, Nishida K, Miyamoto H, Doita M. 2004. Lumbar instability and clinical symptoms: which is the more critical factor for symptoms: sagittal translation or segment angulation? J. Spinal Disord. Tech. 17, 284–290. ( 10.1097/01.bsd.0000102473.95064.9d) [DOI] [PubMed] [Google Scholar]
- 12.Quint U, Wilke HJ. 2008. Grading of degenerative disk disease and functional impairment: imaging versus patho-anatomical findings. Eur. Spine J. 17, 1705–1713. ( 10.1007/s00586-008-0787-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer WO, Spratt KF, Weinstein J, Lehmann TR, Goel V. 1990. 1990 Volvo Award in clinical sciences. The consistency and accuracy of roentgenograms for measuring sagittal translation in the lumbar vertebral motion segment. An experimental model. Spine 15, 741–750. [PubMed] [Google Scholar]
- 14.Reitmaier S, et al. 2013. In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur. Spine J. 23, 19–26. ( 10.1007/s00586-013-2998-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panjabi MM. 1988. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine 13, 1129–1134. ( 10.1097/00007632-198810000-00013) [DOI] [PubMed] [Google Scholar]
- 16.Wilke HJ, Wenger K, Claes L. 1998. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 7, 148–154. ( 10.1007/s005860050045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilke HJ, Claes L, Schmitt H, Wolf S. 1994. A universal spine tester for in vitro experiments with muscle force simulation. Eur. Spine J. 3, 91–97. ( 10.1007/BF02221446) [DOI] [PubMed] [Google Scholar]
- 18.Panjabi M, Abumi K, Duranceau J, Oxland T. 1989. Spinal stability and intersegmental muscle forces. A biomechanical model. Spine 14, 194–200. ( 10.1097/00007632-198902000-00008) [DOI] [PubMed] [Google Scholar]
- 19.Stokes IA. 1988. Mechanical function of facet joints in the lumbar spine. Clin. Biomech. 3, 101–105. ( 10.1016/0268-0033(88)90052-6) [DOI] [PubMed] [Google Scholar]
- 20.Hegewald AA, Knecht S, Baumgartner D, Gerber H, Endres M, Kaps C, Stussi E, Thome C. 2009. Biomechanical testing of a polymer-based biomaterial for the restoration of spinal stability after nucleotomy. J. Orthop. Surg. Res. 4, 25 ( 10.1186/1749-799X-4-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilke HJ, Heuer F, Neidlinger-Wilke C, Claes L. 2006. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur. Spine J. 15, 433–438. ( 10.1007/s00586-006-0177-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaigle AM, Holm SH, Hansson TH. 1995. Experimental instability in the lumbar spine. Spine 20, 421–430. ( 10.1097/00007632-199502001-00004) [DOI] [PubMed] [Google Scholar]
- 23.Guder E, Hill S, Kandziora F, Schnake KJ. 2009. Partial nucleotomy of the ovine disc as an in vivo model for disc degeneration. Z. Orthop. Unfall. 147, 52–58. ( 10.1055/s-2008-1039139) [DOI] [PubMed] [Google Scholar]
- 24.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. 2002. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine 27, 1278–1285. ( 10.1097/00007632-200206150-00007) [DOI] [PubMed] [Google Scholar]
- 25.Lu DS, Shono Y, Oda I, Abumi K, Kaneda K. 1997. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine 22, 1828–1834. ( 10.1097/00007632-199708150-00006) [DOI] [PubMed] [Google Scholar]
- 26.Detiger SE, Hoogendoorn RJ, van der Veen AJ, van Royen BJ, Helder MN, Koenderink GH, Smit TH. 2013. Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J. Orthop. Res. 31, 703–709. ( 10.1002/jor.22296) [DOI] [PubMed] [Google Scholar]
- 27.Jerosch J, Castro WH. 1996. Long-term results in revision surgery following lumbar disk nucleotomy. Z. Orthop. Ihre Grenzgeb. 134, 89–96. ( 10.1055/s-2008-1037423) [DOI] [PubMed] [Google Scholar]
- 28.Adams MA, Hutton WC. 1983. The mechanical function of the lumbar apophyseal joints. Spine 8, 327–330. ( 10.1097/00007632-198304000-00017) [DOI] [PubMed] [Google Scholar]