Abstract
Background: Toxoplasma gondii is the causal agent of toxoplasmosis in which one third of the world's population has been infected. In pregnant women, it may cause abortion and severe damage to the fetal central nervous system. During pregnancy, the prevalence of toxoplasmosis increases throughout the second and third quarter of gestation, simultaneously progesterone and 17β-estradiol also increase. Thus, it has been suggested that these hormones can aggravate or reduce parasite reproduction. The aim of this study was reviewing the relationship between hormones and infection caused by T. gondii in several experimental animal models and humans, focused mainly on: (a) congenital transmission, (b) parasite reproduction, (c) strain virulence, (d) levels of hormone in host induced by T. gondii infection, and (e) participation of hormone receptors in T. gondii infection. Are the hormones specific modulators of T. gondii infection? A systematic review methodology was used to consult several databases (Pub Med, Lilacs, Medline, Science direct, Scielo, Ebsco, Sprinker, Wiley, and Google Scholar) dated from September, 2013 to March, 2014.
Results: Thirty studies were included; eight studies in humans and 22 in animals and cell cultures. In the human studies, the most studied hormones were testosterone, progesterone, prolactin, and 17β-estradiol. Type I (RH and BK) and Type II (Prugniaud, SC, ME49, T45, P78, and T38) were the most frequent experimental strains.
Conclusions: Thirty-five years have passed since the first studies regarding T. gondii infection and its relationship with hormones. This systematic review suggests that hormones modulate T. gondii infection in different animal models. However, given that data were not comparable, further studies are required to determine the mechanism of hormone action in the T. gondii infectious process.
Keywords: Toxoplasma infection, steroids hormones, no steroid hormones, toxoplasmosis, Toxoplasma
Introduction
Toxoplasma gondii (T. gondii) is the causal agent of toxoplasmosis and one third of the world population has been affected by this parasite (el-On and Peiser, 2003). In immunocompetent adults, 80% of the cases can be asymptomatic. On the other hand, in immunocompromised patients, T. gondii is an opportunistic parasite that has been held responsible for mortal encephalitis (Cabrera-Muñoz et al., 2010).
Congenital transmission of T. gondii causes severe consequences in which the degree of damage depends on the time when the mother is infected (Speroff et al., 1999). Infection during early pregnancy can result in apoptosis of placental cells and fetal resorption (Senegas et al., 2009). When pregnant females infected during latter stage of pregnancy and inflammatory responses are low, congenital transmission is likely to occur (Roberts et al., 2001; Pfaff et al., 2008). The transmission frequency of T. gondii is high (80%) at end of pregnancy.
Pregnancy and T. gondii infection
During pregnancy, maternal hormones alter the immune responses of the mother in the presence of fetal antigens. The increases in the susceptibility to infection and a diminished pro-inflammatory response have critical anti-parasitic properties that cause an unfavorable development of toxoplasmosis (Craig et al., 2001; Roberts et al., 2001; Prigione et al., 2006; Dionne et al., 2012). In the second and third trimester of gestation, there is a significant increase of 17β-estradiol and progesterone levels and it is during this period, when the prevalence of Toxoplasma infection increases (Montoya and Remington, 2008; Al-warid and Al-qadhi, 2012).
17β-estradiol and T. gondii infection
17β-estradiol (E2) is synthetized mainly in the ovary, breast, endometrial tissue, and brain. E2 plays a vital role in the menstrual cycle and human reproduction. In the nervous system, the estrogens are neuroprotective (Duenas et al., 1996; Arevalo et al., 2010). It has been reported that the administration of pharmacological doses of 17β-estradiol increases the susceptibility to Toxoplasma infection (Pung and Luster, 1986).
Progesterone
Progesterone is present in the ovary and corpus luteum where it is primarily involved in the second phase of the menstrual cycle and reproductive processes of women. Progesterone is synthetized in breast, endometrial, and brain too (Speroff et al., 1999). In cells infected with tachyzoites of T. gondii, progesterone did not regulate the replication of parasites (Gay-Andrieu et al., 2002). Progesterone levels are reduced during pregnancy in sheep after infection by T. gondii (Aiumalamai et al., 1990; Fredriksson et al., 1990).
Testosterone levels regulation by T. gondii infection in human beings and mice
Testosterone and their derivatives (dihydrotestosterone and dehydroepiandrosterone) are androgens produced mainly in male gonads, adrenal glands and the brain. Testosterone can act directly as a ligand of androgen receptors (AR) found in several target tissues. Androgens stimulate the development of the secondary sexual characters in males, participate in human reproduction and maturation of human fetal testes (O'Shaughnessy and Fowler, 2014). In the brain, it is considered as a neuroprotective hormone (Kurth et al., 2014). IgG anti-Toxoplasma antibodies were significantly correlated to testosterone (Shirbazou et al., 2011), and results are different accord type strain (Kaňková et al., 2011). T. gondii produces high testosterone levels in infected animals and mRNA expression of luteinizing hormone receptor (LHR) (Oktenli et al., 2004; Abdoli et al., 2012; Lim et al., 2013).
Thyroxine (T4) and T. gondii infection
Studies in Nylar female mice infected with T. gondii, exhibited hypogonadotrophic hypogonadism secondary to hypothalamic dysfunction (Stahl et al., 1985, 1994). These mice infected with T. gondii Cornell strain, present atrophy in the thymus, ovaries, and uterus, cessation of cycling, anovulation, and decline of serum thyroxine (T4) levels (Stahl et al., 1985).
Corticosteroids effect on T. gondii
Cortisol is a glucocorticoid hormone secreted by the adrenal cortex. It works through a signal transduction pathway that initiates by hormone linkage to specific cell receptors. Proteins synthesized by the glucocorticoid response inhibit or stimulate the specific tissue (Gardner et al., 2011). Cortisone increased the amount of tachyzoites, cysts and cystozoite, as the breakage of cysts released a higher resistant antigen-cystozoite in mice brains infected with T. gondii (Hulínská et al., 1990).
Anti-parasitic effect of prolactin on T. gondii infection
PRL is capable of inhibiting multiplication of Toxoplasma in murine microglial cell cultures (Benedetto et al., 2001). PRL significantly restricted intracellular growth of Toxoplasma in mice and human cell lines (Dzitko et al., 2010; 2012). Moreover, it been documented that women with hyperprolactinemia showed lower T. gondii prevalence (Dzitko et al., 2008). It has been reported that serum human prolactin (shPRL) has the capacity to bind to live RH tachyzoites (type I) and ME49 (type II) strains in a specific way (Dzitko et al., 2013).
The aim of this study was to review the relationship between hormones and infection by T. gondii in several experimental animal models and humans. Focusing the information on: (a) congenital transmission, (b) parasite reproduction, (c) strain virulence, (d) levels of hormone in host induced by T. gondii infection, (e) participation of hormone receptors in T. gondii infection.
Materials and methods
Database search
Reports from September 2013 to February 2014 were obtained from a total of nine databases (Pub Med, Lilacs, Medline, Science direct, Scielo, Ebsco, Sprinker, Wiley, Google Scholar). Mesh terms were “Toxoplasma or toxoplasmosis or Toxoplasma gondii” combined with progesterone, 17β-estradiol, testosterone, cortisol, cortisone, aldosterone, 11-desoxicorticosterone, dihydrotestosterone, dehydroepiandrosterone, and non-steroid hormones; growth hormone, prolactin, parathyroid hormone, corticotrophin, insulin, glucagon, luteinizing hormone, thyroid stimulating hormone, human chorionic gonadotropin, antidiuretic hormone, oxytocin, melanocyte stimulating hormone, somatostatin, thyrotropin-releasing hormone, gonadotropin-releasing hormone, noradrenaline, adrenaline, melatonin, thyroxine, and triiodothyronine. Toxoplasma and hormones and strain Toxoplasma. The criteria used for including data were: the full text of papers written in English (reviews and case reports not considered), studies performed on humans, animals, and in cell cultures.
Data collection methods
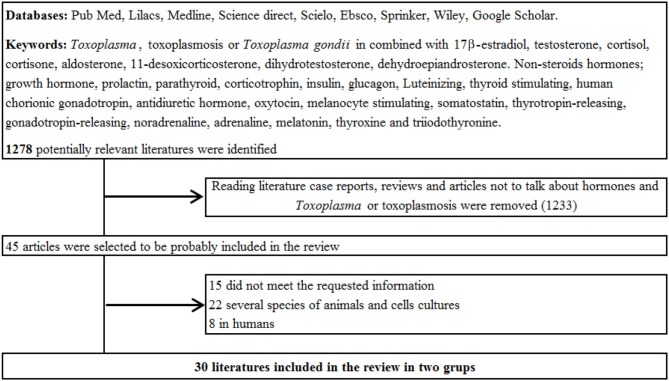
Two reviewers (GRML and GMAF) carefully studied all selected studies. The full text of selected original articles were obtained and reviewed. Inclusion criteria for this analysis were explicit data of all independent variables and at least one dependent variable; data collection and criteria eligibility were established for determining the frequency or proportion of each study. The independent variables were T. gondii strain, hormones, study design, stage of infection and developmental stage of the parasite, post infection evaluation time, age, host, and technical analysis. Dependent variables were increased or decreased of infection and number of parasites. Reference lists of full-text publications were examined for identifying studies not originally selected Figure 1.
Figure 1.
This figure shows the flow of the search obtained, data collection methods, and database search.
From 30 articles meeting inclusion criteria, all results were captured on an Excel database. A number of studies presented frequency distribution of dependent variables; in these cases, the sum of the products of each value by frequency was included for comparison in the database. Some articles presented ranges, mean plus standard deviation; these articles were included in the database using the median.
Results
One thousand two hundred and seventy eight articles potentially related to T. gondii or hormones were found. However, only 45 were selected and of these, 30 met the inclusion criteria for this systematic review. The analysis was divided into three categories: (A) humans in Table 1, (B) several species of animals in Table 2, and (C) Cell cultures in Table 3 and studies conducted in the time period that this research included Figure 2.
Table 1.
Effect of hormones on Toxoplasma gondii infection in humans.
| References | Age of the host (years) | Sexa | Analysis techniqueb | Hormonesc | Diagnostic/groupd | N | Resultse | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Oktenli et al., 2004 | 17–18 | NS | ELISA | Testosterone | T. gondii antibodies | |||||
| Control | 20 | IgM:0.53 ± 0.13 | IgG:0.43 ± 1.08 | – | |||||||
| Normal testosterone levels | 31 | IgM:3.88 ± 1.14* | IgG:4.95 ± 0.91* | ↑ | <0.001 | ||||||
| Low testosterone levels | 9 | IgM:4.00 ± 1.03* | IgG:4.50 ± 1.08* | ↑ | <0.001 | ||||||
| QUIL | Total Testosterone (TT) nM/L ± SD | ||||||||||
| Control | 20 | 17.11 ± 1.01 | – | ||||||||
| Normal testosterone levels | 31 | 17.29 ± 1.38 | – | ||||||||
| Low testosterone levels | 9 | 4.57 ± 0.56* | ↓ | <0.001 | |||||||
| 2 | Hodková et al., 2007 | 21–24 | NS | ELISA | Testosterone | T. gondii antibodies | |||||
| 89 | Positive: 18 | – | |||||||||
| Negative: 71 | – | ||||||||||
| Dom S | Dominance score | ||||||||||
| Infected | 18 | 0.184* | ↑ | =0.051 | |||||||
| Uninfected | 71 | −0.57* | |||||||||
| Masculinity score | |||||||||||
| Mas S | Infected | 18 | 0.17 | ↑ | 0.17 | ||||||
| Uninfected | 71 | −0.03 | |||||||||
| 3 | Flegr et al., 2008a | RIA | Testosterone | Testosterone levels ng/mL | |||||||
| 21.03 | W | 174 | 0.230* | – | <0.0001 | ||||||
| 20.91 | M | 91 | 0.387* | – | |||||||
| ELISA | T. gondii antibodies | ||||||||||
| W | 174 | Positive: 29 | – | ||||||||
| M | 91 | Negative: 23 | ↑ | <0.001 | |||||||
| 4 | Dzitko et al., 2008 | ELISA | Prolactin | Seropositive anti-Toxoplasma antibodies | |||||||
| NS | W | Control | 205 | 93 | |||||||
| M | Control | 76 | 39 | ||||||||
| W | Hyperprolactinaemia | 168 | 57* | ↓ | =0.025 | ||||||
| M | Hyperprolactinaemia | 66 | 31 | ||||||||
| W | Hypoprolactinaemia | 32 | 10 | ||||||||
| M | Hypoprolactinaemia | 9 | 3 | ||||||||
| 5 | Flegr et al., 2008b | RIA | Testosterone | Testosterone levels nM/L | |||||||
| 21.05 | W | 135 | 0.23 | – | <0.001 | ||||||
| 20.94 | M | 106 | 0.41 | – | |||||||
| Digit radio 2D:4D | |||||||||||
| W | ELISA | 194 | Right: 0.315 | Left: 0.587 | |||||||
| M | 106 | Right: 0.167 | Left: 0.002* | – | <0.01 | ||||||
| 6 | Shirbazou et al., 2011 | ELISA | Seropositive T. gondii antibodies | ||||||||
| NS | W | 73 | 24 | – | |||||||
| NS | M | 107 | 39 | – | |||||||
| Cortisol levels in blood | |||||||||||
| NS | W | Cortisol | Uninfected | 12 | – | ||||||
| NS | M | Uninfected | 19 | – | |||||||
| NS | W | Infected | 24 | t: 5.774* | <0.0001 | ||||||
| NS | M | Infected | 39 | – | |||||||
| Testosterone levels in blood | |||||||||||
| NS | W | Testosterone | Uninfected | 12 | – | ||||||
| NS | M | Uninfected | 19 | – | |||||||
| NS | W | Infected | 24 | t: 2.491* | =0.002 | ||||||
| NS | M | Infected | 39 | – | |||||||
| 7 | Al-warid and Al-qadhi, 2012 | ELISA | Anti-Toxoplasma antibodies | ||||||||
| 19–40 | W | Uninfected | 9 | (−) IgG | (−) IgM | – | |||||
| Acute | 10 | (−) IgG | (+) IgM | – | |||||||
| Sub-acute | 9 | (+) IgG | (−) IgM | – | |||||||
| Chronic | 13 | (+) IgG | (+) IgM | – | |||||||
| Progesterone levels ng/dL ± SD | |||||||||||
| W | ELISA | Progesterone (P4) | Uninfected | 9 | 18.3 ± 9.84 | ||||||
| Infected | 32 | 11.19 ± 9.76 | |||||||||
| P4 levels ng/dL ± SD | |||||||||||
| Acute | 10 | 5.35 ± 7.15 | – | ||||||||
| Sub-acute | 9 | 15 ± 9.01 | – | ||||||||
| Chronic | 13 | 14.62 ± 10.38 | – | ||||||||
| Estradiol levels pg/dL ± SD | |||||||||||
| W | ELISA | 17β-estradiol (E2) | Uninfected | 9 | 53.61 ± 76.24 | – | |||||
| Infectadas | 32 | 88.19 ± 101.10 | – | ||||||||
| E2 levels pg/dL ± SD | |||||||||||
| Acute | 10 | 70.66 ± 51.08 | – | ||||||||
| Sub-acute | 9 | 92.51 ± 78.70 | – | ||||||||
| Chronic | 13 | 108.02 ± 138.67 | – | ||||||||
| 8 | de la Torre et al., 2012 | 20–29 | ELISA | DHEAS | Seropositive T. gondii antibodies | ||||||
| 82 | 42 | ||||||||||
| IL | DHEAS levels ug/dL | ||||||||||
| W | Active RC by T. gondii | 26 | 58 | – | |||||||
| M | 206 | ||||||||||
| W | RS of RC by T. gondii | 19 | 95* | =0.12 | |||||||
| M | 199* | =0.79 | |||||||||
| W | Positive of T. gondii w OL | 16 | 113 | ||||||||
| M | 177 | ||||||||||
| W | Negative assay for T. gondii | 21 | 122* | =0.3 | |||||||
| M | 161* | =0.87 | |||||||||
M, Men; W, Woman; NS, Not Specified.
ELISA, Enzyme-Linked ImmunoSorbent Assay; (QUIL), Chemiluminescence; Dom S, Dominance Score; Mas S, Masculinity Score; RIA, Radioimmunoassay; IL, Immunoluminimetric.
DHEAS, Dehydroepiandrosterone Sulphated; E2, 17β-estradiol; P4, Progesterone.
RS, Retinal Scars; RC, Retinochoroiditis; w OL, Without Ocular Lesions.
↑, Increased infection; ↓, Decrement infection; ↑, Increased hormone; ↓, Decrement hormone;
and bold, Statistically Significant. NS, Not specified.
Table 2.
Effect of Toxoplasma gondii infection on hormones in animals.
| References | Type of study | Type of host | Age of the host (weeks) | Way of infectiona | Stage parasite | Strainb | Number of parasites | Days post-infection | Analysis techniquec | Hormonesd | Groupe | N | Resultsf | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kittas and Henry, 1979 | In vivo | Guinea-pigs | NS | SC | Cysts | Bk | 50 | 42 | Number of Toixoplasma cysts ± SD | ||||||
| HIS | 17β-estradiol (E2) | Control F: | 8 | 88.75 ± 21.60 | ||||||||||||
| Control M: | 8 | 82.50 ± 21.1* | <0.001 | |||||||||||||
| Gdt F: | 8 | 63.00 ± 16.5 | ↓ | |||||||||||||
| Gdt M: | 8 | 65.25 ± 10.8 | ↓ | |||||||||||||
| Gdt + Hex F: | 8 | 200.25 ± 16.00 | ↑ | |||||||||||||
| Gdt + Hex M: | 8 | 184.00 ± 36.80 | ↑ | |||||||||||||
| 2 | Kittas and Henry, 1980 | In vivo | Mice | 11 | SC | Cysts | Bk | 30 | 42 | Number of Toxoplasma cysts ± SD | ||||||
| HIS | 17β-estradiol | Control F: | 8 | 222 ± 42 | ||||||||||||
| (E2) | Control M: | 8 | 220 ± 23 | |||||||||||||
| Gdt F: | 8 | 189 ± 22* | ↓ | <0.001 | ||||||||||||
| Gdt M: | 8 | 178 ± 24* | ↓ | <0.001 | ||||||||||||
| Gdt + Hex F: | 8 | 598 ± 64* | ↑ | <0.001 | ||||||||||||
| Gdt + Hex M: | 8 | 599 ± 45* | ↑ | <0.001 | ||||||||||||
| 3 | Pung and Luster, 1986 | In vivo | Mice (B6C3F1) | 8–10 | SC | Cysts | T45 | 30 | 35 | Number of Toxoplasma cysts ± SD | ||||||
| RIA | Control | 6 | 982 ± 194 | |||||||||||||
| DES | 6 | 2244 ± 66* | ↑ | <0.05 | ||||||||||||
| 17β-estradiol | 6 | 1934 ± 198* | ↑ | <0.05 | ||||||||||||
| 5α-Dihydrotesti osterone | 6 | 792 ± 164 | ↓ | |||||||||||||
| Progesterone | 6 | 1012 ± 172 | ↑ | |||||||||||||
| Zeralanol | 6 | 1463 ± 190 | ↑ | |||||||||||||
| a-Dienestrol | 6 | 2405 ± 227* | ↑ | <0.05 | ||||||||||||
| Corticosterone | 6 | 1954 ± 314* | ↑ | <0.05 | ||||||||||||
| Effect of Tamoxifen, number of cysts ± SD | ||||||||||||||||
| RIA | 17β-estradiol (E2) | Control | 6 | 1115 ± 112 | ||||||||||||
| Tamoxifen | 6 | 975 ± 124 | ↓ | |||||||||||||
| 17β-estradiol | 6 | 2220 ± 182* | ↑ | <0.05 | ||||||||||||
| Tamoxifen + E2 | 6 | 1027 ± 167 | ↓ | |||||||||||||
| 4 | Fredriksson et al., 1990 | In vivo | Ewes (Scottish blackface) | NS | Oral | Oocysts | RH | 2000 | 90.5 | Progesterone levels (nM/L) | ||||||
| RIA | Progesterone (P4) | Control | 3 | 10–20 | ||||||||||||
| Infected | 13 | 10 | ↓ | NS | ||||||||||||
| Vaccinated | 15 | 10 | ↓ | NS | ||||||||||||
| 5 | Aiumalamai et al., 1990 | In vivo | Ewes (Swedish Peltsheep) | 52–104 | NS | Oocysts | NS | NS | 90.5 | Progesterone levels (nM/L) | ||||||
| RIA | Progesterone (P4) | 7 | Day 5: 6–8 | |||||||||||||
| Days 10 a 15: 19- | ↑ | <0.05 | ||||||||||||||
| 6 | Hulínská et al., 1990 | In vivo | Mice (H VUFB) | 4–5 | IP | Cysts | P78 | 10 | Number of tachyzoites and stozoites | |||||||
| 5–14 | HIS y MIC | Cortisone | Group 1 | 20 | 10–14 days | ↑ | – | |||||||||
| 12–47 | Group 2 | 20 | ||||||||||||||
| 7 | Engeland et al., 1996 | In vivo | Goat (Norwegian) | NS | SC | Bradyzoites | NS | 1250 | 54–73 | Progesterone levels | ||||||
| ELISA y SF | Progesterone (P4) | Control | 6 | |||||||||||||
| Infected | 5 | |||||||||||||||
| 8 | Stahl and Kaneda, 1998a | In vivo | Mice (Nya: NYLAR) | NS | IP | Cysts | CS | 8 | 3 and 4 | T4 levels (Mean) | ||||||
| RIA | Thyroxine (T4) | Control | 10 | 7.5 | ||||||||||||
| Infected | 10 | 3 | ↓ | <0.01 | ||||||||||||
| 9 | Stahl and Kaneda, 1998a | In vivo | Mice (Nya: NYLAR) | 12 | IP | Cysts | CS | 8 | 4 | Subnormal T4 response to a 1 |ig bolus or TRH (Mean) | ||||||
| RIA | Thyroxine (T4) | Control | 8 | 11 | ||||||||||||
| Infected | 8 | 3 | ↓ | <0.01 | ||||||||||||
| 10 | Liesenfeld et al., 2001 | In vivo | Mice (C57BL/6) | 8–10 | Oral | Cysts | ME 49 | 100 | 7 | Number of parasitophorous vacuoles | ||||||
| NS | Testosterone | Control | 657 ± 399 | |||||||||||||
| Testosteron | 426 ± 282 | ↓ | =0.0141 | |||||||||||||
| 11 | Kaňková et al., 2011 | In vivo | Mice (BALB/c and C57 Black) | 5–6 | Oral | Cysts | T38 | 10 | 60 | Differences in serum testosterone levels | ||||||
| RIA | Testosterone | M. Toxo infected | 12 | Z = −2.32 | ↓ | =0.005 | ||||||||||
| M. Controls | 20 | |||||||||||||||
| F. Toxo infected | 12 | Z = −2.76 | ↓ | =0.020 | ||||||||||||
| F. Controls | 20 | |||||||||||||||
| 12 | Abdoli et al., 2012 | In vivo | Rats (Wistar) | NS | IP | Tachyzoites | RH | 1 × 107 | Effect of T. gondii infection on Serum Testosterone (ST) | |||||||
| 10 | ELISA | Testosterone | Uninfected | 5 | 0.6 ± 0.01 | |||||||||||
| 10 | Infected | 3 | 0.55 ± 0.02* | ↓ | <0.05 | |||||||||||
| Effect of T.gondii infection on IntratesticularTestosteron (ITT) | ||||||||||||||||
| 10 | Uninfected | 5 | 4.07 ± 0.02 | |||||||||||||
| 10 | Infected | 3 | 3.89 ± 0.05* | ↓ | <0.05 | |||||||||||
| 13 | Puvanesuaran et al., 2012 | In vivo | Mice (Swiss) | 3 | Oral | Tachyzoites | RH | 1 × 104 | 4 | Number of tachyzoites (Mean) | ||||||
| MIC | Prednisolone | Control | 3 | 1.48 × 107 | ||||||||||||
| 235 mg/kg | 3 | 2.75 × 107 | ↑ | <0.05 | ||||||||||||
| 470 mg/kg | 3 | 2.92 × 107 | ↑ | <0.05 | ||||||||||||
| 705 mg/kg | 3 | 3.21 × 107 | ↑ | <0.05 | ||||||||||||
| 14 | Lim et al., 2013 | In vivo | Rats (Wistar) | 7 | IP | Tachyzoites | PRU | 5 × 106 | 42–56 | % Increase of Testosterona levels | ||||||
| ELISA | Testosterone | 54 | 60% | ↑ | =0.057 | |||||||||||
| 15 | Mitra et al., 2013 | In vivo | Rats | 6.5 | IP | Tachyzoites | PRU | 10 × 106 | 42–56 | Circuling levels of corticosterone | ||||||
| ELISA | Corticosterone | 126 | 64% | ↓ | <0.05 | |||||||||||
SC, Subcutaneously; IP, Intraperitoneally; NA, Not Applicable.
Type of strain: BK, Beverley; PRU, Prugniaud; CS, Cornell; RH, ME49, T45, P78, T38.
HIS, Histological; RIA, Radioimmunoassay; MIC, Microscopical; SF, Sabin and Feldman; ELISA, Enzyme-Linked ImmunoSorbent Assay.
E2, 17β-estradiol; P4, Progesterone; T4, Thyroxine; DES, Diethylstilbestrol; ST, Serum Testosterone; ITT, Intra testicular testosterone; TRH, Thyrotropin-Releasing Hormone.
M, Male; F, Female; Gdt, Gonadectomy; Hex, Hexoestrol.
↑, Increased infection; ↓, Decrement infection; ↑, Increased hormone; ↓, Decrement hormone;
and bold, Statistically Significant. NS, Not specified; SD, Standard deviation.
Table 3.
Effect of Toxoplasma gondii infection on hormones in cell cultures.
| References | Type of Study | Type of cell culturea | Stage parasite | Strainb | Number of parasites | Days post-infection | Analysis techniquec | Hormoned | Group | N | Resultse | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Benedetto et al., 2001 | In vitro | MGC (C57BL/6) | Tachyzoites | RH | 1 × 104 | 20 h | Intracellular replicaton of T. gondii (Mean ± SD) | ||||||
| ELISA | Prolactin | Control | 7.4 ± 1.0 | |||||||||||
| (PRL) | PRL + rTNF-a | 6.1 ± 1.0 | ↓ | <0.05 | ||||||||||
| 2 | Gay-Andrieu et al., 2002 | In vitro | RAW 264.7 | Tachyzoites | RH | 3.3 × 106 | 3–20 h | Toxoplasma gondii replication | ||||||
| IF, FC | Progesterone | No significant differences | <0.05 | |||||||||||
| y MIC | ||||||||||||||
| 3 | Gets and Monroy, 2005 | In vitro | RAW 264.7 | Tachyzoites | RH | 5 × 105 | 18–24 | Percentage of infected macrophages | ||||||
| MIC | Adrenaline | Control | ||||||||||||
| Adrenaline a | 5.55* | ↑ | <0.05 | |||||||||||
| Adrenaline p | 10* | ↑ | <0.05 | |||||||||||
| 4 | Jones et al., 2008 | In vitro | BmSCs | Tachyzoites | RH | 2 ×106 | 1 | Effect on LPS-induces killing on T. gondii | ||||||
| NS | Progesterone | Control | No significant differences | <0.05 | ||||||||||
| Infected | ||||||||||||||
| 5 | Dzitko et al., 2010 | In vitro | Tachyzoites | BK | 2 x 105 | Influence of rhPRL en la intensidad de multiplication de T.gondii | ||||||||
| L929 | 6 | MTT | Prolactine | No significant differences | ||||||||||
| Hs27 | 1000.0 (ng/mL) | 18 | 8.90 ± 3.46* | ↓ | <0.01 | |||||||||
| HeLa | (No Sig. Diff.) | |||||||||||||
| Inhibition of the proliferation rate (%) of T. gondii | ||||||||||||||
| L929 | 0 (min) | 2.0–100.0 (ng/m L) | 12 | No significant differences | ||||||||||
| 30 | 20.0 (ng/mL) | 12 | 19.87 ± 4.28* | ↑ | <0.05 | |||||||||
| 100.0 (ng/mL) | 12 | 23.66 ± 10.99* | ↑ | <0.05 | ||||||||||
| 60 | 20.0 (ng/mL) | 12 | 19.66 ± 5.73* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 25.53 ± 3.19* | ↑ | <0.01 | ||||||||||
| 180 | 20.0 (ng/mL) | 12 | 26.76 ± 3.02* | ↑ | <0.01 | |||||||||
| Hs27 | 0 (min) | 100.0 (ng/mL) | 12 | 27.00 ± 2.50* | ↑ | <0.01 | ||||||||
| 2.0–100.0 (ng/m L) | 12 | No significant differences | ||||||||||||
| 30 | 20.0 (ng/mL) | 12 | 20.81 ± 4.21* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 21.93 ± 5.48* | ↑ | <0.01 | ||||||||||
| 60 | 20.0 (ng/mL) | 12 | 19.05 ± 2.63* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 23.01 ± 5.93* | ↑ | < 0.01 | ||||||||||
| 180 | 20.0 (ng/mL) | 12 | 21.14 ± 5.62* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 36.15 ± 11.53* | ↑ | <0.01 | ||||||||||
| HeLa | 0 (min) | 2.0–100.0 (ng/mL) | 12 | No significant differences | ||||||||||
| 30 | 20.0 (ng/mL) | 12 | 23.05 ± 4.97* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 31.74 ± 5.79* | ↑ | <0.01 | ||||||||||
| 60 | 20.0 (ng/mL) | 12 | 27.71 ± 7.42* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 31.71 ± 7.06* | ↑ | <0.01 | ||||||||||
| 180 | 20.0 (ng/mL) | 12 | 29.64 ± 6.23* | ↑ | <0.01 | |||||||||
| 100.0 (ng/mL) | 12 | 32.12 ± 3.53* | ↑ | <0.01 | ||||||||||
| 6 | Dzitko et al., 2012 | In vitro | PBMC | Tachyzoites | BK | 2.5 × 10 5 | 3 | % of T. gondii proliferation | ||||||
| ELISA | rhPRL | 0 (ng/mL) | 76.35 ± 10.1 | |||||||||||
| 100.0 (ng/mL) | 81.01 ± 11.6 | |||||||||||||
| sPRL | 0 (ng/mL) | 49.8 ± 4.6 | ||||||||||||
| 100.0 (ng/mL) | 59.6 ± 3.1* | ↓ | <0.01 | |||||||||||
| 7 | Dzitko et al., 2013 | In vitro | L929 | Tachyzoites | 1 × 10 7 | % increse of prolactine Levels | ||||||||
| RH | 30 (min) | ELISA | shPRL | 10.1 | ||||||||||
| 90 (min) | NS | 52.4 | ↑ | =0.056 | ||||||||||
| ME49 | 30 (min) | 16 | ||||||||||||
| 90 (min) | NS | 46.2 | ↑ | =0.056 | ||||||||||
MGC, Microglial cell cultures; RAW 264.7, Murine Macrophage cell line; BmSCs, Bone marrow Stem Cells; L929, Mouse fibroblasts cell line; Hs27, Human foreskin fibroblast; HeLa, Human epithelial cells; PBMC, Peripheral Blood Mononuclear Cells.
Type of strain: Beverley (BK), RH, ME49.
MIC, Microscopical; IF, Immunofluorescence; FC, Flow Cytometry; ELISA, Enzyme-Linked ImmunoSorbent Assay; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
PRL, Prolactin; rhPRL, Recombinant Human Prolactin; sPLR, Serum Prolactin; shPRL, Sheep Prolactin.
↑, Increased infection; ↓, Decrement infection; ↑, Increased hormone; ↓, Decrement hormone;
and bold, Statistically Significant. NS, Not specified; SD, Standard deviation.
Figure 2.
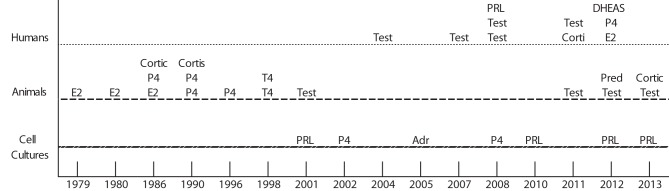
Show studies development in human, animals and cells cultures with different hormones during a time period since 1979–2013. E2, 17β-estradiol; Cortic, Corticosterone; Cortis, Cortisone; Corti, Cortisol; P4, Progesterone; T4, Thyroxine; Test, Testosterone; PRL, Prolactin; Adr, Adrenaline; DHEAS, Dehydroepiandrosterone; Pred, Prednisolone.
Humans
Eight articles were performed with different hormones on humans, from 17 to 40 years old: Testosterone (n = 5) (Oktenli et al., 2004; Hodková et al., 2007; Flegr et al., 2008a,b; Shirbazou et al., 2011), 17β-estradiol and progesterone, dehydroepiandrosterone (DHEA), prolactin, and cortisol and testosterone (n = 1) (Dzitko et al., 2008; Al-warid and Al-qadhi, 2012; de la Torre et al., 2012). These studies used Radioimmunoassay (RIA) or Enzyme-linked ImmunoSorbent assay (ELISA) in 8 studies combined with other analytic methods (Table 1).
Animals
Fifteen articles evaluated the hormone effect in T. gondii infection using different animal models: murine model (n = 12); in guinea-pigs (1) (Kittas and Henry, 1979), in mice (8) (Kittas and Henry, 1980; Pung and Luster, 1986; Hulínská et al., 1990; Stahl and Kaneda, 1998a,b; Liesenfeld et al., 2001; Kaňková et al., 2011; Puvanesuaran et al., 2012), and rats (3) (Abdoli et al., 2012; Lim et al., 2013; Mitra et al., 2013). Two from ewes (2) (Aiumalamai et al., 1990; Fredriksson et al., 1990) and one for goats (1) (Engeland et al., 1996) (Table 2).
Progesterone and testosterone were the most studied hormones (n = 4), estradiol (n = 3), corticosterone and thyroxine (n = 2) and cortisone, adrenaline, and prednisolone (n = 1). Eight T. gondii strains were also analyzed: two Type I (eight RH and four BK) and six Type II (two PRU, ME49 and SC and one T45, P78, T38) and two not specified (Table 2).
The most frequent parasite stage of development studied was the tachyzoite (n = 11), followed by cyst (n = 8), ooquiste (n = 2), and bradizoite (n = 1). The number of parasites used for each experiment depended on the stage of parasite development and the host. In the murine model, tachyzoites from 1 × 104 to 1 × 107 were used (Benedetto et al., 2001; Abdoli et al., 2012; Dzitko et al., 2013). The number of cysts used in different rodent species was from 8 to 100 (Stahl and Kaneda, 1998b; Liesenfeld et al., 2001). In an experiment with goats, 1250 bradyzoytes were used (Engeland et al., 1996) and in another study with sheep infected with ooquistes, the number of ooquistes was not indicated (Aiumalamai et al., 1990) (Table 2).
The post-infection time in each experiment was different, according to each species and parasite stage of development. In guinea pigs, 42 days (Kittas and Henry, 1979); mice, 4 to 60 days (Kittas and Henry, 1980; Pung and Luster, 1986; Hulínská et al., 1990; Stahl and Kaneda, 1998a,b; Liesenfeld et al., 2001; Kaňková et al., 2011; Puvanesuaran et al., 2012); in rats, 10 to 56 days (Abdoli et al., 2012; Lim et al., 2013; Mitra et al., 2013), in a goat, 54 to 73 days (Engeland et al., 1996) and in ewes 90.5 days (Aiumalamai et al., 1990; Fredriksson et al., 1990) (Table 2).
Concerning the route of infection, 15 studies were carried out, four subcutaneous (Kittas and Henry, 1979, 1980; Pung and Luster, 1986; Engeland et al., 1996) and six more by peritoneal administration (Hulínská et al., 1990; Stahl and Kaneda, 1998a,b; Abdoli et al., 2012; Lim et al., 2013; Mitra et al., 2013). In four studies, oral administration was used for infection (Fredriksson et al., 1990; Liesenfeld et al., 2001; Kaňková et al., 2011; Puvanesuaran et al., 2012) and one was not specified (Aiumalamai et al., 1990) (Table 2).
Cell cultures
Seven studies were designed in cell lines; two in RAW 264.7 mouse cell lines (Gay-Andrieu et al., 2002; Gets and Monroy, 2005), one, in bone marrow stem cells (Jones et al., 2008) one in microglial cell cultures (Benedetto et al., 2001) and three with prolactin in Murine L929, Human Hs27, HeLa, and Peritoneal Blood Mononuclear cells (PBMC) (Dzitko et al., 2010, 2012, 2013; Abdoli et al., 2012) (Table 3).
Concerning non-steroid hormones, prolactin and thyroxine hormone have been studied. In this study, other non-steroid hormones such as growth hormone, parathyroid, corticotrophin, insulin and glucagon, luteinizing and follicle hormone, thyroid stimulating, human chorionic gonadotropin, antidiuretic, oxytocin, melanocyte stimulating, somatostatin, thyrotropin-releasing hormone, gonadotropin-releasing hormone, noradrenaline, adrenaline, melatonin, and triiodothyronine were not associated to Toxoplasma infection.
The laboratory analysis methods used were: Radioimmunoassay (RIA) (Pung and Luster, 1986; Aiumalamai et al., 1990; Kaňková et al., 2011). Enzyme-Linked Immunosorbent Assay (ELISA) (Engeland et al., 1996; Abdoli et al., 2012; Dzitko et al., 2012, 2013; Lim et al., 2013). A Morphological Method, (MM), Indirect Immunofluorescence (IFI), Flow Cytometry Analysis (CF) (Gay-Andrieu et al., 2002), Microscopy (Hulínská et al., 1990; Gay-Andrieu et al., 2002), in three histological studies (Kittas and Henry, 1979, 1980; Hulínská et al., 1990) and in two methods. Sabin and Feldman (SF) (Engeland et al., 1996) Inverse Reaction of Polymerase Chain and ELISA (Lim et al., 2013).
Discussion
Congenital toxoplasmosis is one of the most significant burdens of T. gondii infection in humans. Both the maternal–fetal transmission and hormonal levels during pregnancy are poorly understood and yet, may play an important role during the course of the disease. In pregnant women with acute toxoplasmosis, low levels of progesterone and low levels of estrogens can induce severe infection caused by T. gondii (Al-warid and Al-qadhi, 2012). The changes in endocrine phenomena occurring during pregnancy, as well as the size and maturity of the placenta and the embryonic/fetal immune response definitely affect the ability to be protected from invasion or to fight infection (Ortiz-Alegría et al., 2010).
In pregnant women with toxoplasmosis, low levels of progesterone and estrogen can induce severe infection. Nevertheless, the mechanism is unknown (Al-warid and Al-qadhi, 2012). Current studies show that there weren't any statistically significant differences in progesterone levels between infected and uninfected women with T. gondii, although higher progesterone levels were observed in uninfected women compared to low level in infected women. Moreover, estrogen levels in both chronic and uninfected women did not exhibit significant differences, although infected women had a higher level, compared to uninfected women.
The study of 17β-estradiol in T. gondii infection began in 1979, when hexoestrol was administered to mice and increased the number of T. gondii cysts in muscle (Kittas and Henry, 1979). At the same time, the susceptibility to T. gondii infection increased in mice when pharmacological estrogen concentrations were used (Pung and Luster, 1986). Nevertheless, 35 years have passed since these experiments were performed and no further studies regarding 17β-estradiol mechanism in T. gondii infection have been reported.
Progesterone levels are reduced during pregnancy in sheep after infection by T. gondii (Aiumalamai et al., 1990; Fredriksson et al., 1990). This hormonal change could be contributing to the susceptibility to T. gondii infection in sheep.
In RAW 264.7 cells infected with tachyzoites of T. gondii, progesterone did not regulate the replication of parasites (Gay-Andrieu et al., 2002). However, bone marrow stem cells activated with Lippolysaccharide (LPS) and treated with progesterone, while infected with T. gondii tachyzoites, cells exhibited a significant reduction in parasite death compared to activated controls (Jones et al., 2008). These results suggest that progesterone can modulate the survival of parasites in vitro.
The results of this study showed that steroid hormones are the most studied toxoplasmosis interaction. However, the information has a great heterogeneity and is not comparable, due to their different experimental designs. For example, the progesterone has been studied in mice (Pung and Luster, 1986), sheep (Aiumalamai et al., 1990), goats (Engeland et al., 1996), and bone marrow stem cells cultures (Jones et al., 2008). Furthermore, in these experiments, different strains and parasite stage of development were used. Moreover, no study has shown how steroid hormones regulate T. gondii infection.
The first observation of T. gondii infection and its association with testosterone in humans shows that acute infection by this parasite produced temporary hypogondatrophic gonadal insufficiency (Oktenli et al., 2004). On the other hand, there are several human studies analyzing different genders, using portrait pictures of 89 male students, of which 18 were Toxoplasma infected, and 109 female students. When statistically corrected for age, men with latent toxoplasmosis were perceived as more dominant (p = 0.009) and masculine (p = 0.052). These results suggest that the higher level of testosterone could be responsible for at least some of the toxoplasmosis-associated shifts in human and animal behavior (Hodková et al., 2007). In 2008, Flegr showed that the relationship between age, gender and 2D:4D ratio in hands sharply increased with Toxoplasma infection. Infected males had higher testosterone levels, while infected females had lower levels, than Toxoplasma-free males and females, respectively. Toxoplasma-infected males had a lower left hand 2D:4D ratio than Toxoplasma-free males. These results suggest that the relationship between 2D:4D ratio is particularly strong for the left hand and 2D:4D dimorphism will probably be higher in countries with a high prevalence of toxoplasmosis (Flegr et al., 2008b). These results indicate that sexual hormones and gender are key factors determining susceptibility to Toxoplasma infection.
Significantly, lower levels of testosterone in male and female mice with latent toxoplasmosis (strain T38 of T. gondii) were compared to uninfected controls (Kaňková et al., 2011). On the other hand, Liesenfeld in 2001 described the effect of sexual steroids and gender in the susceptibility to infection by T. gondii in mice. Death occurred in female mice before males, and mortality in females was associated to an increase in the number of tachyzoites. Female mice testosterone treatment reduced the number of parasites and pathology.
5α-Dihydrotestosterone reduced the number of cysts in mice infected with T. gondii cysts strain T45. Mice treated with corticosterone increased twice the number of cysts of T. gondii (Pung and Luster, 1986; Hulínská et al., 1990). These results showed that corticosterone could exacerbate the infection process.
The prevalence of T. gondii infection was analyzed in women with hyper and hypoprolactinemia, with a significant increase in this last group (Dzitko et al., 2008). In other studies using peripheral blood mononuclear cells (PBMC) of patients with hyperprolactinemia revealed that exogenous recombinant human prolactin (rhPRL), as well as autologous shPRL from inactivated serum, significantly restricted intracellular growth of Toxoplasma in these cultures (Dzitko et al., 2012). PRL may be one of the potential humoral factors implicated in the limitation of T. gondii invasion. A physiological increase in PRL concentration during pregnancy may significantly reduce the risk of T. gondii proliferating in the expecting mother (Dzitko et al., 2012).
rhPRL reduced T. gondii replication in human cells (Hs27 y HeLa) and murine cells (L929), (Dzitko et al., 2010, 2013). Afterwards in another experimental study, the replication of parasites was reduced in L929 cells treated with prolactin. These results indicate that the inhibition of replication of T. gondii was caused by a limited capacity of the parasites to penetrate host cells, as demonstrated by the reduced number of infected cells. On the other hand, PRL stimulates T cell proliferation (Clevenger et al., 1992) and the release of various protective cytokines as TNF-α which control efficiently the course of T. gondii infection (Benedetto et al., 2001). The possible PRL action could be bidirectional, namely PRL may limit the proliferation of Toxoplasma via surface host cell receptors (Dzitko et al., 2013) leading to the release of protective type-1 cytokines, such as interleukin 12 (IL-12) and IFN-c (Matalka, 2003), and by inhibiting their penetration ability (Dzitko et al., 2010, 2013).
In the last 35 years, researchers worldwide have made a great effort to advance in the field of knowledge on how the hormones are involved in T. gondii infection, however, a major number of studies and the use of modern molecular methods are required to define the mechanistic role of hormones in the regulation of toxoplasmosis.
Implications for research
A crucial factor is the difference in experimental models to study of T. gondii infections and hormones. As well, type's strains and the number limited studies to comparative analysis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdoli A., Dalimi A., Movahedin M. (2012). Impaired reproductive function of male rats infected with Toxoplasma gondii. Andrologia 44, 679–687 10.1111/j.1439-0272.2011.01249.x [DOI] [PubMed] [Google Scholar]
- Aiumalamai S., Fredriksson G., Uggla A., Kindahl H., Edquist L. (1990). The effect of Toxoplasrna gondii Infection in Flunixin Meglumine Treated Pregnant Ewes as Monitored by Plasma Levels of 15-Ketodihydroprostaglandin F2a, progeterone, Oestrone Sulphate and Ultrasound Scanning. J. Vet. Med. 37, 23–34 10.1111/j.1439-0442.1990.tb00872.x [DOI] [PubMed] [Google Scholar]
- Al-warid H. S., Al-qadhi B. N. (2012). Evaluation of progesterone and estrogen hormonal levels in pregnant women with toxoplasmosis. Eur. J. Sci. Res. 91, 515–519 [Google Scholar]
- Arevalo M. A., Santos-Galindo M., Bellini M. J., Azcoitia I., Garcia-Segura L. M. (2010). Actions of estrogens on glial cells: implications for neuroprotection. Biochim. Biophys. Acta 1800, 1106–1112 10.1016/j.bbagen.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Benedetto N., Folgore A., Romano Carratelli C., Galdiero F. (2001). Effects of cytokines and prolactin on the replication of Toxoplasma gondii in murine microglia. Eur. Cytokine Netw. 12, 348–358 [PubMed] [Google Scholar]
- Cabrera-Muñoz E., Escobedo G., Guzmán C., Camacho Arroyo I. (2010). Role of progesterone in HIV and parasitic infections. Open Neuroendocrinol. J. 3, 137–142 23092404 [Google Scholar]
- Clevenger C. V., Sillman A. L., Hanley-Hyde J., Prystowsky M. B. (1992). Requirement for prolactin during cell cycle regulated gene expression in cloned T-lymphocytes. Endocrinology 130, 3216–3222 [DOI] [PubMed] [Google Scholar]
- Craig W. R., Willian W., James A. (2001). Sex-asociated hormones and inmunity to protozoan parasites. Clin. Microbiol. Rev. 14, 476–488 10.1128/CMR.14.3.476-488.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre A., Ríos-Cadavid A. C., Cardozo-García C. M., Padilla L., Gómez-Marín J. E. (2012). Serum levels of dehydroepiandrosterone sulfate (DHEAS) in ocular toxoplasmosis. J. Microbiol. Immunol. 45, 65–68 10.1016/j.jmii.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Dionne P., Robinson S., Klein L. (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 62, 263–271 10.1016/j.yhbeh.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M., Torres-Aleman I., Naftolin F., Garcia-Segura L. M. (1996). Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience 74, 531–539 10.1016/0306-4522(96)00142-X [DOI] [PubMed] [Google Scholar]
- Dzitko K., Dziadek B., Gatkowska J., Długońska H. (2013). Toxoplasma gondii binds sheep prolactin. Exp. Parasitol. 134, 216–219 10.1016/j.exppara.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Dzitko K., Gatkowska J., Płociński P., Dziadek B., Długońska H. (2010). The effect of prolactin (PRL) on the growth of Toxoplasma gondii tachyzoites in vitro. Parasitol. Res. 107, 199–204 10.1007/s00436-010-1849-3 [DOI] [PubMed] [Google Scholar]
- Dzitko K., Lawnicka H., Gatkowska J., Dziadek B., Komorowski J., Długońska H. (2012). Inhibitory effect of prolactin on Toxoplasma proliferation in peripheral blood mononuclear cells from patients with hyperprolactinemia. Parasite Immunol. 34, 302–311 10.1111/j.1365-3024.2012.01359.x [DOI] [PubMed] [Google Scholar]
- Dzitko K., Malicki S., Komorowski J. (2008). Effect of hyperprolactinaemia on Toxoplasma gondii prevalence in humans. Parasitol. Res. 102, 723–729 10.1007/s00436-007-0824-0 [DOI] [PubMed] [Google Scholar]
- el-On J., Peiser J. (2003). Toxoplasma and toxoplasmosis. Harefuah 142, 48–55 [PubMed] [Google Scholar]
- Engeland I. V., Waldeland H., Kindahl H., Ropstad E., Andresen O. (1996). Effect of Toxoplasma gondii infection on the development of pregnancy and on endocrine foetal-placental function in the goat. Vet. Parasitol. 67, 61–74 10.1016/S0304-4017(96)01025-4 [DOI] [PubMed] [Google Scholar]
- Flegr J., Lindová J., Kodym P. (2008a). Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology 135, 427–431 10.1017/S0031182007004064 [DOI] [PubMed] [Google Scholar]
- Flegr J., Lindová J., Pivoñková V., Havlícek J. (2008b). Brief Communication: latent toxoplasmosis and salivary testosterone concentration—important confounding factors in second to fourth digit ratio studies. Am. J. Phys. Anthropol. 137, 479–484 10.1002/ajpa.20888 [DOI] [PubMed] [Google Scholar]
- Fredriksson G., Buxton D., Uggla A., Kindahl H., Edqvist L. E. (1990). The effect of Toxoplasma gondii infection in unvaccinated and iscom-vaccinated pregnant ewes as monitored by plasma levels of 15-ketodihydroprostaglandin F2 alpha, progesterone, and oestrone sulphate. J. Vet. Med. 37, 113–122 10.1111/j.1439-0442.1990.tb00882.x [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Shoback D. M., Greenspan F. S. (2011). Basic and Clinical Endocrinology. New York, NY: McGraw-Hill Medical [Google Scholar]
- Gay-Andrieu F., Cozon G. J. N., Ferrandiz J., Peyron F. (2002). Progesterone fails to modulate Toxoplasma gondii replication in the RAW 2647 murine macrophage cell line. Parasite Immunol. 24, 173–178 10.1046/j.1365-3024.2002.00451.x [DOI] [PubMed] [Google Scholar]
- Gets J., Monroy F. P. (2005). Effects of alpha- and beta-adrenergic agonists on Toxoplasma gondii infection in murine macrophages. J. Parasitol. 91, 193–195 10.1645/GE-3242RN [DOI] [PubMed] [Google Scholar]
- Hodková H., Kolbeková P., Skallová A., Lindová J., Flegr J. (2007). Higher perceived dominance in Toxoplasma infected men–a new evidence for role of increased level of testosterone in toxoplasmosisassociated changes in human behavior. Neuro Endocrinol. Lett. 28, 110–114 [PubMed] [Google Scholar]
- Hulínská D., Sykora J., Zastera M. (1990). Efect of cortisone on Toxoplasma gondii infection studied by electron microscopy. Folia Parasitol. 37, 207–212 [PubMed] [Google Scholar]
- Jones L. A., Jean-Paul A., Henriquez F. L., Lyons R. E., Nickdel M. B., Carter K. C., et al. (2008). Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunol. Lett. 125, 59–69 10.1111/j.1365-2567.2008.02820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaňková S., Kodym P., Flegr J. (2011). Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp. Parasitol. 128, 181–183 10.1016/j.exppara.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Kittas C., Henry L. (1979). Effect of sex hormones on the immune system of guinea-pigs and on the development of toxoplasmic lesions in non-lymphoid organs. Clin. Exp. Immunol. 36, 16–23 [PMC free article] [PubMed] [Google Scholar]
- Kittas C., Henry L. (1980). Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br. J. Exp. Pathol. 61, 590–600 [PMC free article] [PubMed] [Google Scholar]
- Kurth F., Luders E., Sicotte N. L., Gaser C., Giesser B. S., Swerdloff R. S., et al. (2014). Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 6, 454–460 10.1016/j.nicl.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O., Nguyen T. A., Pharke C., Suzuki Y. (2001). Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J. Parasitol. 87, 1491–1493 10.1645/0022-3395(2001)087[1491:IOGASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lim A., Kumar V., Hari Dass S. A., Vyas A. (2013). Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol. Ecol. 22, 102–110 10.1111/mec.12042 [DOI] [PubMed] [Google Scholar]
- Matalka K. Z. (2003). Prolactin enhances production of interferon-gamma, interleukin-12, and interleukin-10, but not of tumor necrosis factor-alpha, in a stimulus-specific manner. Cytokine 21, 187–194 10.1016/S1043-4666(02)00496-9 [DOI] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R. M., Vyas A. (2013). Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis. Models Mech. 6, 516–520 10.1242/dmm.009928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J. G., Remington J. S. (2008). Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47, 554–566 10.1086/590149 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Fowler P. A. (2014). Development of the human fetal testis. Ann. Endocrinol. 75, 48–53 10.1016/j.ando.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Oktenli C., Doganci L., Ozgurtas T., Araz R. E., Tanyuksel M., Musabak U., et al. (2004). Transient hypogonadotrophic hypogonadism in males with acute toxoplasmosis: suppressive effect of interleukin-1 beta on the secretion of GnRH. Hum. Reprod. 19, 859–866 10.1093/humrep/deh161 [DOI] [PubMed] [Google Scholar]
- Ortiz-Alegría L. B., Caballero-Ortega H., Cañedo-Solares I., Rico-Torres C. P., Sahagún-Ruiz A., Medina-Escutia M. E., et al. (2010). Congenital toxoplasmosis: candidate host immune genes relevant for vertical transmission and pathogenesis. Genes Immun. 11, 363–373 10.1038/gene.2010.21 [DOI] [PubMed] [Google Scholar]
- Pfaff A. W., Mousli M., Sénégas A., Marcellin L., Takikawa O., Klein J. P., et al. (2008). Impact of foetus and mother on IFN-gamma-induced indoleamine 2,3-dioxygenase and inducible nitric oxide synthase expression in murine placenta following Toxoplasma gondii infection. Int. J. Parasitol. 38, 249–258 10.1016/j.ijpara.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Prigione I., Chiesa S., Taverna P., Ceccarelli R., Frulio R., Morandi F., et al. (2006). T cell mediated immune responses to Toxoplasma gondii in pregnant women with primary toxoplasmosis. Microbes Infect. 8, 552–560 10.1016/j.micinf.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Pung O. J., Luster M. I. (1986). Toxoplasma gondii: decreased resistance to infection in mice due to estrogen. Exp. Parasitol. 61, 48–56 10.1016/0014-4894(86)90134-7 [DOI] [PubMed] [Google Scholar]
- Puvanesuaran V. R., Nowroji K., Sreenivasan S., Noordin R., Balakrishnan V. (2012). Use of prednisolone to aid propagation of Toxoplasma gondii in mice. Eur. Rev. Med. Pharmacol. Sci. 16, 1028–1032 [PubMed] [Google Scholar]
- Roberts C. W., Walker W., Alexander J. (2001). Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 14, 476–488 10.1128/CMR.14.3.476-488.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senegas A., Villard O., Neuville A., Marcellin L., Pfaff A. W., Steinmetz T., et al. (2009). Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int. J. Parasitol. 39, 481–487 10.1016/j.ijpara.2008.08.009 [DOI] [PubMed] [Google Scholar]
- Shirbazou S., Abasian L., Talebi M. F. (2011). Effects of Toxoplasma gondii infection on plasma testosterone and cortisol level and stress index on patients referred to Sina hospital, Tehran. Jundishapur J. Microbiol. 4, 167–173 [Google Scholar]
- Speroff L., Glass R. H., Kase N. G. (1999). Clinical Ginecologic Endocrinology and Infertility, 6th Edn. Philadelphia, PA: Lippincott Willians&Wilkins [Google Scholar]
- Stahl W., Dias J. A., Turek G. (1985). Hypothalamic-adenohypophyseal origin of reproductive failure in mice following chronic infection with Toxoplasma gondii. Proc. Soc. Exp. Biol. Med. 178, 246–249 10.3181/00379727-178-42006 [DOI] [PubMed] [Google Scholar]
- Stahl W., Kaneda Y. (1998a). Impaired thyroid function in murine toxoplasmosis. Parasitology 117(Pt 3), 217–222 10.1017/S003118209800300X [DOI] [PubMed] [Google Scholar]
- Stahl W., Kaneda Y. (1998b). Aetiology of thyroidal dysfunction in murine toxoplasmosis. Parasitology 117(Pt 3), 223–227 10.1017/S0031182098003035 [DOI] [PubMed] [Google Scholar]
- Stahl W., Kaneda Y., Noguchi T. (1994). Reproductive failure in mice chronically infected with Toxoplasma gondii. Parasitol. Res. 80, 22–28 10.1007/BF00932619 [DOI] [PubMed] [Google Scholar]