Abstract
Purpose.
Ciliary neurotrophic factor (CNTF) protects rod photoreceptors from retinal degenerative disease in multiple nonhuman models. Thus far, CNTF has failed to demonstrate rod protection in trials for human retinitis pigmentosa. Recently, CNTF was found to improve cone photoreceptor function in a canine CNGB3 achromatopsia model. This study explores whether this finding translates to humans with CNGB3 achromatopsia.
Methods.
A five-subject, open-label Phase I/II study was initiated by implanting intraocular microcapsules releasing CNTF (nominally 20 ng/d) into one eye each of CNGB3 achromat participants. Fellow eyes served as untreated controls. Subjects were followed for 1 year.
Results.
Pupil constriction in treated eyes gave evidence of intraocular CNTF release. Additionally, scotopic ERG responses were reduced, and dark-adapted psychophysical absolute thresholds were increased, attributable to diminished rod or rod pathway activity. Optical coherence tomography revealed that the cone-rich fovea underwent structural changes as the foveal hyporeflective zone (HRZ) became diminished in CNTF-treated eyes. No objectively measurable enhancement of cone function was found by assessments of visual acuity, mesopic increment sensitivity threshold, or the photopic ERG. Careful measurements of color hue discrimination showed no change. Nonetheless, subjects reported beneficial changes of visual function in the treated eyes, including reduced light sensitivity and aversion to bright light, which may trace to decreased effective ambient light from the pupillary constriction; further they noted slowed adaptation to darkness, consistent with CNTF action on rod photoreceptors.
Conclusions.
Ciliary neurotrophic factor did not measurably enhance cone function, which reveals a species difference between human and canine CNGB3 cones in response to CNTF. (ClinicalTrials.gov number, NCT01648452.)
Keywords: CNTF, human clinical trial, CNGB3, achromatopsia, cone photoreceptor, rod photoreceptor, ECT implant, CNTF-releasing implant
CNTF protects rod photoreceptors in animal retinal degeneration models. A recent study showed CNTF partially restores cone function in dogs with CNGB3 mutations. Since CNGB3 mutations account for 50% of human achromatopsia, we explored potential benefits of CNTF for CNGB3-achromats.
Introduction
Ciliary neurotrophic factor (CNTF) was discovered as a neurotrophic factor that supported ciliary neurons and hence was named as such.1,2 Shortly thereafter, LaVail and colleagues3 showed that CNTF promotes the rod photoreceptor survival, and as reviewed by Wen and colleagues,4 the CNTF neuroprotective effects on rods was subsequently demonstrated in many animal models across several species. This raised hope that CNTF might act to protect rods in human retinal degenerations. The concurrent development of encapsulated cell technology (ECT) in the form of intraocular implantable microcapsules, that allowed delivery of physiologic levels of CNTF to the vitreous over extended periods of time, made it feasible to mount human CNTF clinical trials.5–8
The first CNTF human phase I clinical trial was conducted in 10 patients with advanced retinitis pigmentosa (RP).9 The ECT devices were well tolerated, and no significant adverse effects were attributed to CNTF. Several participants had an apparent increase in visual acuity during the trial, and three maintained greater than 10-letter improvement attributed to enhanced cone function from CNTF treatment.9 In subsequent phase II CNTF trials for RP, Talcott and colleagues10 directly monitored macular cones using adaptive optics scanning laser ophthalmoscopy and observed that cone numbers remained stable over intervals spanning 12 to 35 months in eyes that received CNTF implants, whereas the sham-treated fellow eyes had a 9% to 24% decrease in cone numbers. Additionally, a phase II clinical trial of CNTF implants for patients with age-related macular degeneration found that visual acuity decline could be prevented in a subset of participants by CNTF treatment.11 These clinical data indicated that CNTF has neurotrophic effects on human cone photoreceptors. Consistent with the human outcomes, CNTF was recently shown to stimulate regeneration of cone outer segments in a rat model of secondary cone degeneration.12
A recent study explored CNTF effects on cone photoreceptors involving dogs with CNGB3-related cone dysfunction.13 The CNGB3 gene encodes the β subunit of the cyclic GMP-gated channels on cone outer segments, the function of which is vital for cone activation by light. Genetic ablation of CNGB3 expression leads to selective loss of cone function but with some preservation of the cones themselves.14 Human CNGB3 mutations cause the condition termed achromatopsia, in which lack of cone function results in total loss of color discrimination and also greatly reduces visual acuity. Mutations of CNGB3 are responsible for approximately 50% of all patients with autosomal recessive achromatopsia.15,16 In the treatment study of CNGB3 dog cone dysfunction, Komaromy and colleagues13 found that concurrent intravitreal application of CNTF allowed successful rescue of cone function in older dogs (aged 14–42 months) by CNGB3 cDNA gene transfer, whereas without CNTF, the gene therapy rescue was limited to younger dogs (aged <0.5 years). Further and quite surprisingly, application of CNTF alone, without gene transfer, effected a transient restoration of cone function. Taken in context with possible neurotrophic effects of CNTF on human cones observed in clinical trials, the restoration of cone function in CNGB3 dogs raised the important question of whether CNTF treatment could augment or partially restore cone function for human CNGB3-achromatopsia. To address this, we initiated an open-label phase I/II trial to evaluate the safety and possible benefit of CNTF on human cone function in five subjects with CNGB3-achromatopsia.
Methods
Protocol Design
This prospective nonrandomized phase I/II study was designed and conducted at the National Eye Institute (NEI) and carried out at the National Institutes of Health Clinical Center in Bethesda, Maryland. Inclusion criteria were: participants aged >18 years; molecular results demonstrating two CNGB3 gene mutations and no CNGA3 disease-associated sequence variations; and best-corrected visual acuity of 20/200 or better (Snellen equivalent; measured on Early Treatment Diabetic Retinopathy Study [ETDRS] charts) in the worse-seeing eye.
Five participants had CNTF-secreting, encapsulated cell implants (NT-501, Neurotech, Inc., Cumberland, RI, USA) surgically inserted into one eye. We did not employ sham manipulation of the fellow untreated eyes, as previous studies noted that CNTF caused pupillary constriction which obviates true masking.17 Structural and functional measures of the retina were made at two baseline visits prior to implantation and afterwards at five follow-up visits of 1 week and 1, 3, 6, and 12 months. Patients were followed for 1 year, and all patients completed their one year scheduled visits.
The study adhered to the tenets of the Declarations of Helsinki. The protocol was reviewed and approved by the NIH Combined Neuroscience Institutional Review Board and by the Recombinant DNA Advisory Committee. Additional oversight was provided by an independent Data and Safety Monitoring Committee. The study was registered under clinicaltrials.gov NCT01648452. Informed consent was obtained prior to study enrollment.
Ophthalmic Assessments
Slit-lamp biomicroscopy with documentation of intraocular pressure, surgical site status and anterior chamber cell activity, lens photography, vitreous biomicroscopy, and fundus exams were performed at each visit.
Vision Function Assessments
Visual function assessments were carried out by three technicians trained in and experienced with clinical research measurements; ETDRS visual acuity was measured under standard conditions. Macular field sensitivity was tested by static perimetry using a 10-2 Humphrey Visual Field Analyzer (Humphrey Instruments, Inc., San Leandro, CA, USA). Dark-adapted absolute psychophysical thresholds were measured using a Goldmann-Weekers dark adaptometer (Haag-Streit AG, Koeniz, Switzerland) after participants had been in complete darkness for 30 minutes. Thresholds were measured to an 11° achromatic patch located centrally or 30° nasally.
Color hue discrimination was tested by the Nagel anomaloscope, American Optical Hardy Rand Rittler (AOHRR) color plates, and a low vision version of the Cambridge Color Test (LvCCT)18 implemented on a ViSaGe System (Cambridge Research Systems Ltd., Rochester, UK) using custom-written software. Hardy Rand Rittler testing followed the guidelines accompanying the test and administered under a Macbeth Lamp at 300 lux.
Electroretinogram
Full-field ERGs were recorded according to International Society of Clinical Electrophysiology of Vision Standards (ISCEV).19 The Naka-Rushton function was fitted to scotopic ERG amplitudes for retinal illuminance of −1.0 to 1.0 log scot td-s to derive the response maximum Vmax and the log retinal illuminance that produces the half-maximum response, K.20 A photoreceptoral response (P3) model was fitted to the leading edge of the ERG A-wave recorded to the brightest flash stimulus used, a rod saturating flash of 3.6 log scot td-s (30 scot cd/m2).21 Derived parameters were: the sensitivity parameter S [(scot td-s)−1 s−2] that scales retinal illuminance, the maximal photoreceptoral response RmaxP3 (μV) and the delay in response onset td (seconds). An estimate of the postreceptoral component of the ERG (P2) was obtained by subtracting the P3 model from ERG responses.22
Optical Coherence Tomography
Macula retinal morphology was imaged using spectral-domain optical coherence tomography (SD-OCT, Cirrus; Carl Zeiss Meditec, Inc., Dublin, CA, USA) with a segmentation algorithm to extract retinal thickness.
CNTF Implant
The ciliary neurotrophic factor–secreting NT-501 device (Neurotech, Inc.) is 1 mm in diameter and 6 mm long and constructed of a semipermeable polymer outer membrane and medical-grade sealant with a titanium anchor at one end to allow suturing to the sclera. Each implant contains an internal polyethylene terephthalate yarn scaffold that supports human cells. These cells (designated NTC-201) were derived originally from a human RPE cell line and have been genetically engineered to continuously produce human CNTF. The devices were loaded with 203,000 cells and secreted CNTF at 20 ng/d in vitro at 2 weeks post manufacture. Similar devices were used in four previous human trials,9–11,23 a dog and a rabbit trial.5,24 The CNTF devices in the present study used a polysulfone membrane with pore size that provided a greater molecular weight cutoff, and thus, a greater release rate than used in previous clinical studies. However, the output level was within the safety dose range previously tested in toxicology studies.
Surgical Procedure
Surgical intraocular implantation required 30 to 60 minutes to perform under intravenous sedation and local anesthesia. Implants were inserted into the vitreous cavity via a 2.0-mm sclerotomy made in the pars plana region and were anchored to sclera with a single 9-0 Prolene suture, with appropriate antibiotic coverage.
Evaluation of the NT-501 Implant After Removal
Twenty days after device implantation, participant 005 developed unexpected ocular inflammation, and the implant was removed and evaluated by functional (CNTF secretion levels) and morphological (histology) analysis. Immediately upon removal, the device was placed into endo–serum-free media conditioned medium (GIBCO BRL, Gaithersburg, MD, USA) at 37°C, 5% CO2, and 95% humidity for 24 hours, and the rate of CNTF secretion was determined using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). Capsules were fixed in 4% paraformaldehyde for 1 hour and embedded in methacrylate. Consecutive longitudinal 4 μm–thick sections were cut through the capsule, stained with hematoxylin and eosin (H&E). The explanted device contained an appropriate high-density of viable cells throughout.
Statistics
Data at each follow-up visit were compared to the mean of the two baseline visits. The level of significance (P < 0.05) was adjusted using Holm-Bonferroni sequential correction for multiple comparisons.25 Briefly, the P value for each comparison is adjusted according to: p[Bonferroni], i|C = (C − i + 1) × P value, where C = number of comparisons and i = the rank of P value for each visit in ascending order.
Results
Study Subjects and Safety of the Implants
The study enrolled five patients with confirmed biallelic CNGB3 gene mutations and with no known CNGA3 mutations (Table). Three participants were homozygous for the common c.1148delC mutation and two participants were compound heterozygotes. The c.607C>T; p.R203* mutation is located in the cyclic nucleotide-gated cation channel beta-3 and results in premature termination of protein translation.15,16 The c.467C>T; p.S156F mutation is located in the amino-terminus near an amino-terminal Ca2+/calmodulin binding site.16,26 It has been observed in trans with clear pathogenic mutations in a number of individuals with achromatopsia16 (Kohl S, unpublished observations, 2014). Three participants were males and two were females, all Caucasians, aged 24 to 54 years. Their baseline Snellen acuities were between 20/100 and 20/200 and were quite symmetrical between the two eyes of each individual.
Table.
Demographic Information
|
Participant ID |
Age* |
Sex |
CNGB3
Allele 1 |
CNGB3
Allele2 |
CNGA3 |
| 001 | 54 | Male | c.1148delC | c.1148delC | Negative |
| 002 | 36 | Female | c.607C>T | c.1148delC | Negative |
| 003 | 29 | Male | c.1148delC | c.1148delC | Negative |
| 004 | 35 | Male | c.1148delC | c.1148delC | Negative |
| 005 | 24 | Female | c.1148delC | c.467C>T | Negative |
Age at study entry.
The implant device was removed from participant 005 after 20 days due to intense ocular inflammation which resolved rapidly. Visual acuity returned to preimplantation values (see Supplementary Table S1 for adverse events). Test results from participant 005 were excluded from subsequent monitoring and from outcomes analyses except where indicated. The explanted device was tested, cultured, and found to be free from inflammatory cells, pathogen, mycoplasma, and endotoxin. There was no evidence of breakage or defect.
The explanted device was evaluated for CNTF output and found to release 33 ng of CNTF per day, which was expected based upon in vitro testing with this device. This output level was within the safety dose range previously tested in toxicology studies. Participant 002 experienced minor inflammation 24 days after implantation which resolved with systemic and topical antibiotics and corticosteroids (Supplementary Table S1).
We detected no CNTF, nor antibodies specific to CNTF or NTC-201-6A cells in serum, and no significant change in clinical chemistry or hematological values occurred for any participant.
Pupils, Visual Acuity, and Macular Visual Field Sensitivity
Pupil constriction was observed in all CNTF implanted eyes by 3 months except participant 005, whose device was explanted at 20 days. Participants 003 and 004 showed pupil constriction by 1 month (see Supplementary Table S2). Pupil constriction persisted in the four remaining participants up to 12 months, the end of the study period.
Visual acuity remained within three letters of baseline for each eye during the study in both the treated and untreated eyes of all participants (Supplementary Fig. S1).
The difference in mean macular sensitivity (Humphrey HVF 10-2) between treated and untreated eyes was not significantly different at 3 months (P = 0.45) or 6 months (P = 0.5) compared with baseline.
Color Discrimination
Color vision was evaluated by three methods: Nagel Anomaloscope, a modified LvCCT,18 and American Optical Hardy Rand Rittler (AOHRR) color test plates.27 No participant demonstrated any change or improvement of chromatic discrimination. On the anomaloscope, as expected for achromatic subjects, all participants accepted a chromatic match across a wide range of red-green color mixtures and required increased brightness as the red-green ratio decreased (Fig. 1A). The slopes of these matching functions did not change in either treated or control eyes over the course of the study. With the LvCCT test, color discrimination thresholds were measured along eight axes, separated by 45° in CIE 1976 L*u*v* space. As expected for achromats, the participants were unable to discriminate chromatic differences even at the maximum saturation along any color axis in either eye (Fig. 1B). Inconsistent responses were obtained to all color plates with AOHRR testing, except that all participants could consistently identify the triangle on plate 21 at baseline and across all visits.
Figure 1.
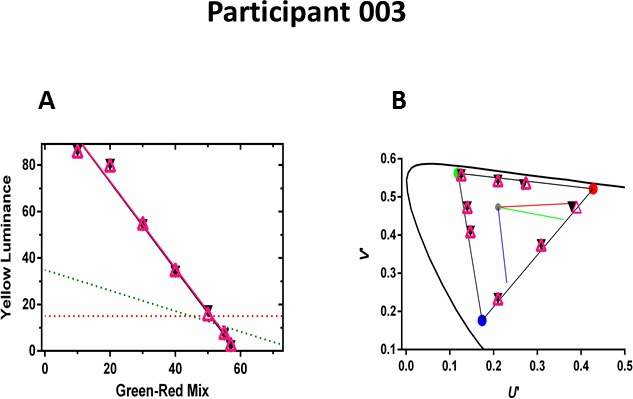
Color discrimination of participant 003 at 12 months. (A) Anomaloscope matches from the treated (open purple triangles) and untreated eyes (black circles) along with linear regressions over the matching range. Red and green dashed lines show expected matches for protans and deutans, respectively. (B) Low-vision version of the Cambridge Color Test: color discrimination thresholds from the treated (open purple triangles) and untreated eyes (black circles). All thresholds are at or adjacent to the maximum gamut (triangle) that could be produced by the monitor. Curved line shows CIE 1976 L*u*v* space. Red, green, and blue lines indicate protan, deutan, and tritan confusion lines, respectively.
ERG
Reduced amplitudes of rod-mediated scotopic ERG responses compared with baseline were found for all CNTF treated eyes (Fig. 2A) as has been observed previously in human and animal CNTF studies.4,13,28–30 An average of 43% reduction was found with dimmer scotopic stimuli across postimplant times of 3, 6, and 12 months for the aggregate four combined participants (Fig. 2B). Naka-Rushton analysis of ERG B-waves indicated a 40% reduction of Vmax, the maximal rod response, and a 0.2 log unit elevation of retinal sensitivity (Supplementary Fig. S2). These results indicate changes in retinal function associated with sustained intravitreal CNTF release. Further analysis of the ERG response into photoreceptor (P3) and postreceptoral (P2) components (see Methods section) found that rod phototransduction parameters (RmaxP3 and S) from treated eyes were not different from untreated eyes (Supplementary Fig. S3). The onset of the P2 responses was delayed in the CNTF-treated eyes compared with contralateral untreated eyes for each of the four subjects at postimplant times of 1 to 12 months (Supplementary Fig. S4). However, neither maximal P2 amplitude nor the rate of rise of the saturated P2 response (3.6 log scot td-s ERG flash) was altered in the treated eyes. Based on models of the ERG response, these results suggest that rod photoreceptor function is not affected directly by sustained intravitreal release of CNTF. The isolated delay in the onset of the P2 response suggests a delay in signal transmission between photoreceptors and bipolar cells.
Figure 2.
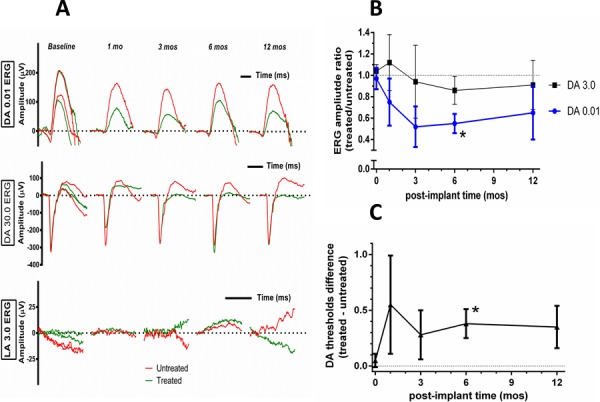
Retinal function of participant 002 following CNTF. (A) Rod- and cone-mediated ERGs from participant 002. Dark-adapted dim flash rod responses (0.01 ERG, upper panel), from the CNTF-treated eye (green) are consistently smaller at 1, 3, 6, and 12 months post implantation compared with the untreated eye (red). The decrease in the CNTF treated eye is less pronounced for the dark-adapted bright flash response (30.0 ERG, middle panel). No cone-mediated responses were obtained to the light-adapted standard flash 3.0 ERG, (lower panel) at any visit. Two baseline evaluations were performed. (B) Mean ERG amplitude ratios (treated/nontreated) at 1 to 12 months post surgery normalized to presurgery baseline (0 months) for the ISCEV dark-adapted dim flash (DA 0.01) and maximal (DA 3.0) responses. Asterisk indicates that the reduction in the ratio for the dim flash amplitude was borderline significant (p[Bonferroni] < 0.075) at 6 months post surgery. (C) Logarithmic difference in absolute psychophysical dark-adapted thresholds were elevated between 1 to 12 months post surgery; asterisk indicates borderline significance at 6 months (p[Bonferroni] < 0.055).
No improvement in light adapted photopic ERG responses was detected in CNTF-treated eyes. The cone ERG responses were non-detectable at baseline and remained nondetectable after CNTF treatment. In no case was there evidence of cone driven activity before or after implant (Fig. 2A, bottom panel).
Dark-Adapted Threshold
Dark-adapted psychophysical absolute thresholds were increased in all four participants with CNTF implants. Between 3 and 12 months post surgery, thresholds were elevated by approximately 0.3 log (50% increase) compared with fellow untreated eyes (Fig. 2C), consistent with reduced scotopic ERG responses and with self-report of altered night vision (see below).
Retinal Morphology by OCT
All five participants had bilateral foveal hyporeflective zones (HRZ) at baseline with absent inner segment ellipsoid (ISe), as previously observed in achromats.31–35 By 3 months post device implantation, the HRZ was altered in the treated eyes, as the lucent zone appeared to be filled or collapsed or otherwise no longer observable, and it remained so to the 12-month exam (Figs. 3C–E). These changes were not observed in the untreated eyes nor in participant 005, implicating CNTF as responsible for the changes in retinal foveal morphology. As has been reported previously, OCT scans indicated that the retina outside the central foveal zone became progressively thicker after CNTF exposure and stabilized by 6 and 12 months after implantation (see Supplementary Table S3).11
Figure 3.
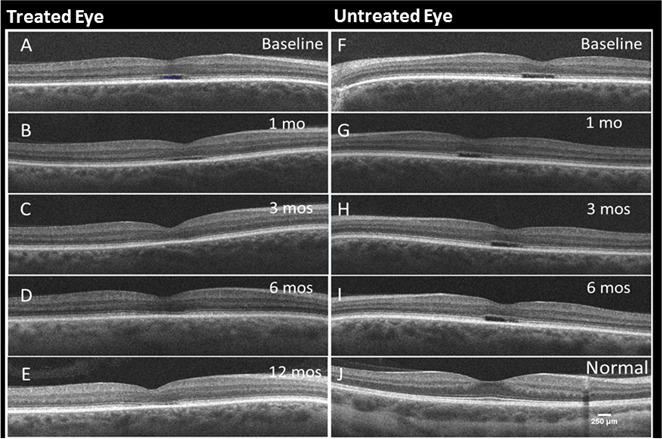
Serial foveal SD-OCT scans from participant 001 over the duration of the trial (A–E). As previously described for achromats (24–28), all eyes at baseline and untreated have a foveal HRZ deep in the retina; this is limited exclusively to the center of the fovea, extends approximately 400 μm and lies just above the bright RPE line. In untreated eyes, this HRZ has an abrupt squared-off edge at each end. The HRZ architecture changes and is no longer apparent in CNTF treated eyes. Treated eye at baseline (A), 1 month (B), 3 months (C), 6 months (D), and 12 months (E), respectively. (F–I) Untreated eye at baseline, months 1, 3, and 6, respectively. (J) Control scan from a normal eye.
Subjective Reporting of Visual Perception
Open-ended questions were asked at every examination regarding changes to visual perception and quality of life. All participants with CNTF implants (except participant 005) reported perceived changes in visual function by the 1-month examination. These subjective observations were difficult to quantify and must be handled cautiously, but they might indicate possible, mild, perceptual changes for the CNTF eye. Implanted eyes were reported as less sensitive to light and to adapt to transitions from dark to light more quickly than the fellow eyes. Patients also reported that the treated eyes tolerated brighter rooms better. They opened their eyes wider and depended less on sunglasses and squinted less in brighter light. Some reported discerning “details and textures” better with the implanted eye, and the vision was “more clear” and provided better detail at distance, such as seeing “leaves of a tree” or “shingles on a roof.” The treated eye “became the more dominant eye during the day.” The benefits in daylight were offset by more difficult perception in dim light. All participants also complained of slowed adaptation to darkness by the first postoperative week and throughout the study.
Discussion
There is ample evidence that ECT intraocular implants delivered CNTF to the study eyes: the pupils were constricted in treated eyes; rod–scotopic ERG amplitudes were diminished; psychophysical absolute threshold of dark-adapted perception was increased; and on OCT, the foveal hyporeflective zone was diminished, and the perimacular retina was thickened. Furthermore, the device explanted from participant 005 gave an output of 33 ng/d, confirming CNTF release after implantation. However, we found no objective evidence that CNTF enhanced the cone activity in these individuals with CNGB3 mutations. Testing twice before implanting the CNTF-releasing devices indicated no cone mediated vision at baseline, and no change or improvement was subsequently measured on four examinations over 12 months post implantation for any subject. Visual acuity was unchanged; color discrimination was not observed by three independent metrics; and cone-driven photopic ERG responses remained below the limit of detection.
However, within 1 month post implantation, all subjects reported that they preferred the vision of the implanted eye. Statements, always pertaining to the treated eye, included experiencing less photoaversion in daylight environments and definite but difficult to quantify claims of actual benefit to perception. These subjective claims could reflect real but subtle improvements too small to be detectable by current technologies. The diminished photophobia likely results from reduced rod sensitivity, as CNTF is known to downregulate the rod phototransduction machinery.4,36
This is one of the few human therapy trials to monitor color discrimination as a critical outcome variable. Cones respond to hue differently than rods, and color discrimination is attractive for a trial aimed at modifying and possibly enhancing cone function. Color discrimination proved an important metric in a gene therapy trial for dichromatic new world nonhuman primates.37 Upon receiving the missing L-opsin human color pigment gene delivered by adeno-associated virus (AAV) vector, the red-green color deficient monkeys exhibited enhanced trichromatic hue discrimination and demonstrated behavioral responses to colors previously invisible to them.37 Studying our human CNGB3 achromat participants for new ability to discriminate hues using the modified low vision Cambridge color test18 and with AOHRR color plates19 requires only that after treatment their cone photoreceptors can signal neural activity across even a limited but new color space on psychophysical testing. It would not depend on participants accurately naming colors with which they had no previous experience.
Two participants suffered intraocular inflammation at 3 weeks after ECT devices were implanted, one judged sufficiently severe to warrant surgical removal. This was not anticipated, as similar CNTF-releasing NT501 ECT devices previously had been implanted in nearly 200 participants in one phase I and two phase II trials without manifesting inflammation9–11 (ClinicalTrials.gov number, NCT01408472). In the present study, the CNTF devices used a polysulfone membrane with pore size that provided a greater molecular weight cutoff than previous implants, which might allow release of additional, unknown factors from the sequestered cells. Alternatively, this may be an idiosyncratic allergenic response to the membrane materials at a very low occurrence rate.
To understand why human CNGB3 cones apparently failed to respond to CNTF, two factors should be considered: the mode of delivery and subject age. Cones of the CNGB3 mutation dogs responded to a large excess of CNTF protein given in a bolus by intravitreal injection,13 but the present CNGB3 human study failed to show an effect using continuous CNTF release in physiologic amounts by the implanted devices. Similar implants were subsequently used in three dogs and caused a transient augmentation of cone function (Komaromy AM, unpublished observation, 2013), suggesting that, unlike humans, CNGB3-achromatopsia dogs retain the ability to restore some cone function above baseline. Age is also a potential factor. Gene therapy studies delivering wild-type CNGB3 cDNA to cones in mouse and dog demonstrated clear age-dependence functional restoration, indicating a declining therapeutic window with age.38,39 So perhaps even the youngest individual enrolled in the present trial (aged 25 years) is beyond some required stage of developmental plasticity or damaged beyond repair.
It is also possible that cones in human, dog, and mouse have different molecular requirements for cyclic nucleotide–gated (CNG) subunit trafficking, channel assembly, and activity. Cyclic nucleotide–gated channels are heterotetramers composed of α and β subunits.40,41 Cone CNG channels have two α and two β subunits,42 while rod CNG channels contain three α and one β subunits.43,44 One function of β subunits is to facilitate targeting the channels to particular organelles, such as cilia on olfactory sensory neurons or outer segments of photoreceptors.45–47 Subunit β may be dispensable for function, as the CNGB3 knockout mouse retains approximately 20% of cone ERG function at 1 year,14 whereas function is lost entirely in CNGA3 knockout mice within the first month of age.48 By comparison, we selected our five participants for CNGB3 mutations on both alleles, and none had CNGA3 mutations or variations, but none had evidence of cone photopic ERG responses at baseline.
In dogs with CNGB3 mutations, the α subunit is expressed but not detectable in cone outer segments, and no cone-driven ERG is detectable.39 However, when the β subunit was introduced via an AAV vector, the α subunits arrived at the cone outer segments, implicating a chaperone function for the β subunit.39 The finding that CNTF transiently restores cone-driven ERGs in CNGB3 dogs suggests that α subunit trafficking to cone outer segments is improved and forms functional α homotetramer channels.13
The present study leads to the important conclusion that human cones with CNGB3 mutations respond differently to CNTF than do canine cones. The results of this study and other clinical trials with CNTF are important to learn whether the beneficial effects found for other species also extend to human retinal diseases.
Acknowledgments
The authors thank Jennifer Sarchet, Patrick Lopez, Leanne Reuter, and John Rowan for their assistance and technical expertise; Xinjing Wang and Christian Antolik for molecular screening study participants; and Matthew McMahon and Cesar Perez-Gonzalez for their critical reading of the manuscript. This study was conducted at the NIH under a Cooperative Research and Development Agreement (CRADA) with Neurotech, Inc. We thank Neurotech for providing the CNTF devices.
Supported by the National Institutes of Health Intramural Research Programs of the National Eye Institute and the National Institute on Deafness and Other Communication Disorders. This work also was supported by the National Institutes of Health Intramural Program Funding for Human Protocol 12-EI-0167 and by Contract N01-EY-1-2113.
Disclosure: W.M. Zein, None; B.G. Jeffrey, None; H.E. Wiley, None; A.E. Turriff, None; S.J. Tumminia, None; W. Tao, Neurotech USA, Inc. (E, C); R.A. Bush, None; D. Marangoni, None; R. Wen, None; L.L. Wei, None; P.A. Sieving, None
References
- 1. Adler R, Landa KB, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979; 204: 1434–1436 [DOI] [PubMed] [Google Scholar]
- 2. Varon S, Manthorpe M, Adler R. Cholinergic neuronotrophic factors: I. Survival, neurite outgrowth and choline acetyltransferase activity in monolayer cultures from chick embryo ciliary ganglia. Brain Res. 1979; 173: 29–45 [DOI] [PubMed] [Google Scholar]
- 3. LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992; 89: 11249–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen R, Tao W, Li Y, Sieving PA. CNTF. and retina. Prog Retin Eye Res. 2012; 31: 136–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002; 43: 3292–3298 [PubMed] [Google Scholar]
- 6. Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006; 6: 717–726 [DOI] [PubMed] [Google Scholar]
- 7. Tao W, Wen R, Laties AM, Aguirre GD. Cell-based delivery systems: development of encapsulated cell technology for ophthalmic applications. In: Jaffe JJAP, Pearson PA. eds Intraocular Drug Delivery. New York, NY: Taylor & Francis; 2006: 11–128 [Google Scholar]
- 8. Tao W, Wen R. Application of encapsulated cell technology for retinal degenerative diseases. In: Tombran-Tink J, Barnstable C. eds Retinal Degenerations: Biology, Diagnostics, and Therapeutics. Totowa, NJ: Humana Press; 2007: 401–413 [Google Scholar]
- 9. Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006; 103: 3896–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talcott KE, Ratnam K, Sundquist SM, et al. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2011; 52: 2219–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011; 108: 6241–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Tao W, Luo L, et al. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Komaromy AM, Rowlan JS, Corr ATP, et al. Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Ther. 2013; 21: 1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Morris L, Fliesler SJ, Sherry DM, Ding X-Q. Early-onset, slow progression of cone photoreceptor dysfunction and degeneration in CNG channel subunit CNGB3 deficiency. Invest Ophthalmol Vis Sci. 2011; 52: 3557–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohl S, Baumann B, Broghammer M, et al. Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet. 2000; 9: 2107–2116 [DOI] [PubMed] [Google Scholar]
- 16. Kohl S, Varsanyi B, Antunes GA, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005; 13: 302–308 [DOI] [PubMed] [Google Scholar]
- 17. Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 2013; 156: 283–292.e281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simunovic MP, Votruba M, Regan BC, Mollon JD. Colour discrimination ellipses in patients with dominant optic atrophy. Vision Res. 1998; 38: 3413–3419 [DOI] [PubMed] [Google Scholar]
- 19. Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach MISCEV. Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009; 118: 69–77 [DOI] [PubMed] [Google Scholar]
- 20. Fulton AB, Rushton WA. The human rod ERG: correlation with psychophysical responses in light and dark adaptation. Vision Res. 1978; 18: 793–800 [DOI] [PubMed] [Google Scholar]
- 21. Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci. 1995; 36: 1603–1614 [PubMed] [Google Scholar]
- 22. Hood DC, Birch DG. Beta wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am A. 1996; 13: 623–633 [DOI] [PubMed] [Google Scholar]
- 23. Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2012; 53: 7484–7491 [DOI] [PubMed] [Google Scholar]
- 24. Thanos CG, Bell WJ, O'Rourke P, et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004; 10: 1617–1622 [DOI] [PubMed] [Google Scholar]
- 25. Abdi H. Holm's sequential Bonferroni procedure. In: Salkind N. ed Encyclopedia of Research Design. Thousand Oaks, CA: SAGE Publications, Inc.; 2010: 574–578 [Google Scholar]
- 26. Peng C, Rich ED, Thor CA, Varnum MD. Functionally important calmodulin-binding sites in both NH2- and COOH-terminal regions of the cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit. J Biol Chem. 2003; 278: 24617–24623 [DOI] [PubMed] [Google Scholar]
- 27. Hardy LH, Rand G, Rittler MC. The H-R-R polychromatic plates. I. A. test for the detection, classification, and estimation of the degree of defective color vision. Arch Ophthalmol. 1954; 51: 216–228 [PubMed] [Google Scholar]
- 28. Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001; 4: 461–472 [DOI] [PubMed] [Google Scholar]
- 29. Bush RA, Lei B, Tao W, et al. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci. 2004; 45: 2420–2430 [DOI] [PubMed] [Google Scholar]
- 30. Rhee KD, Ruiz A, Duncan JL, et al. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2007; 48: 1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thiadens AA, Roosing S, Collin RW, et al. Comprehensive analysis of the achromatopsia genes CNGA3 and CNGB3 in progressive cone dystrophy. Ophthalmology. 2010; 117: 825–830 e821 [DOI] [PubMed] [Google Scholar]
- 32. Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011; 118: 882–887 [DOI] [PubMed] [Google Scholar]
- 33. Genead MA, Fishman GA, Rha J, et al. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci. 2011; 52: 7298–7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Proudlock F, Gottlob I. Foveal development and nystagmus. Ann N Y Acad Sci. 2011; 1233: 292–297 [DOI] [PubMed] [Google Scholar]
- 35. Sundaram V, Wilde C, Aboshiha J, et al. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology. 2014; 121: 234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wen R, Song Y, Kjellstrom S, et al. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci. 2006; 26: 13523–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mancuso K, Hauswirth WW, Li Q, et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009; 461: 784–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho LS, Xu J, Pearson RA, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011; 20: 3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Komaromy AM, Alexander JJ, Rowlan JS, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010; 19: 2581–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996; 19: 235–263 [DOI] [PubMed] [Google Scholar]
- 41. Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002; 82: 769–824 [DOI] [PubMed] [Google Scholar]
- 42. Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004; 42: 401–410 [DOI] [PubMed] [Google Scholar]
- 43. Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002; 36: 881–889 [DOI] [PubMed] [Google Scholar]
- 44. Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002; 36: 891–896 [DOI] [PubMed] [Google Scholar]
- 45. Huttl S, Michalakis S, Seeliger M, et al. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci. 2005; 25: 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jenkins PM, Hurd TW, Zhang L, et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006; 16: 1211–1216 [DOI] [PubMed] [Google Scholar]
- 47. Michalakis S, Reisert J, Geiger H, et al. Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping. J Biol Chem. 2006; 281: 35156–35166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biel M, Seeliger M, Pfeifer A, et al. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci U S A. 1999; 96: 7553–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]


