Abstract
Magnetoreception remains one of the few unsolved mysteries in sensory biology. The upper beak, which is innervated by the ophthalmic branch of the trigeminal nerve (V1), has been suggested to contain magnetic sensors based on ferromagnetic structures. Recently, its existence in pigeons has been seriously challenged by studies suggesting that the previously described iron-accumulations are macrophages, not magnetosensitive nerve endings. This raised the fundamental question of whether V1 is involved in magnetoreception in pigeons at all. We exposed pigeons to either a constantly changing magnetic field (CMF), to a zero magnetic field providing no magnetic information, or to CMF conditions after V1 was cut bilaterally. Using immediate early genes as a marker of neuronal responsiveness, we report that the trigeminal brainstem nuclei of pigeons, which receive V1 input, are activated under CMF conditions and that this neuronal activation disappears if the magnetic stimuli are removed or if V1 is cut. Our data suggest that the trigeminal system in pigeons is involved in processing magnetic field information and that V1 transmits this information from currently unknown, V1-associated magnetosensors to the brain.
Keywords: bird migration, magnetic sense, trigeminal system, immediate early gene, homing
1. Introduction
To find their way during migration, birds need robust and precise navigational abilities. Behavioural experiments have proved that the Earth's magnetic field is one of several reference systems birds use to find their way [1,2]. However, the exact mechanisms how the Earth's magnetic field is perceived and processed is only starting to be understood. Currently, three main magnetoreception hypotheses are discussed: a light-dependent chemical compass sense associated with the visual system [3–10], a recently suggested involvement of the vestibular system [11–15] but see [16], and an iron-mineral-based sense located in the upper beak [17–20]. The last hypothesis was associated with claims of the existence of iron-mineral structures in six defined dendritic fields within the subepidermal layer of the upper beak [17–20]. Treiber et al. [21,22], however, showed that the previously described iron-oxide deposits are much more likely to be macrophages than V1 dendrites containing a magnetic sensor [21–23]. Does that mean that the upper-beak hypothesis is obsolete? Not necessarily.
Several studies provide strong evidence for an involvement of the ophthalmic branch of the trigeminal nerve (V1) in avian magnetoreception. V1 is the only non-olfactory nerve innervating the upper beak in pigeons [24]. Mora et al. [25] could show that homing pigeons trained to distinguish between the presence and absence of a strong magnetic anomaly lost this ability after sectioning V1; Heyers et al. [26] showed that constantly changing magnetic fields (CMFs) activate the trigemino-recipient brainstem complex in a migratory songbird species, the European robin, and that this activation disappeared when either the magnetic field was compensated (ZMF) or V1 was cut; and Kishkinev et al. [27] showed that Eurasian reed warblers were unable to compensate for a 1000 km east–west displacement when V1 was cut. In addition to these studies where V1 was actually cut, a large number of studies using anaesthetics applied onto the upper beak also reported significant effects [28–30, but see critique in 9]. These studies indicate an involvement of the trigeminal system in magnetoreception, possibly to determine the bird's geographical position. Nevertheless, the recent findings of Treiber et al. [21,22] force the field to reconsider whether V1 is involved in magnetoreception in homing pigeons at all.
To answer this central question, the aim of this study was to investigate whether the two brain areas receiving neuronal input from V1 are activated by magnetic stimuli in pigeons and whether such an activation depends on intact V1s. V1 sends its afferents into an ascending trigeminal tract, which terminates in the principal trigeminal sensory nucleus (PrV), and a descending tract, which terminates in the spinal trigeminal sensory nuclei (SpV) [31]. Using an antibody raised against ZENK protein (acronym for zif268, Egr-1, NGFI-A, Krox 24) [4,6,26,32–36], we compared the neuronal activation patterns in PrV and SpV after (i) magnetic stimulation of birds with intact V1s (reference group), (ii) zero magnetic field stimulation of birds with intact V1s (the nerve remains intact, but there is no magnetic information to process) and (iii) magnetic stimulation of birds with cut V1s (magnetic information present as in (i), but no information from V1-associated sensors can reach the brain). In addition, neuronal tracing was used to test where V1 afferents terminate in the brain and whether they show spatial proximity to activated neurons.
2. Results
2.1. Neuronal activation
First, we consider the neuronal activation seen in pigeons with intact trigeminal nerves experiencing different magnetic field conditions. Magnetic stimulation increased the number of ZENK-positive cells in both PrV and SpV. When birds had experienced a zero magnetic field, we observed an average of 69 ± 48 (s.d.) ZENK-expressing neurons in PrV and 144 ± 65 ZENK-expressing neurons in SpV. For birds that experienced a constantly CMF, we observed a significantly increased number of ZENK-positive neurons in PrV. We counted 181 ± 119 ZENK-expressing neurons within PrV and 502 ± 286 ZENK-expressing neurons in SpV (statistical evaluation, see below). Thus, in the CMF, we observed a 249% increase of ZENK-positive cells in SpV and a 162% increase of ZENK-positive cells in PrV compared with the ZMF condition (figure 1).
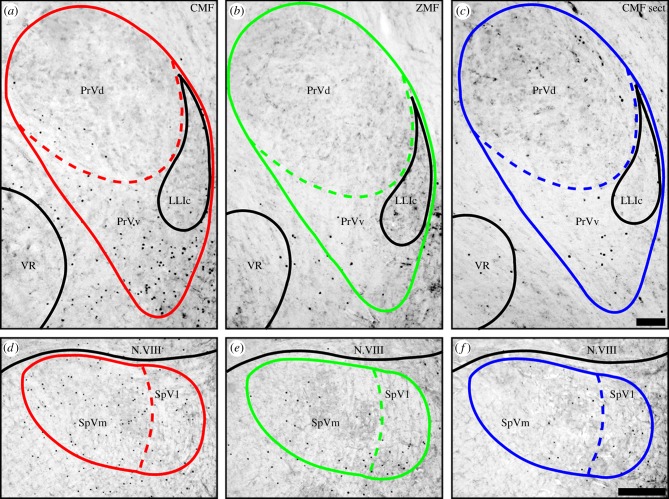
Figure 1.
Magnetic field changes induce expression of ZENK in PrV (a–c) and SpV (d–f). Dorsal is up, lateral is right. Frontal brain sections show strongly increased nuclear ZENK expression (black dots) under CMF conditions (CMF; red; a,c). ZENK expression is mainly confined to a crescent-shaped region (PrVv) ventral to PrV proper (PrVd). The number of ZENK-expressing neurons dropped significantly when the magnetic field stimulus was removed (ZMF; green; b,e) or when the ophthalmic branch of the trigeminal nerve was cut (sect.; blue; c,f). Scale bars, 100 µm in c (for a–c); 200 µm in f (for d–f). LLIc, nucleus of the lateral lemniscus, caudal part; N.VIII, vestibulo-cochlear nerve; PrVd, principal sensory nucleus of the trigeminal nerve; PrVv, ventral (to) PrV; VR, motor nucleus of the trigeminal nerve; SpVl, lateral part of the spinal trigeminal sensory nucleus; SpVm, medial part of the spinal trigeminal sensory nucleus.
Similar to the pattern observed in European robins [26], the vast majority of ZENK-positive neurons within PrV occurred in the so-called ventral PrV (PrVv), and the differences in SpV were mainly owing to an increase in ZENK-positive cells in the medial parts of SpV (SpVm) rather than in the lateral parts (SpVl). Consequently, the number of ZENK-positive neurons in SpV was analysed in two separate parts, namely SpVl and SpVm. We found a statistically significant difference in SpVm where a 347% increase was observed (86 ± 41 ZENK-positive neurons under ZMF conditions compared with 384 ± 276 ZENK-positive neurons in the CMF condition, statistical data are given below).
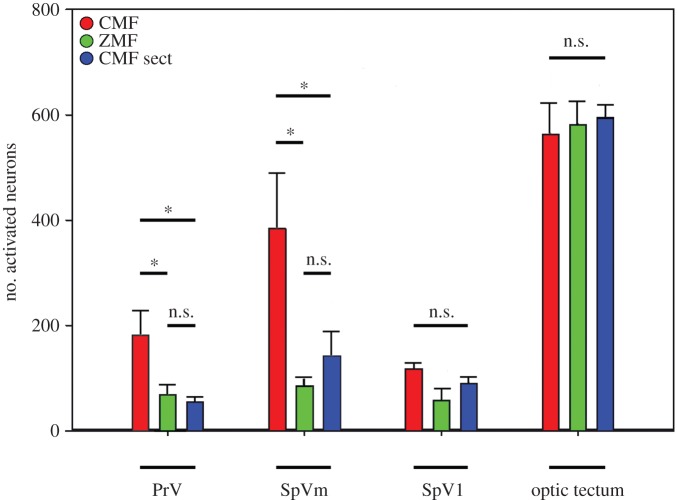
Second, we consider the effects of bilaterally sectioning V1. When pigeons were exposed to the CMF condition, bilateral sectioning of V1 led to a significant decrease of ZENK-labelled neurons in both PrV and SpV compared with pigeons with intact nerves. Bilaterally sectioned birds showed 55 ± 23 ZENK-positive neurons within PrV, 142 ± 110 ZENK-positive neurons in SpVm, and 90 ± 37 ZENK-positive neurons in SpVl. A detailed statistical analysis revealed a significant increase of ZENK-positive neurons in the CMF condition when the birds had intact V1s compared with the ZMF condition with intact V1s and with the V1 sectioned CMF condition (PrV total: one-way ANOVA F = 5.354, p = 0.018, followed by an all pair wise multiple comparison using the Holm–Sidak method: CMF contra ZMF t = 2.561, p = 0.043; CMF contra CMF sect. t = 3.044, p = 0.024. SpVm: one-way ANOVA F = 6.407, p = 0.010, followed by an all pairwise multiple comparison using the Holm–Sidak method: CMF contra ZMF t = 3.464, p = 0.010; CMF contra CMF sect. t = 2.513, p = 0.047). The number of ZENK-positive neurons observed in pigeons experiencing the ZMF was not significantly different from the number of ZENK-positive neurons observed in the V1-sectioned pigeons exposed to the CMF (PrV total: one-way ANOVA F = 5.354, p = 0.018, followed by an all pairwise multiple comparison using the Holm–Sidak method: ZMF contra CMF sect. t = 0.483, p = 0.636. SpVm: one-way ANOVA F = 6.407, p = 0.010, followed by an all pairwise multiple comparison using the Holm–Sidak method: ZMF contra CMF sect. t = 0.951, p = 0.357). Within SpVl, no significant differences were observed between the different treatments (SpVl: one-way ANOVA F = 2.214, p = 0.144). In addition, no significant differences in the number of ZENK-positive neurons were observed between the three magnetic field groups in a 500 × 500 µm measuring slice of the optic tectum (CMF: 562 ± 141; ZMF: 581 ± 113; CMF sect. 595 ± 60; one-way ANOVA, F = 0.127; p = 0.88; figure 3). In the optic tectum, ZENK-expressing neurons were mainly confined to layers 7–10 of the stratum griseum fibrosum superficialis and stratum griseum centrale.
Figure 3.
Quantification of ZENK-activated neurons in PrV, SpV, and the optic tectum of all investigated pigeons. Birds experiencing a CMF are shown in red, birds experiencing a zero magnetic field (ZMF) are shown in green, and birds with sectioned V1 experiencing CMF conditions (CMF sect.) are shown in blue. Since we counted ZENK-positive neurons in every second slide bilaterally throughout the relevant areas, the numbers indicated on the y-axis reflect the approximate absolute number of ZENK-activated neurons in one side of brain, or when multiplied by 2, the total number of bilaterally activated neurons in PrV and SpV. Error bars indicate s.e.m.
2.2. Neuronal tract tracing
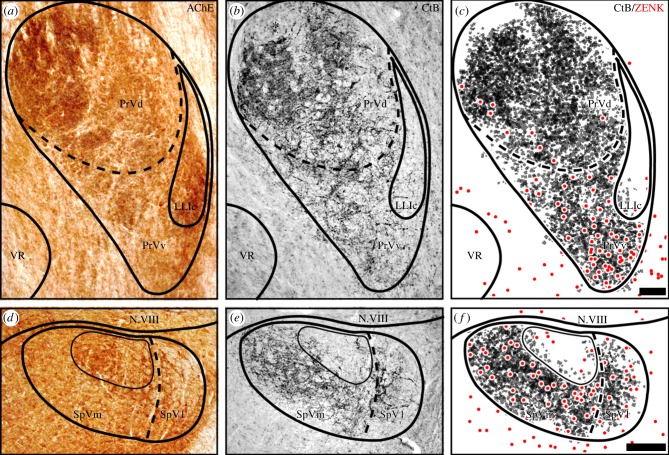
After tracer injection into V1, massive fibre labelling was observed in the ascending trigeminal tract terminating in both dorsal/principal PrV and ventral PrV portions of the ipsilateral side. Terminations in PrVv appeared to be less dense than in PrVd, probably reflecting a lower neuron density in PrVv. Representative neuronal-tracing patterns are depicted in figure 2b,e. These results are almost identical to the neuronal tracing patterns that were observed in previous studies (compare 31; figure 3c) including a dorsomedially located subregion in PrVd showing no tracer labelling (asterisk in figure 2b). Labelled fibres travelling via the descending trigeminal tract/SpVl curved medially to terminate in SpVm. Here, a dorsally located subnucleus, which is characterized by acetylcholinesterase (AChE) staining, did not contain V1 fibre terminations (figure 2d,e). Because we could not exclude the possibility that tracer application into the nerve would affect its functionality and thus ZENK expression in the brain, neuronal tracing patterns were compared with the regional distribution of ZENK-positive neurons in corresponding sections from other pigeons with intact V1s experiencing CMF conditions. We observed clear spatial proximity and regional overlap in PrVv (figure 2c) and throughout SpV (figure 2f).
Figure 2.
Neuronal tracing reveals spatial proximity between ZENK-expressing neurons and V1 fibres. Dorsal is up, lateral is right. (a,c) Enzymatic acetylcholine esterase activity (AChE) was used to determine the anatomical boundaries of PrV and SpV. (b,e) Neuronal tracing of V1 afferents to PrV (b) and SpV (e) (CtB; black immunosignal). (c,f) Schematic colour-coded overlay of CtB (black) and ZENK immunosignal (red) in corresponding sections of a specimen experiencing CMF conditions show close regional overlap both in PrVv and SpV. Scale bar, 100 µm in c (for a–c); 200 µm in f (for d–f). For anatomical abbreviations, see legend to figure 1.
2.3. Video analysis
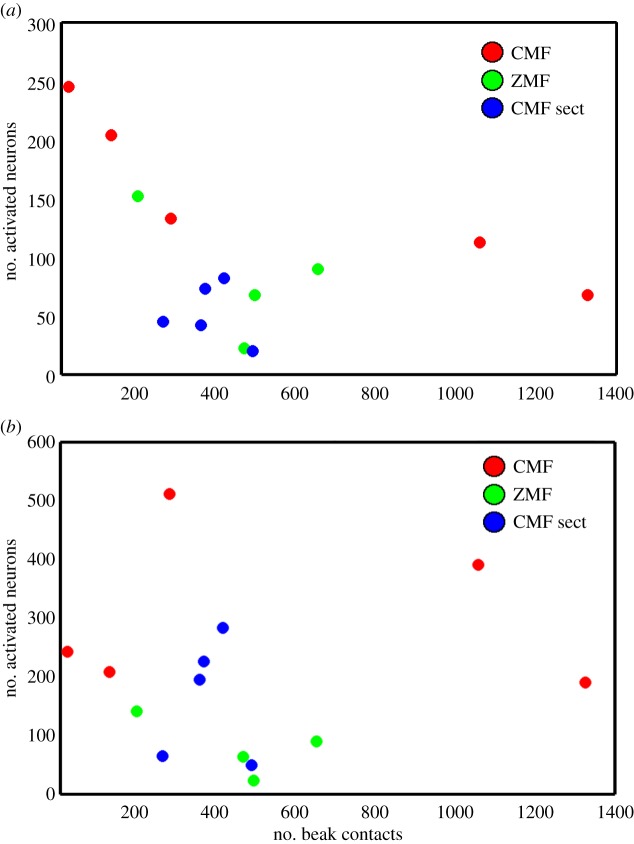
To investigate whether differences in motor behaviour [37] and/or somatosensory stimulation of the beak could have led to the differences in ZENK activation observed in PrV and SpV, we carefully analysed video recordings of the pigeons' behaviour before they were collected for brain analyses. No systematic differences in motor behaviour between the groups were observed (ANOVA, d.f. = 2, F = 0.8731, p = 0.445). Furthermore, the exact numbers of beak contacts, which might have activated mechanoreceptors in the upper beak and which occurred within the last hour before the bird was taken for brain activation analysis, were counted (pecking, grooming, scratching and contact with the surroundings). No correlations between the number of beak contacts and the number of ZENK-activated neurons in the groups were found by Spearman's test of correlation: PrV/ZMF (n = 4, Spearman's correlation coefficient (rs) = −0.2, p = 0.917), PrV/CMF sect. (n = 5, rs = −0.1, p = 0.95), SpV/ZMF (n = 4, rs = −0.6, p = 0.417), SpV/sect. (n = 5, rs = 0, p = 1), SpV/CMF (n = 5, rs = 0.3, p = 0.683) except for a negative correlation for PrV/CMF (n = 5, rs = −1, p = 0.02; figure 4). This clearly indicates no systematic relationship between the number of beak contacts and the number of ZENK-activated neurons in PrV and SpV. The only correlation observed would suggest that fewer beak contacts lead to a stronger trigeminal activation, which is highly unlikely to be true.
Figure 4.
No obvious relationship exists between mechanical beak contacts and the number of activated neurons in PrV (a) or SpV (b). The number of activated neurons is shown as a function of the number of beak contacts (grooming, pecking, contact with the arena walls and/or the net) made by any given bird during the last hour before the bird was collected (CMF, red circles, n = 5; ZMF, green circles, n = 4; CMF sect., blue circles, n = 5).
3. Discussion
Based on the presented ZENK expression data, we show that the two brain regions (PrV and SpV) which receive neuronal input through the ophthalmic branch of the trigeminal nerve (V1) are activated by strongly CMFs and that this activation requires intact V1s. ZENK expression decreases significantly when the magnetic field stimuli are removed (activation in CMF compared with ZMF; figure 1a,b), and also when the CMF condition remains present, but the connection between V1-related sensors and the brain is cut (activation in CMF compared with CMF sect.; figure 1a,c). Based on the analysis of ZENK expression in the optic tectum showing no differences between the magnetic treatments, general neuronal activation through magnetic fields can be excluded. It should be noted that light-dependent magnetic sensing is unlikely to be processed through the tectofugal pathway even though this has been claimed [38]. It has been shown that ‘cluster N’ [32,34,39], a forebrain region that is required for magnetic compass orientation [7], receives input from the thalamofugal, not the tectofugal, visual pathway [6]. Thus, this study neither supports nor questions the light-dependent magnetoreception hypothesis for pigeons.
The presented neuronal-tracing results show that V1 fibres terminate in the regions showing high magnetic ZENK activation, both in the ventral parts of PrV (figure 2b,c) and in the SpVm (figure 2e,f). Thereby, our findings in homing pigeons closely resemble previous results [26,31].
The absolute numbers of magnetically ZENK-activated neurons in PrV and SpV are about five-times lower than the equivalent numbers we previously found in European robins [26] using exactly the same experimental protocol. Another previous study, which analysed c-fos expression in one of the trigeminal brainstem regions (PrV) in pigeons after magnetic stimulation found comparatively low numbers of c-fos expressing neurons (50 000 nT magnetic field: 3 ± 1 neurons; 150 000 nT magnetic field: 24 ± 2 neurons [11]). The difference between this study and that of Wu & Dickman [11] might be that Wu & Dickman [11] used c-fos as a neuronal activity marker and did not count the entire PrV, whereas we used ZENK and counted throughout PrVd and PrVv. It could be tempting to speculate that the higher number of ZENK-activated neurons detected in the migratory European robins might reflect a stronger selective pressure and thus adaptation to navigate than in the mostly resident rock pigeon (Columba livia). However, it is too early to tell whether this hypothesis is correct or not.
The existence of magnetically activated neurons in trigemino-recipient brain regions in pigeons has important implications for the concepts of magnetoreception in homing pigeons, because the results of Treiber et al. [21,22] seriously questioned that the iron-mineral-containing structures in the upper beak, previously thought to be potential magnetoreceptors [17–19], are involved in magnetoreception and thus indirectly questioned the whole trigeminal nerve-related magnetic sensing hypothesis. However, as pointed out by Mouritsen [23], it is important to stress that the results of Treiber et al. [21,22] cannot exclude the possibility that there are iron-mineral-based sensors in the upper beak or in other regions innervated by V1. Only a few single-domain magnetite crystals might be needed to sense the geomagnetic field, and a magnetite-based sensory cell containing only a few magnetite crystals will evade detection by Prussian-blue staining, the method used in all previous studies [17,18,21–23,40]. Our findings suggest that there are magnetosensory structures associated with V1 and are thus in agreement with data in Mora et al. [25], Heyers et al. [26] and Kishkinev et al. [27]. In these studies, nerve sectioning led to significant decreases in the birds' ability to detect and/or to react to magnetic field changes [25,27], or found a correlation between the magnetic field stimulation and neuronal responses at brain level [26]. Thus, the most parsimonious explanation for the present data is that V1 carries magnetic field information. But, could there be an alternative explanation for our findings?
Currently, as described in the Introduction, three different magnetoreception hypotheses have been suggested. It seems unlikely that birds actually possess three completely independent magnetoreception systems. We therefore specifically considered the possibility that the suggested vestibular [11,15] and trigeminal [25–27] magnetic senses could be two components of the same system.
What would actually be the prediction if we imagine a scenario where the vestibular system, not the trigeminal system, would be the source of the magnetic activation we have observed in PrV and SpV? That would mean (i) that the activation of PrV and SpV observed in the CMF condition would represent a combination of vestibular magnetic input and non-magnetic trigeminal input (mechanical [41,42] or maybe even olfactory [43,44]); (ii) that the activation of PrV and SpV in the ZMF condition would represent only non-magnetic trigeminal input and (iii) that the activation of PrV and SpV in the CMF-sect. group would represent only vestibular magnetic input (figure 5).
Figure 5.
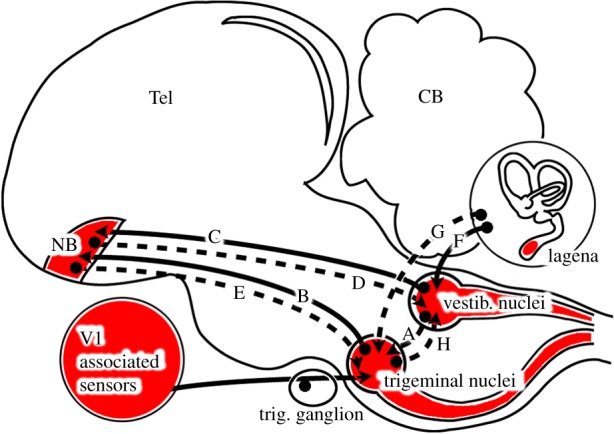
Neuronal connections between the trigeminal and vestibular sensory systems in birds. Putative magnetosensory locations, afferents, efferents and intratelencephalic connections. Known connections in pigeons are depicted as solid lines. Dashed lines indicate currently unknown, putative connections. The trigeminal sensory brain nuclei (PrV, SpV) receive direct input from V1-associated sensors located in or near the upper beak. PrV sends afferents to the telencephalic N. basalis via the quintofrontal tract (B). As shown by retrograde neuronal tracing, N. basalis receives input from superior vestibular nuclei (C). However, reciprocal projections originating from N. basalis have neither been shown for the vestibular nuclei (D) nor for the trigeminal nuclei (E). All major vestibular nuclei receive afferents from all semicircular and otolithic organs including the lagena (F). A recent study reported a direct connection between the lagena and SpV in chicken (G). No connections in either direction between the vestibular and trigeminal brainstem nuclei have been described at present (A,H). References: see main text.
A putative trigemino-vestibular combination hypothesis would require neuronal integration in PrV and SpV of information from V1 and from the vestibular hindbrain nuclei. This could either be achieved through a direct hindbrain connection between the trigeminal and vestibular sensory nuclei, which neighbour each other along almost the whole brainstem, or through an indirect connection involving other brain parts. To date, no evidence suggests a direct connection between the vestibular and trigeminal hindbrain nuclei (A in figure 5). By contrast, connectivity studies have shown that the trigeminal and vestibular systems interconnect at a higher brain level. PrV directly projects (B in figure 5) via the quintofrontal tract to the telencephalic nucleus basalis in pigeons [45] and zebra finches [41], and neurons in the superior vestibular hindbrain nuclei can be retrogradely traced from N. basalis (C in figure 5) in pigeons [46] and zebra finches [41]. Thus, N. basalis receives input from both trigeminal and vestibular hindbrain regions. The problem for a hypothesis suggesting that the magnetic activation in PrV and SpV could have come from putative magnetoreceptors in the vestibular system (lagena) is that, although the vast majority of neuronal connections within the brain show reciprocal innervations, no projections backwards from the N. basalis to either the superior vestibular nuclei (D in figure 5) or to PrV (E in figure 5) have been described yet. However, a recent neuronal tracing study in domestic chicken reported a direct projection from the lagena not only to medial and spinal vestibular nuclei [47] (F in figure 5), but also to SpV (G in figure 5). Thus, a putative neuronal connection seems to exist in a different bird species, which could support an explanation, where the magnetically induced neuronal ZENK activation we observed in PrV or SpV would be owing to primary magnetic sensors located in the lagena. However, to date, no data supporting the existence of such a neuronal connection in pigeons or any migratory bird species have been published. Our data as well as the electrophysiological responses reported by Wu & Dickman [15] would be consistent with an alternative combination hypothesis in which the lagena provides gravity information only that is combined with magnetic information detected in sensors associated with V1. Such a hypothesis would require a connection from PrV and/or SpV to the vestibular hindbrain nuclei (e.g. H in figure 5).
Do our data provide any hints, supporting one of these possibilities? If we make the simple assumption that the thresholds of ZENK induction would be identical between neuronal subpopulations of different sensory systems, irrespective of the stimulus (mechanical or magnetic), one prediction of this scenario would be that the number of ZENK-activated neurons in the ZMF and CMF-sect. groups should approximately add up to the number of ZENK-activated neurons in the CMF condition. This, however, is not the case. For PrV, we find 69 (ZMF) + 55 (CMF sect.) = 124 < 181 (CMF), i.e. the sum of the mean number of activated neurons in the ZMF and CMF-sect. conditions only adds up to about two-thirds of the mean number of activated neurons in the CMF condition. For SpVm, we find 142 (ZMF) + 86 (CMF Sect.) = 228  502 (CMF), i.e. the sum of the mean number of activated neurons in the ZMF and CMF sect. conditions only adds up to less than half of the mean number of activated neurons in the CMF condition. Thus, the simplest combined vestibular/trigeminal hypothesis leaves 33–55% of the ZENK-activated neurons in the CMF condition unexplained. However, we are well aware that this calculation might be too ‘simplistic’, because neurons can be multimodal and/or have different thresholds for ZENK expression.
502 (CMF), i.e. the sum of the mean number of activated neurons in the ZMF and CMF sect. conditions only adds up to less than half of the mean number of activated neurons in the CMF condition. Thus, the simplest combined vestibular/trigeminal hypothesis leaves 33–55% of the ZENK-activated neurons in the CMF condition unexplained. However, we are well aware that this calculation might be too ‘simplistic’, because neurons can be multimodal and/or have different thresholds for ZENK expression.
Our data would, in principle, also be consistent with the olfactory activation hypothesis of Jorge et al. [43,44]. This would require that only the trigeminal nerve would carry the activational olfactory information and the vestibular system the magnetic information.
In conclusion, we have shown that strongly changing magnetic stimulation leads to ZENK activation of neurons in the trigeminal hindbrain nuclei PrVv and SpVm in pigeons, and that the number of activated neurons in these regions significantly decreases when either the magnetic field stimulation is removed or when the ophthalmic branch of the trigeminal nerve is cut. The most parsimonious explanation of these results is that the ophthalmic branch of the trigeminal nerve carries primary magnetic information from currently unknown magnetic sensors associated with this nerve, but we cannot exclude hypotheses for instance involving integration of trigeminal and vestibular input.
4. Material and methods
4.1. Study animals
Twenty-four pigeons (C. livia, 18 for ZENK analysis, six for neuronal tracing) were obtained from local breeders and used in this study. The birds were kept outdoors in a sheltered aviary with food and water provided ad libitum. All animal procedures were approved by the animal care and use committees at LAVES (‘Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit’).
4.2. Nerve sectioning
We bilaterally cut and removed ca 3 mm of V1, because only when V1 is surgically cut, one can be sure that no information reaches the brain through V1. The importance of actually cutting the nerve is strongly supported by a study by Wallraff [48], who investigated the individual effects of different techniques used to deprive pigeons from olfaction. In contrast to bilateral olfactory nerve section, which successfully eliminated perception of olfactory stimuli, spraying the nasal cavity with the same surface anaesthetic that has been used in many magnetoreception studies [28–30] led to highly variable effects depending on the kind of application [48]. We therefore consider nerve sectioning as the only valid ‘loss-of-function’ technique and therefore used it to surely prevent V1 information from reaching the brain [9].
The nerve-sectioning procedures in this study were identical to those used in Zapka et al. [7], Heyers et al. [26] and Kishkinev et al. [27]. Each bird was anaesthetized and immobilized in a custom-built head holder. Above each eye, an incision along the dorsal rim of the orbit was made with a scalpel, and the eyeball was carefully retracted to expose V1, which runs along the inside of each orbit [49]. Then, approximately 3 mm of the nerve was cut and removed to prevent refusion of the nerve endings. After the surgery, the skin was resealed using cyanoacrylate surgical glue. The birds were given at least 3 days to recover from the surgery before they participated in any experiment.
4.3. Magnetic stimulation
Pigeons were placed individually in a custom-built arena (width 80 cm, length 80 cm, height 40 cm) covered with black netting. The inner walls were painted in a black and white stripe pattern to provide neutral visual cues [50]. The floor of the arena was covered with wooden flakes. During the experiment, the pigeon was free to move within the arena but it was not asked to perform any orientation task. The magnetic field conditions were generated by a double-wrapped, three-axial, Merritt 4-coil system [7,51,52] of ca 2 × 2 × 2 m inside a wooden hut operated by high-precision, constant current power supplies (KEPCO BOP 50-4M, Kepco Inc., Flushing, NY). The testing cabin was lined with aluminium shields and grounded to act as a Faraday cage which shielded time-dependent electromagnetic disturbances with frequencies up to at least 20 MHz by about two orders of magnitude while leaving static fields unaffected [52]. The power supplies were placed outside the experimental cabin and remained switched on all the time. Thus, any auditory noise influence was the same in all the magnetic field conditions.
4.4. Experimental procedure
The pigeons were divided into three groups (six individuals per group). The first group experienced a compensated (zero) magnetic field (strength: 0 ± 200 nT), in which the local geomagnetic field was compensated.
The second group was exposed to a constantly CMF, which was controlled and generated by a computer using a custom-written script (Matlab, Mathworks, Natick, MA). The magnetic field stimulation protocol was identical to the one used in Heyers et al. [26]: the CMF condition consisted of two types of magnetic stimulation, which alternated every 5 min. During the first 5 min, the magnetic field turned 90° every 30 s around the horizontal axis with approximately the same inclination (67.6 ± 0.8°) and field strength (48 800 ± 400 nT) as the local geomagnetic field in Oldenburg. During the next 5 min, every 30 s, each of the three axes of the magnetic field was varied randomly and independently between −70 000 nT and + 70 000 nT resulting in a magnetic field that varied strongly in strength (18 500–111 000 nT), horizontal direction (0–359°) and inclination (−84.9° to +76.6°). The randomized aspects of the stimuli were newly generated once for each 5 min period. After that, the same stimulus sequence was used for all tested animals. This alternating procedure was repeated continuously for at least 3 h. We intentionally chose this stimulus design to include large and small changes in any of the three magnetic parameters (horizontal direction, inclination and field strength), because the ideal stimulus for any putative magnetosensory system associated with V1 is unknown. Furthermore, providing a highly variable stimulus helps prevent sensory adaptation effects, and the same stimulus design has previously been shown to successfully activate trigeminal brainstem nuclei in European robins [26]. Finally, it is potentially relevant to point out that seen over 10 min or an hour, neither the ZMF nor the CMF condition provided consistent orientation relevant information. So even if we would have performed orientation experiments with the pigeons, both the CMF and the ZMF group should have been disoriented.
The third group of birds had their trigeminal nerves cut bilaterally, but otherwise underwent the same CMF condition. Each individual animal was exposed to a given magnetic stimulus for 3 h. Incandescent light bulbs (spectrum can be found in the electronic supplementary material of [7]) produced light with an intensity of approximately 20 mW m−2 within the arena.
4.5. Video analysis
Motor activity leads to brain activation [37]. Furthermore, because the ophthalmic branches of the trigeminal nerve in birds transmit information from mechanical sensors in the upper beak, palate and nasal cavity [53], any mechanical contact between the beak and any object in the cage potentially leads to neuronal activation in PrV and/or SpV. We therefore continuously monitored the behaviour of each bird in the test arena in real-time using infrared cameras (840 nm) above and besides the test arena. Only birds that did not fly to the covering net were analysed for brain activation. We have video recordings of the behaviour of 14 of the 18 birds, whose brains we analysed (the remaining four birds were observed live but the video tape malfunctioned). For the 14 birds, an observer, who was blind to the magnetic field condition and the surgery the bird had undergone, used a stopwatch to document how much time each bird spent moving within the arena and quantified the number of mechanical contacts experienced by the beak. Mechanical beak contacts included pecking, grooming, scratching and contacts with objects in its surroundings.
4.6. Processing of brain tissue
After exposure to a given magnetic stimulus (described above), the birds were deeply anesthetized by an overdose of narcoren or ketamine and domitor and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde (PFA) dissolved in 0.12 M phosphate-buffered saline (PBS). The brains were extracted from the skull, post-fixed in 4% PFA and stored for at least 24 h in 30% sucrose dissolved in PBS for cryoprotection. The caudal parts of the brains (approximately up to the level of the midbrain posterior commissure) were cut on a freezing microtome (Leica 1850, Solms, Germany) in six parallel series of 40 µm thick sections in the frontal plane and stored free-floating in PBS containing 0.1% Na-azide at 4°C until being subjected to immunohistochemistry.
4.7. Behavioural molecular mapping
Increases in neuronal activity in a bird's brain can be detected by the expression of immediate early genes such as ZENK [54] (1). As a result of increased neuronal firing, ZENK is expressed in roughly two-thirds of the bird's brain [55,56], including the trigeminal brainstem complex [26]. As a result of exposure to a highly variable stimulus, increased ZENK protein expression can be detected after ca 15 min onwards. ZENK expression peaks after 60–120 min [33,55]. A high level of ZENK protein expression can be kept for several hours, given that the stimulus is not too monotonous. In line with the successful protocol used for European robins in Heyers et al. [26], we exposed our birds to the given magnetic stimulus for 3 h, to ensure that any ZENK activation from placing the bird into the set-up had subsided by the time that brain tissue was collected.
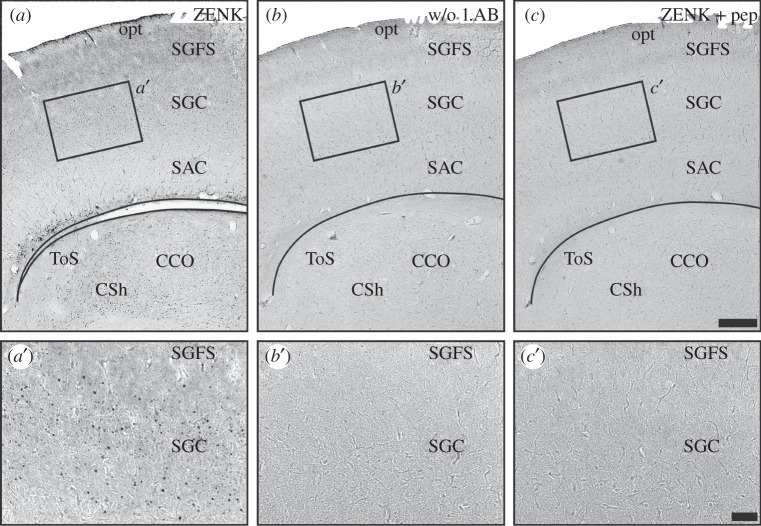
Every second series of the brain slices was stained free floating according to the immuno-ABC technique described previously [6,26,32,56]. The endogenous peroxidases were inactivated by 30 min incubation with 0.3% hydrogen peroxide dissolved in distilled water. Unspecific binding sites were blocked by incubation in 10% normal goat serum (Kraeber, Ellerbek, Germany) dissolved in PBS containing 0.3% Triton-X100 (PBS-T, Sigma, Diessenhofen, Germany) for 30 min. Slices were incubated with a polyclonal rabbit Egr-1/ZENK antibody (sc-189, Santa Cruz, CA, 1 : 1000 in PBS-T) for 3 days at 4°C with gentle agitation. Following this, slices were sequentially incubated for 60 min each with a biotinylated secondary polyclonal goat anti-rabbit IgG antibody and avidin-coupled peroxidase complex (Vector ABC elite kit, Vector Laboratories, Burlingame, CA). Thereafter, activity of peroxidase was detected using a 3′3-diaminobenzidine (Sigma) reaction under usage of β-d-glucose/glucose–oxidase (Sigma) instead of hydrogen peroxidase [57]. The substrate reaction was stopped by transferring the sections into 0.1 M sodium acetate. Sections were mounted on glass slides, dehydrated and cover-slipped with Eukitt (c) (Sigma). Because AchE has previously been shown to label PrV and SpV substructures [26,58], in one corresponding serial set of sections, AchE activity was mapped to facilitate determination of the anatomical boundaries of PrV and SpV. To test for specificity of the reagents and antibody, control sections from one bird were stained in exactly the same way in parallel, thereby either omitting the first antibody or pre-incubating the antibody with the respective blocking peptide encoding for ZENK protein. No immunosignal was observed anywhere in the brain under these conditions (figure 6).
Figure 6.
Control stainings used to test the specificity of the reagents and antibodies used in this study. (a–c) Tectal sections from the same pigeon stained in parallel against (a) ZENK; (b) same procedure but without the primary antibody (rabbit polyclonal ZENK); (c) As A but with the primary ZENK antibody preadsorbed with the immunizing peptide. a′, b′and c′are blow-ups of the area indicated by a box in a, b and c, respectively. ZENK signal is only observed using the complete staining procedure we used in the study (figure 1a,a′). Omission of the primary antibody (figure 1b,b′) and pre-adsorption of the primary antibody with the immunizing peptide (figure 1c,c′) leads to no specific immunosignal. Scale bars: 300 µm in c (for a–c); 100 µm in c′ (for a′–c′). CCO, central core of the midbrain auditory torus; CSh, central shell of the midbrain auditory torus; opt, optic tract; SAC, stratum album centrale of the optic tectum; SGC, stratum griseum centrale of the optic tectum; SGFS, stratum griseum et fibrosum superficiale of the optic tectum; ToS, torus semicircularis (midbrain auditory torus).
4.8. Neuronal tract tracing
Nerve terminations of V1 in the brain were mapped using neuronal tract tracing. Six birds received, under general anaesthesia, a manual injection of approximately 200 nl 0.5% cholera toxin subunit B (CtB) and 5% biotinylated dextran amine dissolved in PBS directly into the nerve using a microinjector (Nanoliter 2000, World Precision Instruments Inc., Hertfordshire, UK) and glass micropipettes with bevelled tips (P-1000 Micropipette puller/BV-10 micropipette beveller, Sutter Instrument, Novato, CA). Access to the nerve was gained in the same way as described for nerve sectioning. Each bird was given 5–7 days to recover from the surgery and to let the tracer transport. After transcardial perfusion, the birds were treated in the same way as described in the ‘behavioural molecular mapping’ section except for using a polyclonal rabbit CtB antibody (1 : 1000 in PBS-T, C-3062, lot no. 084K4763, Sigma-Aldrich, Diessenhofen, Germany; [6]) incubated overnight. The avidin-coupled peroxidase complex (Vector ABC Elite kit, Vector Laboratories) allowed us to detect both CtB and the biotinylated dextrane amine simultaneously.
4.9. Quantification/analysis
ZENK-expressing neurons in all stained sections which contained PrV (six to nine sections per side of the brain) and all stained sections which contained SpV at intermediate levels (i.e. at the level of the vestibulo-cochlear nerve, 12–18 sections per side of the brain) of all pigeons were counted on both sides of the brain, resulting in a total of 689 analysed brain slices. To exclude ‘wishful thinking’ artefacts from our analyses, we blinded the counting procedures: blindness to the magnetic and surgery conditions was achieved by mounting brain slices on glass slides, which were blindly assigned numbers from 1 to 126, and the number of ZENK-expressing neurons was counted independently by two researchers who were unaware of the experimental conditions the birds underwent. To avoid a potential bias based on different staining intensities [26,59], slices from birds belonging to each of the experimental groups were stained together. Thus, three sets of brain slices from a given individual were placed on three different microscope slides and underwent the above-mentioned staining procedure and analysis three independent times. Before each counting of ZENK-positive cells in PrV and SpV, the staining intensity was estimated by studying the ZENK expression levels in the optic tectum, which showed consistent activation in all birds, and the threshold for what was to be counted as a positive cell was defined accordingly. No quantitative differences between the two hemispheres were observed and the relative number of ZENK-expressing neurons in a given brain region was highly consistent between individual counts and between the brain slices from a given individual which were stained independently three times. This indicates that our staining quality and counting results were highly consistent. To further validate our analysis method, ZENK expression in a defined part of the optic tectum (500 × 500 µm) slice at the level of the thalamic isthmo-optic nucleus) in all specimens was chosen. We intentionally chose this region since tectofugal visual input should have been similar, irrespective of the magnetic condition in all birds.
Acknowledgements
The authors cordially thank the University's workshop for building top-quality technical and electronic devices, the animal keeping facility for taking care for our birds and Felix Ströckens, Martina Manns, Ariane Schwartz and Onur Güntürkün for help with establishing the immunohistochemical techniques. H.M. and D.H. designed research; N.L. and D.H performed experiments; N.L., D.H., S.E. and D.E. performed analysis; N.L.S. wrote the magnetic stimulus protocol; N.L., D.H., and H.M. wrote the manuscript, which all authors commented on.
Funding statement
Generous financial support was provided by the VolkswagenStiftung (Lichtenberg Professorship to H.M.) and the Deutsche Forschungsgemeinschaft (DFG MO1408/1-2, FOR701 to H.M. and HE6221/1-1 to D.H.).
References
- 1.Wiltschko W, Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64 (doi:10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- 2.Cochran WW, Mouritsen H, Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408 (doi:10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- 3.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 (doi.org/10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, Dirks P, Weiler R. 2004. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14 294–14 299 (doi:10.1073/pnas.0405968101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouritsen H, Ritz T. 2005. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–414 (doi:10.1016/j.conb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 6.Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 (doi:10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapka M, et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277 (doi:10.1038/nature08528) [DOI] [PubMed] [Google Scholar]
- 8.Hore PJ. 2012. Are biochemical reactions affected by weak magnetic fields? Proc. Natl Acad. Sci. USA 109, 1357–1358 (doi:10.1073/pnas.1120531109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouritsen H, Hore PJ. 2012. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin. Neurobiol. 22, 343–352 (doi:10.1016/j.conb.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 10.Ritz T, et al. 2009. Magnetic compass of birds in based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457 (doi:10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu LQ, Dickman JD. 2011. Magnetoreception in an avian brain in part mediated by inner ear lagena. Curr. Biol. 21, 418–423 (doi:10.1016/j.cub.2011.01.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada Y. 2008. The relation between the migration function of birds and fishes and their lagenal function. Acta Otolaryngol. 128, 432–439 (doi:10.1080/00016480701724920) [DOI] [PubMed] [Google Scholar]
- 13.Zakir M, Wu LQ, Dickman JD. 2012. Morphology and innervation of the vestibular lagena in pigeons. Neuroscience 209, 97–107 (doi:10.1016/j.neuroscience.2012.02.014.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauwers M, et al. 2013. An iron-rich organelle in the cuticular plate of avian hair cells. Curr. Biol. 23, 924–929 (doi:10.1016/j.cub.2013.04.025) [DOI] [PubMed] [Google Scholar]
- 15.Wu LQ, Dickman JD. 2012. Neural correlates of a magnetic sense. Science 336, 1054–1057 (doi:10.1126/science.1216567) [DOI] [PubMed] [Google Scholar]
- 16.Wallraff HG. 1972. Homing of pigeons after extirpation of their cochleae and lagenae. Nat. New Biol. 236, 223–224 (doi:10.1038/newbio236223a0) [DOI] [PubMed] [Google Scholar]
- 17.Fleissner G, Holtkamp-Rotzler E, Hanzlik M, Winklhofer M, Fleissner G, Petersen N, Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360 (doi:10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- 18.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. 2007. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 94, 631–642 (doi:10.1007/s00114-007-0236-0) [DOI] [PubMed] [Google Scholar]
- 19.Falkenberg G, et al. 2010. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE 5, e9231 (doi:10.1371/journal.pone.0009231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eder SHK, Cadiou H, Muhamad A, McNaughton PA, Kirschvink JL, Winklhofer M. 2012. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl Acad. Sci. USA 109, 12 022–12 027 (doi:10.1073/pnas.1205653109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treiber CD, Salzer M, Breuss M, Ushakova L, Lauwers M, Edelman N, Keays DA. 2013. High resolution anatomical mapping confirms the absence of a magnetic sense system in the rostral upper beak of pigeons. Commun. Integr. Biol. 6, e24859 (doi:10.4161/cib.24859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treiber CD, et al. 2012. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484, 367–370 (doi:10.1038/nature11046) [DOI] [PubMed] [Google Scholar]
- 23.Mouritsen H. 2012. Search for the compass needles. Nature 484, 320–321 (doi:10.1038/484320a) [DOI] [PubMed] [Google Scholar]
- 24.Williams MN, Wild JM. 2001. Trigeminally innervated iron-containing structures in the beak of homing pigeons, and other birds. Brain Res. 889, 243–246 (doi:10.1016/S0006-8993(00)03114-0) [DOI] [PubMed] [Google Scholar]
- 25.Mora CV, Davison M, Wild JM, Walker MM. 2004. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432, 508–511 (doi:10.1038/nature03077) [DOI] [PubMed] [Google Scholar]
- 26.Heyers D, Zapka M, Hoffmeister M, Wild JM, Mouritsen H. 2010. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc. Natl Acad. Sci. USA 107, 9394–9399 (doi:10.1073/pnas.0907068107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishkinev D, Chernetsov N, Heyers D, Mouritsen H. 2013. Migratory reed warblers need intact trigeminal nerves to compensate for a 1000 km displacement. PLoS ONE 8, e65847 (doi:10.1371/journal.pone.0065847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiltschko R, Schiffner I, Fuhrmann P, Wiltschko W. 2010. The role of the magnetite-based receptors in the beak in pigeon homing. Curr. Biol. 20, 1534–1538 (doi:10.1016/j.cub.2010.06.073) [DOI] [PubMed] [Google Scholar]
- 29.Wiltschko R, Munro U, Ford H, Stapput K, Wiltschko W. 2008. Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. J. Exp. Biol. 211, 3344–3350 (doi:10.1242/jeb.020313) [DOI] [PubMed] [Google Scholar]
- 30.Wiltschko W, Munro U, Ford H, Wiltschko R. 2009. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. R. Soc. B 276, 2227–2232 (doi:10.1098/rspb.2009.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wild JM, Zeigler HP. 1996. Central projections and somatotopic organisation of trigeminal primary afferents in pigeon (Columba livia). J. Comp. Neurol. 368, 136–152 (doi:10.1002/(SICI)1096-9861(19960422)3) [DOI] [PubMed] [Google Scholar]
- 32.Zapka M, Heyers D, Liedvogel M, Jarvis ED, Mouritsen H. 2010. Night-time neuronal activation of cluster N in a day- and night-migrating songbird. Eur. J. Neurosci. 32, 619–624 (doi:10.1111/j.1460-9568.2010.07311.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarvis ED, Nottebohm F. 1997. Motor-driven gene expression. Proc. Natl Acad. Sci. USA 94, 4097–4102 (doi:10.1073/pnas.94.8.4097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344 (doi:10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemec P, Burda H, Oelschlager HHA. 2005. Towards the neural basis of magnetoreception: a neuroanatomical approach. Naturwissenschaften 92, 151–157 (doi:10.1007/s00114-005-0612-6) [DOI] [PubMed] [Google Scholar]
- 36.Hein CM, Zapka M, Heyers D, Kutzschbauch S, Schneider N-L, Mouritsen H. 2010. Night-migratory garden warblers can orient with their magnetic compass using the left, the right or both eyes. J. R. Soc. Interface 7(Suppl. 2), 227–233 (doi:10.1098/rsif.2009.0376.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Wada K, Mouritsen H, Jarvis ED. 2008. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE 3, e1768 (doi:10.1371/journal.pone.0001768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semm P, Demaine C. 1986. Neurophysiological properties of magnetic cells in the pigeon's visual system. J. Comp. Physiol. A 159.5, 619–625 (doi:10.1007/BF00612035) [DOI] [PubMed] [Google Scholar]
- 39.Jarvis ED, et al. 2013. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J. Comp. Neurol. 521, 3614–3665 (doi:10.1002/cne.23404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker MM, Diebel E, Haugh CV, Pankhurst PM, Montgomery JC, Green CR. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376 (doi:10.1038/37057) [DOI] [PubMed] [Google Scholar]
- 41.Wild JM, Farabaugh SM. 1996. Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. J. Comp. Neurol. 365, 306–328 (doi:10.1002/(SICI)1096-9861(19960205)365:2<306::AID-CNE8>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 42.Pettigrew JD, Frost BJ. 1985. A tactile fovea in the Scolopacidae? Brain Behav. Evol. 26, 185–195 (doi:10.1159/000118775) [PubMed] [Google Scholar]
- 43.Jorge PE, Marquis AE, Phillips JB. 2009. Activational rather than navigational effects of odors on homing of young pigeons. Curr. Biol. 19, 650–654 (doi:10.1016/j.cub.2009.02.066) [DOI] [PubMed] [Google Scholar]
- 44.Jorge PE, Marques PA, Phillips JB. 2010. Activational effects of odours on avian navigation. Proc. R. Soc. B 277, 45–49 (doi:10.1098/rspb.2009.1521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild JM, Arends JJA, Zeigler HP. 1985. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): atrigeminal sensorimotor circuit. J. Comp. Neurol. 234, 441–464 (doi:10.1002/cne.902340404) [DOI] [PubMed] [Google Scholar]
- 46.Schall U, Güntürkün O, Delius J. 1986. Sensory projections to the nucleus basalis prosencephali of the pigeon. Cell Tissue Res. 245, 539–546 (doi:10.1007/BF00218555) [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud A, Reed C, Maklad A. 2013. Central projections of lagenar primary neurons in the chick. J. Comp. Neurol. 521, 3524–3540 (doi:10.1002/cne.23369) [DOI] [PubMed] [Google Scholar]
- 48.Wallraff HG. 1988. Olfactory deprivation in pigeons: examination of methods applied in homing experiments. Comp. Biochem. Physiol. A Comp. Physiol. 89, 621–629 (doi:10.1016/0300-9629(88)90844-4) [DOI] [PubMed] [Google Scholar]
- 49.Zeigler HP, Witkovsky P. 1968. The main sensory trigeminal nucleus in the pigeon: a single-unit analysis. J. Comp. Neurol. 134, 255–263 (doi:10.1002/cne.901340302) [DOI] [PubMed] [Google Scholar]
- 50.Stapput K, Güntürkün O, Hoffmann KP, Wiltschko R, Wiltschko W. 2010. Magnetoreception of directional information in birds requires nondegraded vision. Curr. Biol. 20, 1259–1262 (doi:10.1016/j.cub.2010.05.070) [DOI] [PubMed] [Google Scholar]
- 51.Kirschvink JL. 1992. Uniform magnetic fields and double-wrapped coil systems: improved techniques for the design of bioelectromagnetic experiments. Bioelectromagnetics 13, 401–411 (doi:10.1002/bem.2250130507) [DOI] [PubMed] [Google Scholar]
- 52.Engels S, et al. 2014. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509, 353–356 (doi:10.1038/nature13290) [DOI] [PubMed] [Google Scholar]
- 53.Stingelin W. 1965. Qualitative und quantitative Untersuchungen an Kerngebieten der medulla oblongata bei Vögeln Bibl. Anat. S 6, 1–116 Basel, Sweitzerland: Karger [PubMed] [Google Scholar]
- 54.Mello CV, Vicario DS, Clayton DF. 1992. Song presentation induces gene expression in the songbird forebrain. Proc. Natl Acad. Sci. USA 89, 6818–6822 (doi:10.1073/pnas.89.15.6818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mello CV, Ribeiro S. 1998. ZENK protein regulation by song in the brain of songbirds. J. Comp. Neurol. 393, 426–438 (doi:10.1002/(SICI)1096-9861(19980420)393:4<426::AID-CNE3>3.0.CO;2-2) [DOI] [PubMed] [Google Scholar]
- 56.Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H. 2008. Calcium-binding proteins label functional streams of the visual system in a songbird. Brain Res. Bull. 75, 348–355 (doi:10.1016/j.brainresbull.2007.10.029) [DOI] [PubMed] [Google Scholar]
- 57.Shu S, Ju G, Fan L. 1988. The glucose oxidase–DAB–nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 85, 169–171 (doi:10.1016/0304-3940(88)90346-1) [DOI] [PubMed] [Google Scholar]
- 58.Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S. 2007. The chick brain in stereotaxic coordinates: an atlas featuring neuromeric subdivisions and mammalian homologies. New York, NY: Academic Press [Google Scholar]
- 59.Shimizu T, Bowers AN, Budzynski CA, Kahn MC, Bingman VF. 2004. What does a pigeon (Columba livia) brain look like during homing? Selective examination of ZENK expression. Behav. Neurosci. 118, 845–851 (doi:10.1037/0735-7044.118.4.845) [DOI] [PubMed] [Google Scholar]