Abstract
Purpose.
Interphotoreceptor retinoid-binding protein's (IRBP) role in facilitating the exchange of retinoids between rod and cone photoreceptors, RPE, and Müller cells in the visual cycle remains a mystery. Interphotoreceptor retinoid-binding protein's ability to bind the pericellular matrix of the cone outer segment and Müller cell villi suggests a function in all-trans and 11-cis retinol targeted trafficking in the cone visual cycle. We hypothesize that IRBP facilitates delivery and uptake of all-trans retinol to and release of 11-cis retinol from rat Müller cells (rMC-1).
Methods.
Rat Müller cells were incubated with all-trans retinol and BSA or bovine IRBP (bIRBP). Retinoids in the cell homogenates and conditioned media were analyzed by high performance liquid chromatography (HPLC).
Results.
Cells incubated with 10 μM retinol and BSA had 2100 pmol of all-trans retinol per milligram homogenate protein compared with 3450 pmol when retinol was delivered by bIRBP; these cells also had 450 pmol all-trans retinyl ester per milligram when retinol was delivered by BSA compared with 270 pmol when retinol was delivered by bIRBP. Conditioned media from cells incubated with retinol delivered by BSA did not contain11-cis retinol. However, cells with retinol delivered by bIRBP released 130 pmol/mL of 11-cis retinol into the cell media. Incubation with 5.0 mM deferoxamine (an iron chelator) reduced IRBP-dependent 11-cis retinol retrieval by 60%.
Conclusions.
Promoting Müller cell uptake of all-trans retinol and release of 11-cis retinol is a previously unrecognized function of IRBP that may be critical to cone function and integrity.
Keywords: retina, Müller cell, visual cycle, IRBP, photoreceptors
Our study is the first to show that interphotoreceptor retinoid-binding protein facilitates the accumulation of all-trans-retinol in and the retrieval of 11-cis-retinol from cultured Müller cells. This function is critical to the understanding of the cone visual cycle.
Introduction
Interphotoreceptor retinoid-binding protein (IRBP) has long been thought to have a role in the classical visual cycle involving retinoid exchange between rods and RPE.1–4 However, understanding IRBP's function in this process has proved to be more challenging than initially anticipated.5–8 Recently, experimental evidence has implicated IRBP to have an important function in the cone visual cycle, involving retinoid exchange between cones and Müller glial cells.9–16
The mechanism(s) responsible for IRBP's role(s) in the cone visual cycle are only beginning to be uncovered. We recently reported that IRBP binds to the cone outer segment and Müller cell microvilli pericelluar matrices.17 Such binding could target and/or facilitate delivery/uptake of its retinol ligands. Furthermore, we reported that IRBP has a free radical scavenging activity18 and can protect all-trans and 11-cis retinol from photodegradation (Tsin AT, et al. IOVS 2013;54:ARVO E-Abstract 3765). In the present study, we have collected experimental evidence to show that these properties may contribute to IRBP's ability to enhance delivery of all-trans retinol to and retrieval of 11-cis retinol from Müller cells in culture. The IRBP-dependent retrieval of 11-cis retinol also confirms a possible isomerase activity in rat Müller cells (rMC-1) in culture. Results from inhibition studies using the iron-chelator deferoxamine, suggest that these Müller cells may express an iron-dependent enzyme with an isomerase activity. This is the first report to show IRBP's role in promoting the delivery and retrieval of retinols in Müller cells.
Methods
Our research was approved by the Research & Development and Biosafety Committees of the University of Texas at San Antonio, Buffalo Veterans Affairs, Medical Center and State University of New York at Buffalo. All chemicals were of highest quality and obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless otherwise stated.
Bovine IRBP (bIRBP)
The purification strategy is discussed in section three and illustrated in Figure 1 of Gonzalez-Fernandez et al.18 Based on that study, the following protocol was followed: bovine retinas were collected under dim red light by WL Lawson Co. (Lincoln, NE, USA) and held at −80°C until use. All procedures were carried out at 4°C in the presence of 0.56 mM Dithiothreitol, as well as protease inhibitors. Extracellular proteins were extracted from detached bovine retinas by gentle saline wash. For each purification, 200 retinas were thawed and soaked for 15 minutes in PBS (2 mM potassium phosphate, 7 mM sodium phosphate, 13.4 mM KCl, 136 mM NaCl, pH 7.4) containing 0.5 mM phenyl sulfonyl fluoride, and centrifuged at 2000g for 5 minutes. The retinas were gently resuspended in PBS for 10 minutes with gentle agitation and centrifuged for 10 minutes at 3000g. The pooled washes were then cleared at 20,000g for 30 minutes. A broad-spectrum protease inhibitor cocktail was then added along with 50% concavallin A (ConA) Sepharose 4B slurry (GE Healthcare, Piscataway, NJ, USA) in 50 mM Tris-HCl (150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, and 1 mM MnCl2; pH 7.5). The bIRBP was allowed to bind to the ConA finally eluted in 10% methyl a-D-mannopyranoside with 50 mM Tris-HCl, pH 7.5. The ConA-binding proteins were subjected to a Q-sepharose high performance (QHP) column (GE Healthcare) equilibrated with 20 mM Tris-HCl, pH 7.5, 50 mM NaCl on an Akta Fast Protein Liquid Chromatography system. Bovine IRBP was eluted at 60 mM NaCl with a linear gradient. The protein was concentrated to 5 mL using an Amicon centrifugal filter and subjected to size exclusion using Sephacryl S-300HR in a 2.6 × 100 cm column with running buffer w 20 mM Tris-HCl, pH 7.5, and 100 mM NaCl. The pooled S300 bIRBP fractions were subjected to second QHP column (1.6 × 12 cm) and eluted with a NaCl gradient as before. The bIRBP containing fractions were pooled and the concentration determined by both absorbance spectroscopy and amino acid analysis. Purity was determined by SDS-PAGE analysis. Approximately 0.25 mg of 98% pure bIRBP was obtained per retina.
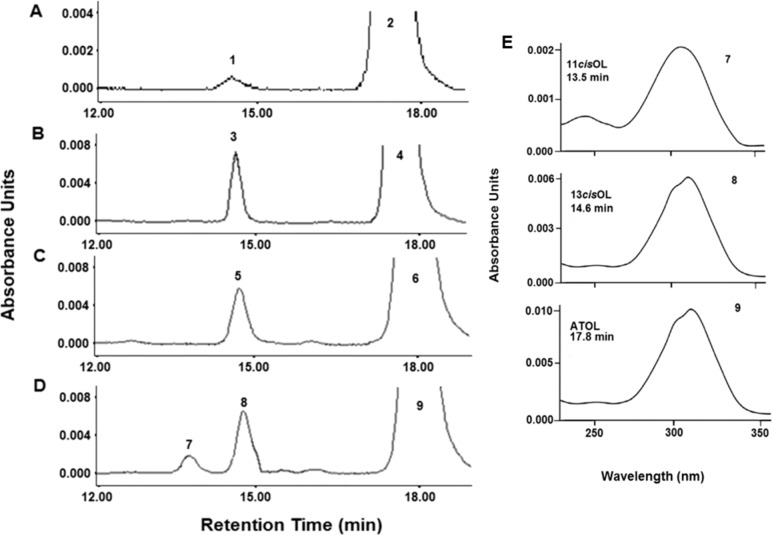
Figure 1.
Comparison of the time-dependent decrease of all-trans retinol in the conditioned media of rMC-1 incubated with bIRBP or BSA at 0, 12, and 24 hours. (A) Amount of all-trans retinol remaining in the conditioned media of cells incubated with 10 μM all-trans retinol and 2 μM BSA (♦) or bIRBP (▪). (B) Representative HPLC chromatograms of retinoid extracts from conditioned media of cells incubated with 10 μM all-trans retinol + 2uM bIRBP; retention time of all-trans retinol = 17.8 minutes (n = 9 for all data points, mean ± SE; seeding density: 250,000 cells/mL).
Cell Culture
SV40 transformed rat Müller cells (rMC-1) were obtained from Vijay Sarthy (Northwestern University, Chicago, IL, USA). Cells were seeded into T75 flasks and maintained at 37°C + 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM) containing 1 g/L glucose and 10% fetal bovine serum (FBS). At confluence, cells were seeded into 6-well plates for experimental assays. Seeding densities were 250,000 cells/mL for Figures 1 through 6 and 50,000 cells/mL for Supplementary Figure S1.
Time-Dependent Change of Retinoids in Müller Cell and in Conditioned Media
To determine optimal incubation time for all-trans retinol delivery, 250,000 cells/mL were seeded into 6-well plates (total volume 1-mL each) for 24 hours at 37°C +5% CO2. Cells were serum starved 6 hours before the addition of all-trans retinol (under dim red light) and incubated at 37°C + 5% CO2. In dim red light, retinoids were dissolved in ethanol, dispersed in BSA or bIRBP, added to serum free culture media and incubated at 4°C for 6 hours before being added to the cells19; final retinoid concentration in the culture media was 10 μM, BSA concentration was 2 μM, and bRIBP concentration was also 2 μM. Cells with respective treatments were incubated for a subsequent total of 24 hours. Cells and conditioned media were harvested at 0, 12, and 24 hours. Retinols and retinyl esters were extracted using 1:2 ethanol/hexane, redissolved in respective mobile phase, and analyzed by high performance liquid chromatography (HPLC). Cells were washed three times and harvested by cell scraper using 1 mL tris-maleate buffer. Cells in 1 mL buffer were homogenized using glass-glass homogenizers (Radnoti glass), 30 μL removed for protein determination, and the remaining sample was used for extraction of retinoids for analysis by HPLC.
Determination of Optimal All-Trans Retinol Concentration and Cell Density
To determine the appropriate concentration of all-trans retinol in the conditioned media, rMC-1 cells were seeded at 250,000 cells/mL and incubated for 24 hours. Growth media was then aspirated and media replaced with 1, 2, 4, or 10 μM all-trans retinol containing 2 μM BSA or 2 μM bIRBP in serum free DMEM prepared, as noted previously. Cells with respective treatments were incubated for 24 hours. At the end of treatment, conditioned media and cells were harvested, retinoids extracted, and then analyzed by HPLC. To determine the optimal cell density, cells were seeded at 50,000, 250,000, and 500,000 cells/mL into 6-well plates (1 mL per well) for 24 hours under conditions mentioned previously. At the end of treatment, conditioned media and cells were harvested, retinoids were extracted, and then analyzed by HPLC (Supplementary Table S1).
Deferoxamine Inhibition of Enzyme Activity
Cells were seeded into 6-well plates at 250,000 cell/ml. After 24 hours, cells were pre-incubated with 0, 2.5, or 5.0 mM deferoxamine mesylate for 24 hours. Media containing deferoxamine was aspirated, the cells washed three times with 1X HBSS, and were then moved to dim red light. Serum free growth media containing 10 μM all-trans retinol + 2 μM bIRBP was then added to each well (total volume, 1 mL per well) and incubated for a final 24 hours in darkness. Plates were then removed under dim red light and media collected from each sample. Retinols were extracted from conditioned media samples and analyzed by HPLC.
HPLC
All experiments involving retinoids were carried out under dim red light. Extracted retinoids were analyzed by 5 μm, 4.6 × 250-mm column (Agilent Technologies, Santa Clara, CA, USA) connected to Waters HPLC equipped with a 2996 photodiode array detector and Empower Software (Waters, Inc., Milford, MA, USA). Peaks were identified by retention time and spectral analysis in comparison with authentic standards. Eleven-cis retinol was derived by NaBH4 reduction of 11-cis retinal (provided by Rosalie Crouch; Medical University South Carolina, Charleston, SC, USA). Acylation were performed by reacting retinol with acyl chloride to produce 11-cis retinyl ester, and 13-cis retinyl ester20 that were then purified by HPLC. Analysis of retinols was carried out by isocratic method using 10% dioxane in hexane at a flow rate of 1 mL/min. Analysis of retinyl esters was carried out by gradient method using 0.2% to 10% dioxane in hexane at a flow rate of 2 mL/min. Quantification of retinoids was carried out by comparison of peak area to retinoid standards.
Protein Determination
Total protein concentration was determined by Bradford method using BioRad Protein Assay Dye Reagent and Helios Spectrophotometer equipped with Vision32 software (ThermoScientific, Waltham, MA, USA).
Statistical Analysis
Statistical analyses were performed using student's t-test and values where P less than or equal 0.05 were considered significant.
Results
Time-Dependent Changes in Extra- and Intra- All-Trans-Retinol Concentrations of Müller cells
There was a significant, time-dependent, decrease of all-trans retinol in the conditioned media after Müller cells were incubated with serum free media containing 10 μM all-trans retinol and 2 μM BSA or bIRBP (Figs. 1A, 1B). Conditioned media from cells incubated with retinol containing BSA yielded 2200 pmol of all-trans retinol per milliliter at 0 hour, 1746 pmol at 12 hours, and 1132 pmol at 24 hours (Fig. 1A). After incubation with retinol delivered by bIRBP, there was 2200 pmol all-trans retinol per milliliter at 0 hour, 1200 pmol at 12 hours, and 704 pmol at 24 hours (Fig. 1A; n = 9 for all samples; mean ± SE). High performance liquid chromatography chromatograms show purity of all-trans retinol extracted from conditioned media at 0, 12, and 24 hours (Fig. 1B).
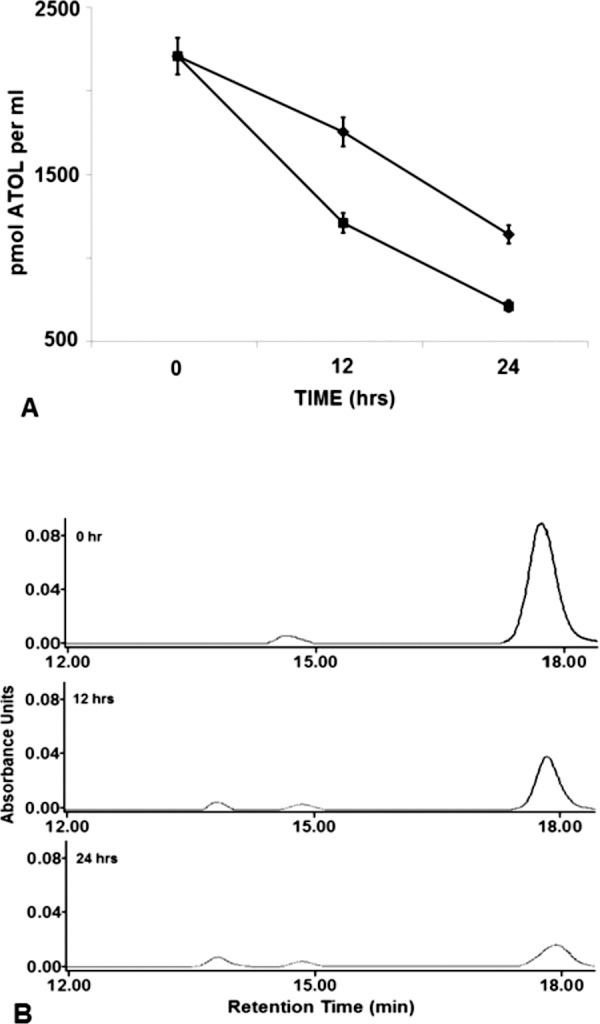
In contrast to a decrease in retinol concentration in the conditioned medium, there was a time-dependent increase of all-trans retinol in cultured rMC-1 incubated with 10 μM all-trans retinol and 2 μM BSA or bIRBP (Figs. 2A, 2B). Cells incubated with retinol and BSA yielded no retinol at 0 hour, 1750 pmol retinol per milligram of homogenate protein at 12 hours, and 2100 pmol per milligram of protein at 24 hours (Fig. 2A). After incubation of retinol containing bIRBP, there was no retinol in cell proteins at 0 hour, 1850 pmol retinol per milligram of homogenate protein at 12 hours, and 3450 pmol/mg of protein at 24 hours (Fig. 2A; n = 9 for all samples; mean ± SE).
Figure 2.
Comparison of the time-dependent increase of all-trans retinol in rMC-1 incubated with 10 μM all-trans retinol + 2 μM IRBP at 0, 12, and 24 hours. (A) Change in the amount of all-trans retinol with time in cells incubated with10 μM all-trans retinol and 2 μM BSA (♦) or bIRBP (▪). (B) High performance liquid chromatography chromatograms of representative retinol extracts from cells at 0, 12 and 24 hours; retention time of all-trans retinol = 17.8 minutes, 13-cis retinol = 14.6 minutes (n = 9 for all data points, mean ± SE; seeding density: 250,000 cells or 0.4 mg homogenate protein/mL).
Time-Dependent Accumulation in All-Trans Retinyl Ester in Müller Cells
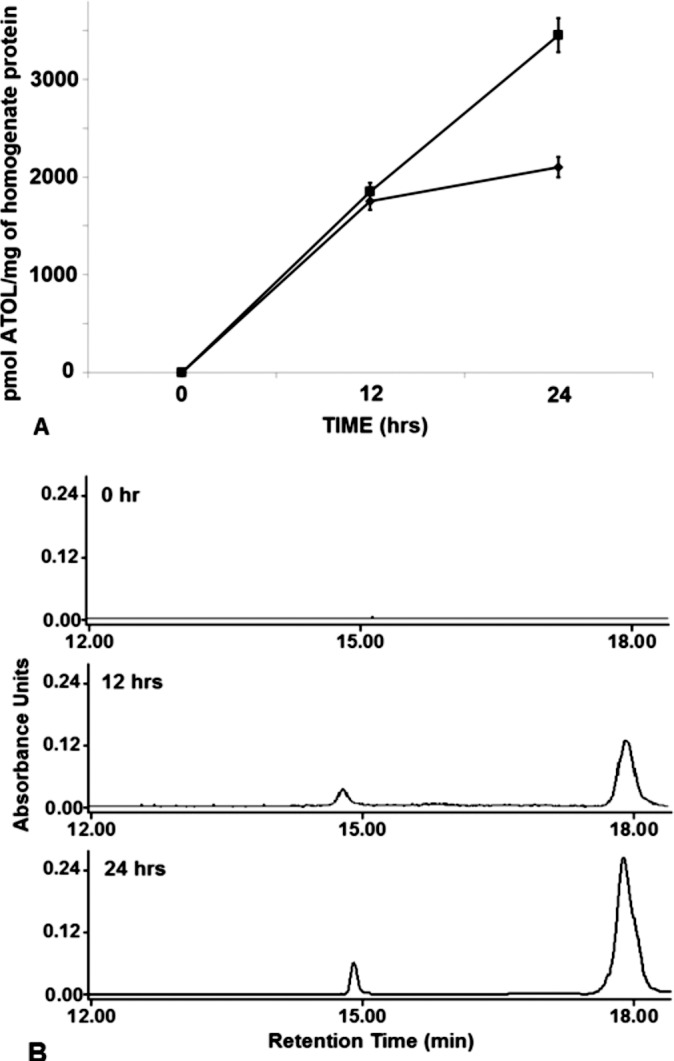
Figure 3 shows that cultured rMC-1 incubated with 10 μM all-trans retinol and 2 μM BSA or bIRBP accumulated all-trans retinyl ester over time. Cells incubated with all-trans retinol and BSA, no retinyl esters were found at 0 hour. However, 325 pmol all-trans retinyl ester per milligram of homogenate protein was found at 12 hours, and 450 pmol/mg of protein was found at 24 hours (Fig. 3A). Cells incubated with all-trans retinol and bIRBP, no retinyl esters were found in samples at 0 hour. However, 150 pmol all-trans retinyl ester per milligram of homogenate protein was found at 12 hours, and 270 pmol/mg of protein was found at 24 hours (Fig. 3A). High performance liquid chromatography chromatograms show no retinyl ester at 0 hour and an increase of all-trans retinyl ester at 12 and 24 hours of incubation (Fig. 3B).
Figure 3.
Comparison of the time-dependent increase of all-trans retinyl ester in rMC-1 cells incubated with 10 μM all-trans retinol + 2 μM IRBP at 0, 12, and 24 hours. (A) Change in the amount of all-trans retinyl ester with time after incubation with 10 μM all-trans retinol and 2 μM BSA (♦) or bIRBP (▪). (B) High performance liquid chromatography chromatograms of representative retinoid extract at 0, 12 and 24 hours; retention time of all-trans retinyl ester = 5.9 minutes. Change in the amount of all-trans retinyl ester with time n = 9 for all data points, mean ± SE (seeding density 250,000 cells or 0.4 mg homogenate protein/mL).
Time-dependent Increase of 11-Cis Retinol in the Conditioned Media Associated With IRBP
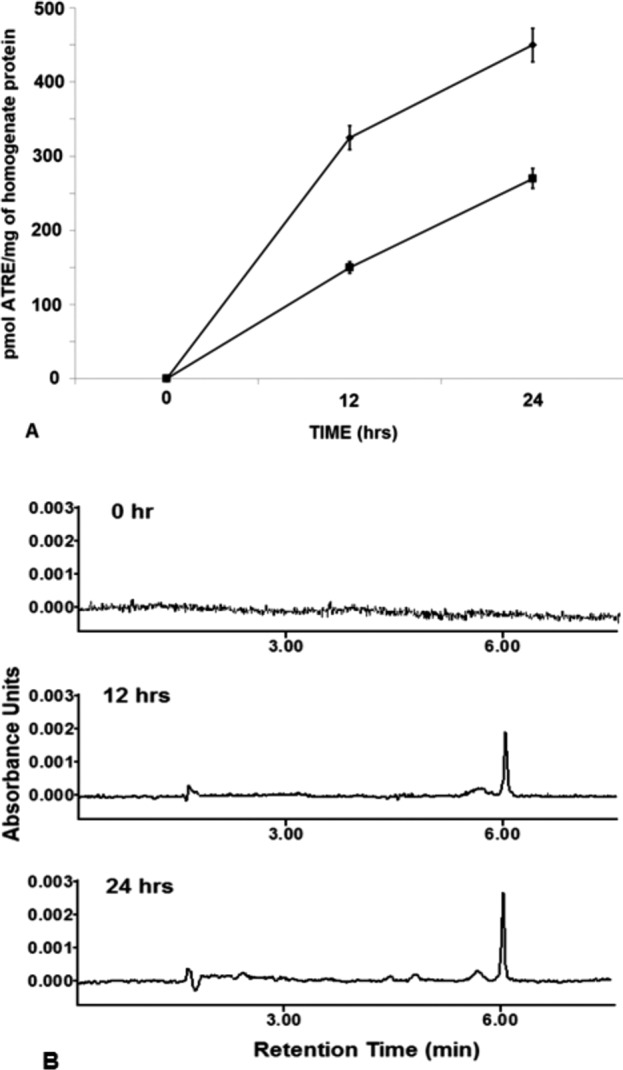
We observed for the first time 11-cis retinol in the conditioned media of Müller cells incubated with 10 μM all-trans retinol and 2 μM bIRBP (Fig. 4). Figure 4A shows HPLC chromatogram of a sample of authentic and purified all-trans retinol (peak 2 in Fig. 4A) with small amount of 13-cis retinol (peak 1 in Fig. 4A). After 24 hours incubation of all-trans retinol without Müller cells, retinols extracted from the conditioned media contained a higher amount of 13-cis retinol (peak 3 in Fig. 4B), in addition to all-trans retinol (peak 4 in Fig. 4B). In comparison, retinols extracted from conditioned media of cells incubated with all-trans retinol and BSA show similar profiles of 13-cis retinol (peak 5 in Fig. 4C) and all-trans retinol (peak 6 in Fig. 4C). However, retinols extracted from conditioned media of cells incubated with all-trans retinol and bIRBP had a distinct, additional 11-cis retinol component. Its identity was confirmed by retention time and spectral analysis by the online photodiode array detector (peak 7 in Fig. 4D) in comparison to authentic retinoid standards. Peaks for 13-cis retinol (peak 8 in Fig. 4D) and all-trans retinol (peak 9 in Fig. 4D) were also noted. Figure 4E shows UV spectra of HPLC peaks from the online photodiode array detector identified in Figures 4A through 4D. Results in Figure 5 shows a time-dependent increase of 11-cis retinol in the conditioned media of Müller cells incubated with all-trans retinol and IRBP. There was no detectable 11-cis retinol at 0 hr, 15 pmol 11-cis retinol per milliliter at 12 hours, and 137 pmol per milliliter at 24 hours (Fig. 5). In comparison, no 11-cis retinol was noted in conditioned media of Müller cells incubated with all-trans retinol and BSA.
Figure 4.
High performance liquid chromatography chromatograms and UV spectra of retinols. (A) High performance liquid chromatography purified all-trans retinol with 1.1% 13-cis retinol. (B) Retinols in conditioned media with all-trans retinol + 2 μM bIRBP after 24 hours at 37°C + 5% CO2 (similar results were obtained without cells and with osteosarcoma cells; sm63- ATCC). (C) Retinol in conditioned media from cells incubated with all-trans retinol and 2 μM BSA for 24 hours. (D) Retinols in conditioned media from cells incubated with all-trans retinol and 2 μM bIRBP for 24 hours. (E) Ultraviolet spectra from online photodiode array detector for peaks 7, 8, and 9 in (D). Peak 7 is 11-cis retinol with a λmax of 318 nm. Peak 8 is identified as 13-cis retinol with a λmax of 330 nm and is consistent with spectral scans of peaks 1, 3, and 5 in (A–C). Peak 9 is identified as all-trans retinol with a λmax of 325 nm and is consistent with UV spectra of peaks 2, 4, and 6 in ([A–C]; n = 9 for all data points, mean ± SE).
Figure 5.
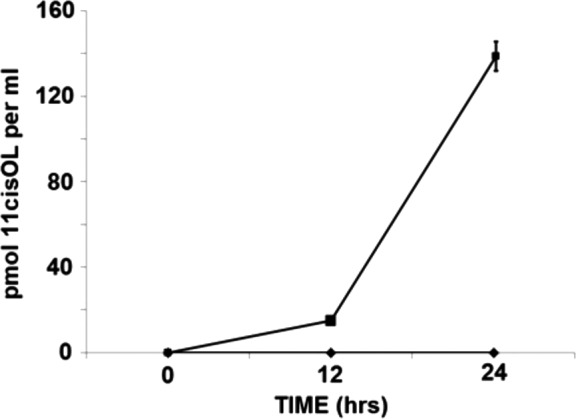
Comparison of time-dependent increase of 11-cis retinol in conditioned media (CM) from cells incubated with 10 μM ATOL + 2μM bIRBP (▪) and all-trans retinol + 2 μM BSA (♦; n = 9 for all data points, mean ± SE).
Inhibition of Isomerase Activity by Iron Chelator Deferoxamine
Figure 6 shows no significant change in 11-cis retinol between control and 2.5 mM deferoxamine. However there was a 60% loss of 11-cis retinol in the conditioned media from cells (incubated with all-trans retinol and bIRBP) treated with 5.0 mM deferoxamine compared with control without this inhibitor (Fig. 6).
Figure 6.
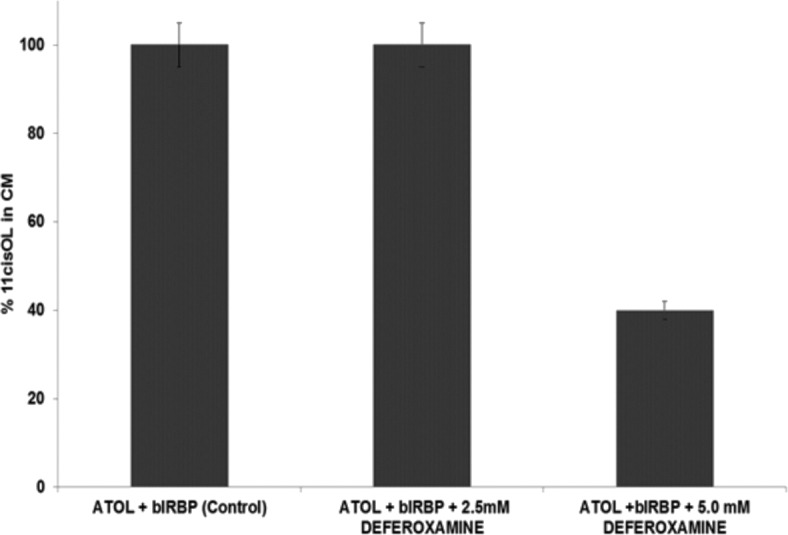
Inhibition of 11-cis retinol release from Müller cells by deferoxamine. Deferoxamine was added to the conditioned media at the indicated concentrations and cells were pretreated for 24 hours prior to the addition of all-trans retinol and IRBP. No difference of 11-cis retinol in the conditioned media was detected between control and deferoxamine at 2.5 mM. However a 60% reduction of 11-cis retinol was observed in the conditioned media containing 5.0 mM deferoxamine, compared with control. n = 3 for all data points, mean ± SE. Deferoxamine did not alter cell morphology or affect viability; viability was determined by dye-exclusion of trypan blue (data not shown).
Discussion
We have previously showed that cultured Müller cells synthesize and accumulate retinyl esters when all-trans retinol is delivered by BSA.21 However, it was not clear if IRBP, a normal component of the interphotoreceptor matrix, has such an activity. Although IRBP is known to promote 11-cis retinal release from RPE cells,10 no study has asked whether IRBP can promote retinol uptake and/or release of retinol from Müller cells. This question is relevant to the cone visual cycle as IRBP was recently shown to bind the cone outer segment and Müller microvilli pericellular matrices.17
The present study shows that bIRBP is more effective than BSA in facilitating retinol accumulation in cultured Müller cells (Fig. 2). We observed a corresponding time-dependent decrease in retinol concentration in the conditioned media (Fig. 1), suggesting that intracellular retinol may be derived from this extracellular source. Consistent with prior observations,21 Müller cells in the present study also synthesized retinyl ester from exogenous retinol (Fig. 3). A recent report from Kaylor et al.22 identified multifunctional O-acyltransferase (MFAT) as the 11-cis specific retinyl ester synthase in retinal Müller cells. Interestingly, higher levels of all-trans retinyl ester were recovered in cells incubated with all-trans retinol and BSA compared with bIRBP (Fig. 3) despite the higher intracellular all-trans retinol concentration in cells incubated with bIRBP (Fig. 2). A mass action effect may explain this finding. Since all-trans retinyl ester is a known substrate of Isomerase I, it is possible that the observed lower level of all-trans retinyl ester in cells (incubated with all-trans retinol and IRBP) was due to the enzymatic conversion of all-trans retinyl ester to 11-cis retinol by the isomerase. This is consistent with the result that 11-cis retinol was recovered in the conditioned media of cells incubated with all-trans retinol and bIRBP but not BSA (Figs. 4, 5; Supplementary Table S1 and Fig. S1). Together, our results suggest that IRBP, in comparison to BSA, facilitates all-trans retinol uptake by Müller cells as well as the retrieval of 11-cis retinol from these cells. The observation that only 11-cis retinol was detected in the conditioned media with bIRBP (but not BSA) suggests that IRBP may interact specifically with Müller cells and/or protects retinol from degradation (Tsin AT, et al. IOVS 2013;54: ARVO E-Abstract 3765).
Using deferoxamine, we have observed a significant reduction in the level of 11-cis retinol in the conditioned media of cells incubated with all-trans retinol and IRBP (Fig. 6). The inhibitory effect is attributable to the existence of an iron-dependent enzyme in the rat Müller cell that promotes the production of 11-cis retinol.23–25 Additional experiments will be needed to identify the isomerase in Müller cells that synthesizes 11-cis retinol. Since both Isomerase I and II are known to be iron-dependent,23–27 it is possible that this isomerase activity in the rMC-1 is from either one of these two enzymes. Kaylor et al.23 reported that chicken Müller cell homogenates (after incubation with all-trans retinol) synthesized more 9-cis than 11-cis retinol (fig. 9C). However, we did not detect 9-cis retinol in cell homogenates nor in conditioned media from rat Müller cells incubated with all-trans retinol and IRBP or BSA. It is possible that this apparent difference in isomerase activity is because all-trans retinol was isomerized in situ in cultured Müller cells in the present study, an assay condition that is different than the in vitro activity assay of the retinol isomerase enzyme in the cell homogenate in Kaylor et al.23 Nevertheless, the present study is the first to show that cultured Müller cells have the ability to synthesize and release 11-cis retinol, thus, providing an opportunity for future biochemical studies on the activity of the isomerase enzyme in Müller cells. Our studies may help to understand the role of IRBP in the cone visual cycle. The early literature assumed that the main role of IRBP is simply to solubilize visual cycle retinoids in the aqueous environment of the subretinal space allowing these lipid molecules to cross the interphotoreceptor matrix. However, in vitro studies monitoring the translocation of retinol between lipid vesicles8 and in vivo observations using IRBP−/− mice7,28 studies failed to show dependency of retinol transport in the presence of IRBP. However, a significant reduction in cone photoresponse has more recently been documented in IRBP−/− mice.12–14 Based on our conclusion of IRBP's role in the retinol delivery and removal from Müller cells as well as its protective effect for retinol in the interphotoreceptor matrix, a role beyond solubilizing retinoids should be considered if we are to understand IRBP's function in the visual cycle. Our study, which is the first to demonstrate a time-dependent delivery and retrieval of retinols in rat Müller cells in culture, could explain the reduced cone photoresponse in IRBP−/− mice and point to a novel and important role of IRBP in maintaining cone integrity and function.
Acknowledgments
The authors thank Vijay Sarthy who provided SV-40 transformed rMC-1 cells, Rosalie Crouch (Medical University of South Carolina) who provided 11-cis retinal with support from the National Eye Institute, Mary Alice Garlipp and Molly Sprada who assisted in purification of bIRBP, and Isidro “Daniel” Obregon, Andrew S. Mendiola, Samantha Cervantes, Aimee Mirande, Antonio Pena, and Nancy A. Pina who provided technical assistance.
Supported by grants from the National Institutes of Minority Health and Health Disparities (G12MD007591; Bethesda, MD, USA), Merit Review Award I01BX007080 from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development (NW Washington, DC, USA), National Institutes of Health (Bethesda, MD, USA) RO1 EY09412, and an Unrestricted Research Grant from Research to Prevent Blindness (New York, NY, USA) to the Department of Ophthalmology at SUNY at Buffalo.
Disclosure: B.S. Betts-Obregon, None; F. Gonzalez-Fernandez, None; A.T. Tsin, None
References
- 1. McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001; 20: 469–529 [DOI] [PubMed] [Google Scholar]
- 2. Pepperberg DR, Okajima TL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993; 7: 61–85 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein–an old gene for new eyes. Vision Res. 2003; 43: 3021–3036 [DOI] [PubMed] [Google Scholar]
- 4. Saari JC. Retinoids in Photosensitive Systems. The Retinoids. New York: Raven Press; 1994: 351–385 [Google Scholar]
- 5. Gonzalez-Fernandez F. Interphotoreceptor retinoid binding protein; myths and mysteries. J Ophthalmic Vis Res. 2012; 7: 100–104 [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez-Fernandez F, Ghosh D. Focus on molecules: interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res. 2008; 86: 169–170 [DOI] [PubMed] [Google Scholar]
- 7. Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999; 38: 12012–12019 [DOI] [PubMed] [Google Scholar]
- 8. Ho MT, Massey JB, Pownall HJ, Anderson RE, Hollyfield JG. Mechanism of vitamin A movement between rod outer segments, interphotoreceptor retinoid-binding protein, and liposomes. J Biol Chem. 1989; 264: 928–935 [PubMed] [Google Scholar]
- 9. Okajima TI, Pepperberg DR, Ripps H, Wiggert B, Chader GJ. Interphotoreceptor retinoid-binding protein promotes rhodopsin regeneration in toad photoreceptors. Proc Natl Acad Sci U S A. 1990; 87: 6907–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlson A, Bok D. Promotion of the release of 11-cis-retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992; 31: 9056–9062 [DOI] [PubMed] [Google Scholar]
- 11. Kolesnikov AV, Ala-Laurila P, Shukolyukov SA, et al. Visual cycle and its metabolic support in gecko photoreceptors. Vision Res. 2007; 47: 363–374 [DOI] [PubMed] [Google Scholar]
- 12. Parker RO, Fan J, Nickerson JM, Liou GI, Crouch RK. Normal cone function requires the interphotoreceptor retinoid binding protein. J Neurosci. 2009; 29: 4616–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parker RO, Crouch RK. The interphotoreceptor retinoid binding (IRBP) is essential for normal retinoid processing in cone photoreceptors. Adv Exp Med Biol. 2010; 664: 141–149 [DOI] [PubMed] [Google Scholar]
- 14. Parker R, Wang JS, Kefalov VJ, Crouch RK. Interphotoreceptor retinoid-binding protein as the physiologically relevant carrier of 11-cis-retinol in the cone visual cycle. J Neurosci. 2011; 31: 4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ala-Laurila P, Kolesnikov AV, Crouch RK, et al. Visual cycle: dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol. 2006; 128: 153–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qtaishat NM, Wiggert B, Pepperberg DR. Interphotoreceptor retinoid-binding protein (IRBP) promotes the release of all-trans retinol from the isolated retina following rhodopsin bleaching illumination. Exp Eye Res. 2005; 81: 455–463 [DOI] [PubMed] [Google Scholar]
- 17. Garlipp MA, Gonzalez-Fernandez F. Cone outer segment and Muller microvilli pericellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res. 2013; 113: 192–202 [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Fernandez F, Sung D, Haswell KM, Tsin A, Ghosh D. Thiol-dependent antioxidant activity of interphotoreceptor retinoid-binding protein. Exp Eye Res. 2014; 120: 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das SR, Gouras P. Retinoid metabolism in cultured human retinal pigment epithelium. Biochem J. 1988; 250: 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bridges C, Alvarez R. Measurement of the vitamin A cycle. In: Parcker L. ed Methods in Enzymology. San Diego, CA: Academic Press; 1982: 463–485 [DOI] [PubMed] [Google Scholar]
- 21. Betts BS, Obregon I, Tsin AT. Cultured Müller cells from mammals can synthesize and accumulate retinyl esters. Exp Eye Res. 2012; 101: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaylor JJ, Cook JD, Makshanoff J, Bischoff N, Yong J, Travis GH. Identification of the 11-cis-specific retinyl-ester synthase in retinal Müller cells as multifunctional O-acyltransferase (MFAT). Proc Natl Acad Sci U S A. 2014; 111: 7302–7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaylor JJ, Yuan Q, Cook J, et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat Chem Biol. 2013; 9: 30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi Y, Moiseyev G, Chen Y, Nikolaeva O, Ma JX. An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species. FEBS J. 2011; 278: 2913–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moiseyev G, Takahashi Y, Chen Y, et al. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem. 2006; 281: 2835–2840 [DOI] [PubMed] [Google Scholar]
- 26. Muniz A, Betts BS, Trevino AR, et al. Evidence for two retinoid cycles in the cone-dominated chicken eye. Biochemistry. 2009; 48: 6854–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007; 85: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolesnikov AV, Tang PH, Parker RO, Crouch RK, Kefalov VJ. The mammalian cone visual cycle promotes rapid M/L-cone pigment regeneration independently of the interphotoreceptor retinoid-binding protein. J Neurosci. 2011; 31: 7900–7909 [DOI] [PMC free article] [PubMed] [Google Scholar]