Abstract
Purpose.
Proper refractive eye growth depends on several features of the visual image and requisite retinal pathways. In this study, we determined the contribution of rod pathways to normal refractive development and form deprivation (FD) myopia by testing Gnat1−/− mice, which lack functional rods due to a mutation in rod transducin-α.
Methods.
Refractive development was measured in Gnat1−/− (n = 30–36) and wild-type (WT) mice (n = 5–9) from 4 to 12 weeks of age. FD was induced monocularly from 4 weeks of age using head-mounted diffuser goggles (Gnat1−/−, n = 9–10; WT, n = 7–8). Refractive state and ocular biometry were obtained weekly using a photorefractor, 1310 nm optical coherence tomography, and partial coherence interferometry. We measured retinal dopamine and its metabolite, DOPAC, using HPLC.
Results.
During normal development, the refractions of WT mice started at 5.36 ± 0.68 diopters (D) and became more hyperopic before plateauing at 7.78 ± 0.64 D. In contrast, refractions in Gnat1−/− mice were stable at 7.39 ± 1.22 D across all ages. Three weeks of FD induced a 2.54 ± 0.77 D myopic shift in WT mice, while Gnat1−/− mice did not respond to FD at any age. Axial lengths of Gnat1−/− and WT mice increased with age, but differences between genotypes or with goggling did not reach statistical significance and fell within the precision of the instruments. The DOPAC levels were significantly lower in Gnat1−/− mice from 2 to 12 weeks of age with DOPAC/dopamine ratio peaking earlier in Gnat1−/− compared to WT mice. No differences in dopamine were seen in response to FD or between genotypes.
Conclusions.
Functional rod photoreceptors are critical to normal refractive development and the response to FD in mice. Dopamine levels may not directly modulate the refractive state of the mouse eye, but tonic levels of dopamine during development may determine susceptibility to myopia.
Keywords: refractive development, myopia, rod photoreceptors, dopamine
Gnat1−/− mice with nonfunctional rod photoreceptors had abnormal refractive development and lacked a response to form deprivation. Dopamine levels may not directly modulate the refractive state of the eye, but tonic levels of dopamine during development may determine susceptibility to myopia.
Introduction
Visually-driven eye growth is responsible for matching the power of the eye with ocular length to acquire in-focus images; a process called emmetropization. Local signaling by the retina mediates refractive development, as shown by the selective effect of partial occluders altering growth in only the corresponding region of the globe in chickens1–3 and primates,4 preservation of the response to form deprivation (FD)5 or lens-induced defocus6 after optic nerve section in chickens, or pharmacologically blocking retinal ganglion cell transmission in tree shrews7 (see review8). However, it should be noted that the response to lens defocus is altered after blocking input from higher visual processing areas in chickens.5,9–11
Since an in-focus image is the ultimate goal of refractive development, it may be presumed that the visual image should be of high acuity and temporal resolution,12 qualities attributed to cone-mediated visual processing. Thus, there is an assumption that cone pathways likely underlie the signaling needed for proper eye growth control. However, results from a few studies suggest that cone pathways may not dominate the signaling of mammalian eye growth: (1) Laser ablation of the cone-rich fovea region in monkeys did not prevent the development of FD myopia13,14 and (2) imposing FD on the rod-dominated peripheral regions of the monkey eye produced similar magnitudes of FD myopia as when the entire visual field was affected.15
The limitation of these studies is that rods and cones are present in the periphery of the retina and, thus, a small population of cones still could be contributing signals for eye growth. In addition, these experiments were performed under photopic conditions in which cones would be functionally predominant. Nonetheless, they do suggest that the rod-rich peripheral regions of the retina may be important for visually-guided eye growth and a few other studies have suggested a role for photoreceptors in refractive development.16 In fact, spatial frequency thresholds of mice without functional cones (cyclic nucleotide-gated cation channel subunit A3 knock-out, CNGA3−/−, mice), are the same as those of wild-type (WT) mice, suggesting that rod and/or rod pathways are capable of providing visual signals under photopic conditions for the optokinetic response. In contrast, mice with nonfunctional rods (CNGB1−/− mice) have much poorer spatial resolution.17
Abnormal refractive development also is associated with retinal diseases involving rod photoreceptors and/or pathways. For instance, patients with the complete form of congenital stationary night blindness (cCSNB) have disrupted visual transmission between rods and ON bipolar cells due to a mutation in Nyx gene and also present with high myopia.18 In addition, patients with cone–rod dystrophy19,20 or retinitis pigmentosa21 have increased incidence of myopia. Similarly, we found that mice with the Nyx mutation22 and mouse models of retinitis pigmentosa with a mutation in the Pde6b gene (Pde6brd1/rd1 [rd1] or Pde6brd10/rd10 [rd10])23 are more susceptible to FD myopia. Finally, in the retinopathy of prematurity (ROP) rat model, myopia is present (although paradoxically with shorter than normal axial length)24 and abnormal rod photoreceptor function has been implicated.25
Dopamine (DA), a key neuromodulator in the retina that regulates circadian rhythms and mediates adaptation to different lighting conditions, has been proposed as a stop signal for visually-driven eye growth.26 In the retina, DA is synthesized from L-3,4-dihydroxyphenylalanine (L-DOPA) and metabolized into 3,4-dihydroxyphenylacetate (DOPAC). Synthesis and release of DA are stimulated by light via the ON pathway.27–30 Initial light exposure increases retinal DA synthesis, release, and metabolism; however, the system then reaches equilibrium, such that the steady state level of DA does not change appreciably and only DOPAC levels vary during the light phase.31 Thus, an increase in the amount of DOPAC is an indicator of DA turnover (often reported as DOPAC/DA ratio) and use. Light regulation of dopamine levels is mainly through rods, cones, and possibly melanopsin cells.32–34 Dopamine increases in a log-linear relationship with illuminance,35–37 although these studies did not examine rod-isolating illuminance levels. Moreover, rod pathways and the dopaminergic system interact structurally and functionally; for instance, DA neurons synapse onto AII and A17 amacrine cells in the rod pathway, rod-driven ON pathways stimulate DA release, which in turn decrease rod function as the retina adapts to daylight function, and loss of rods results in decreased DA levels in the retina.32,38
The mouse recently has been adopted as an experimental model for myopia, offering the ability to manipulate genes and environment (see review39). The mouse eye responds with myopic shifts when exposed to FD40–45 or negative lens defocus.43,46 In addition, a number of studies using mice have confirmed signaling pathways implicated in previous chicken studies as influencing refractive development, such as the early growth response protein-1,47–49 muscarinic receptors,50 adenosine receptors,51 retinoic acid,52 and dopamine23,53 (Zhou X, et al. IOVS 2014;55:ARVO E-abstract 3038).
To more fully explore the contributions of rod photoreceptors to emmetropization and myopia development, we tested mice with nonfunctional rod photoreceptors, carrying alleles for the gene of the rhodopsin-associated G protein, transducin α1 (Gnat1) under normal and form deprived visual conditions. We then evaluated DA and DOPAC levels in the retina across postnatal development and following FD.
Methods
Animals and Experimental Design
The Gnat1−/− mice were a generous gift from Janis Lem, PhD (Tufts-New England Medical Center, Boston, MA, USA). Importantly, loss of Gnat1 renders the rods nonfunctional, but does not induce rod degeneration until 13 weeks of age.54 Mice were maintained at the Atlanta Veterans Affairs Medical Center, on 12:12-hour light cycles (~17 lux; lights on at 6 AM) and housed in typical shoe box cages with mouse chow and water available ad libitum.
Refractive development (RD) was characterized with weekly measurements of refractive error and axial length from 4 to 12 weeks of age in Gnat1−/− and age-matched WT control mice without any visual manipulation (see Table for animal numbers used). The response to FD was characterized by subjecting separate cohorts of WT and Gnat1−/− mice to monocular diffuser goggles at 4 weeks of age. Goggles were held in place using head-mounted frames, as described previously, for up to 8 weeks (12 weeks of age).41 All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the local Institutional Animal Care and Use Committee.
Table.
Animal Numbers Used in the Experiments Described in This Study
|
Experiment |
Measurement |
Strain |
Weeks of Age |
||||||||||
|
1 |
2 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|||
| Refractive development | Refraction | WT | 8 | 9 | 9 | 8 | 9 | 9 | 5 | 9 | 9 | ||
| Gnat1−/− | 31 | 30 | 36 | 35 | 35 | 36 | 33 | 35 | 35 | ||||
| Axial length | WT | 8 | 8 | 8 | 7 | 8 | 8 | 5 | 8 | 8 | |||
| Gnat1−/− | 10 | 10 | 10 | 9 | 10 | 10 | 8 | 10 | 10 | ||||
| Form deprivation | Refraction | WT naive | 8 | 8 | 8 | 9 | 9 | ||||||
| WT FD | 14 | 13 | 12 | 10 | 7 | ||||||||
| Gnat1−/− naive | 13 | 11 | 13 | 12 | 13 | ||||||||
| Gnat1−/− FD | 10 | 9 | 9 | 10 | 9 | ||||||||
| Axial length | WT naive | 10 | 10 | 10 | 9 | ||||||||
| WT FD | 12 | 12 | 11 | 8 | |||||||||
| Gnat1−/− naive | 9 | 9 | 8 | 7 | |||||||||
| Gnat1−/− FD | 14 | 12 | 12 | 9 | |||||||||
| Dopamine | ZT3 and ZT22 | WT ZT3 | 7 | ||||||||||
| WT ZT22 | 6 | ||||||||||||
| Gnat1−/− ZT3 | 3 | ||||||||||||
| Gnat1−/− ZT22 | 3 | ||||||||||||
| Across age | WT | 6 | 3 | 11 | 11 | 10 | 9 | 8 | |||||
| Gnat1−/− | 12 | 13 | 10 | 7 | 9 | 7 | 7 | ||||||
| Form deprivation | WT naive | 4 | |||||||||||
| WT FD | 10 | ||||||||||||
| Gnat1−/− naive | 6 | ||||||||||||
| Gnat1−/− FD | 5 | ||||||||||||
Mice were derived from two or more separate litters for each experiment.
It is important to note that we found the rd8 gene55 in the Gnat1−/− mice, as well as in some of the WT animals (31% as homogenous mutant of rd8, 26% as heterozygotes). It is unlikely that the slow rod photoreceptor degeneration caused by the rd8 mutation had any significant contributions to our results as the mice reported here were younger than 6 months, when electroretinograms still are normal in rd8 mice,56 and no statistically significant differences were found between the different rd8 genotypes (WT mice 2-way repeated ANOVA on myopic shift, main effect for rd8 genotype F(2,48) = 0.125, P = 0.88).
Refractive State and Axial Length Measurements
Each mouse underwent the following experimental measurements of refractive error and ocular biometry, as described previously.57 First, eyes were dilated with 1% tropicamide and measurements of refractive state obtained using an automated photorefractor.23,42,58 Axial length measurements of a subset of mice (see Table for animal numbers) were acquired using a custom-built 845-nm time-domain partial coherence interferometer (PCI) after being placed in an open-ended conical tube with the mouse's head pedestal stabilized by a clip.58,59
Mice then were anesthetized (ketamine 80 mg/kg and xylazine 16 mg/kg) and refractions repeated to obtain a more stabilized measurement with standard deviations of less than 0.5 diopters (D).57 While still anesthetized, axial length measurements were obtained using 1310 nm spectral-domain optical coherence tomography (SD-OCT; Bioptigen, Inc., Durham, NC, USA), as described previously.58 After measurements, mice were given yohimbine (2.1 mg/kg) to reverse the effects of anesthesia and reduce the development of corneal lesions.60 Mice recovered on a warming pad with saline drops applied to the eyes. The entire measurement routine lasted approximately 30 minutes.
Retinal Dopamine Quantification
Separate cohorts of 8-week-old WT and Gnat1−/− mice were sacrificed at Zeitgeber time 3 (ZT3) and ZT22 (or 3 hours after and 2 hours before light onset, respectively) to confirm abnormal DOPAC/DA ratio phenotype in Gnat1−/− mice33 (see Table for animal numbers). To determine the levels of retinal DA during development, retinas from both genotypes were collected at 1, 2, 4, 6, 8, 10, and 12 weeks of age between 10 and 12 AM (see Table). In addition, retinal DA and DOPAC levels also were measured from mice after the final endpoint of the FD experiments (see Table). Retinas were collected 48 hours after ocular parameters assessment to provide a recovery period from any effects of anesthesia. In brief, DA and DOPAC levels were quantified using HPLC with coulometric detection as described previously.31 Retinas were homogenized in 0.1 N HClO4 solution (0.01% sodium metabisulfite and 50 ng/mL internal standard 3,4 dihydroxybenzylamine hydrobromide) and centrifuged. The HPLC conditions included an Ultrasphere ODS 5 μm 250 × 4.6 mm column (HiChrom, Berkshire, England, or Beckman Coulter, Fullerton, CA, USA) for separation with a mobile phase containing 0.1 M phosphoric acid, 0.1 mM EDTA, 0.3 to 0.35 mM sodium octylsulfate, 6% acetonitrile, adjusted to pH 2.7 with NaOH. The retinal DA and DOPAC levels were quantified using standard curves generated with 0.1 to 1 ng DA and DOPAC. All FD cohort retinas were homogenized individually, while the right and left retinas were pooled together in the RD cohort for DA and DOPAC quantification.
Data Analysis
All statistical analyses were performed using commercial software (SigmaStat 3.5; Aspire Software International, Ashburn, VA, USA). Data plotted in Figures are presented as mean ± SEM, underwent repeated-measures 2-way ANOVA, and Holm-Sidak post hoc tests for statistical significance. For RD refraction and axial length data both eyes received the same treatment and the values from the two eyes were averaged to represent a single value from each individual mouse. In a selection of animals that underwent PCI and SD-OCT measurements, the axial length values were averaged together, since measurements by the two techniques were in good agreement (interclass correlation coefficient = 0.94).58 For FD results, refractive errors are presented as “myopic shift” (difference between right and left eyes), since the refractive errors of untreated opposite eyes were not statistically different from those of naïve control eyes. Axial lengths of FD cohort were normalized to 4-week-old values (baseline) to eliminate individual variability in eye size due to differences in body size.61 “Axial shift” represents the difference in length between the right and left eye after values had been normalized to baseline. The DA and DOPAC levels across age were normalized to the Gnat1−/− and WT values obtained at ZT3. The DA and DOPAC values from FD cohorts were analyzed by taking the difference between the two eyes. When normality failed for DA analysis, Student's t-test was used with P value corrected with the rough false discovery rate method (calculated as P*[#tests + 1]/[2 * #tests]).62
Results
Abnormal Refractive Development in Gnat1−/− Mice
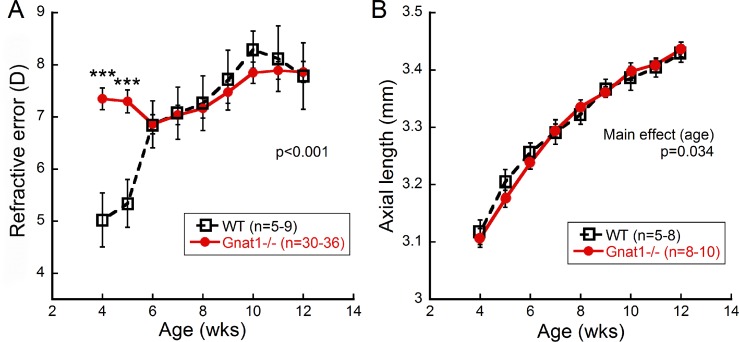
Nonfunctional rod photoreceptors had the most profound effect on normal refractive development at young ages. At 4 weeks of age, WT animals had refractive errors of 5.02 ± 0.52 D (mean ± SEM, n = 12) and became more hyperopic with age, reaching a refractive error of 7.78 ± 0.64 D at 12 weeks old (n = 12, Fig. 1A). In contrast, the eyes of Gnat1−/− mice did not have a period of growth toward relative hyperopia, but had stable refractive errors in the range from 6.85 to 7.88 D during the entire study period (n = 31–38/timepoint). This produced significant differences between Gnat1−/− and WT mice at 4 and 5 weeks of age (Fig. 1A; 2-way repeated ANOVA, F(7,336) = 9.33, P < 0.001).
Figure 1.
Refractive development of Gnat1−/− mice. (A) Refractive error plotted across age for WT and Gnat1−/− mice shows that Gnat1−/− mice refractions change little across the experimental period compared to WT (2-way repeated ANOVA, F(7,336) = 9.33, P < 0.001). (B) Axial length measurements in WT and Gnat1−/− mice increased with age, but were not statistically different (2-way repeated ANOVA, main effect of age, F(8,154) = 2.17, P = 0.034). Holm-Sidak post hoc comparisons ***P < 0.001. Symbols represent mean ± SEM.
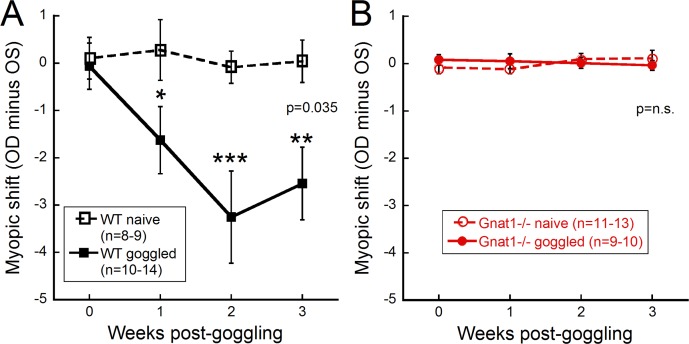
Figure 2.
Use of FD has no effect on Gnat1−/− mice. (A) WT mice showed a significant shift (goggled minus opposite eye) with FD goggling, while the nongoggled naïve mice showed no change between eyes (right eye minus left eye; 2-way repeated ANOVA, F(3,77) = 3.1, P = 0.035). (B) The Gnat1−/− mice did not respond to FD and showed no change in refractive error across the goggling period. Holm-Sidak post hoc comparisons, *P < 0.05, **P < 0.01, ***P < 0.001. Data shown are mean ± SEM.
While axial length significantly increased with age for WT and Gnat1−/− mice, there were no differences between Gnat1−/− and WT mice (Fig. 1B; 2-way repeated ANOVA, main effect of age F(8,154) = 2.17, P = 0.034; n = 5–8/timepoint for WT and 9–15 mice/timepoint for Gnat1−/−).
Gnat1−/− Mice Unresponsive to FD
First, regardless of genotype, eyes in naïve control mice (not goggled) had similar refractive errors (Fig. 2; myopic shift [difference between right and left eyes] in WT mice, 0.04 ± 0.45 D, n = 9; Gnat1−/− mice, 0.11 ± 0.18 D, n = 12). Conversely, the response to FD differed depending on the genotype. The WT mice exhibited a relative myopic shift (−2.54 ± 0.77 D, n = 10) compared to the contralateral eye after 3 weeks of goggle wear (Fig. 2A; 2-way repeated ANOVA, F(3,77) = 3.1, P = 0.035). In contrast, Gnat1−/− mice did not respond to FD. After 3 weeks of goggle wear, the refractive errors of Gnat1−/− form-deprived eyes did not shift and remained similar to the contralateral eye (−0.04 ± 0.10 D, n = 10; Fig. 2B; 2-way repeated ANOVA, F(3,86) = 0.9, P = 0.447). Therefore, results from form-deprived Gnat1−/− mice were not significantly different from the naïve nongoggled Gnat1−/− animals (Fig. 2B). At 8 weeks after goggling, Gnat1−/− animals still had no significant myopic shift (−0.12 ± 0.14 D, n = 9) compared to WT mice (−2.15 ± 1.27 D, n = 7). A direct comparison of WT and Gnat1−/− mice at 3 weeks after goggling showed significant differences in response to FD between the genotypes (data not shown; 2-way ANOVA, F(1,42) = 7.36, P = 0.01).
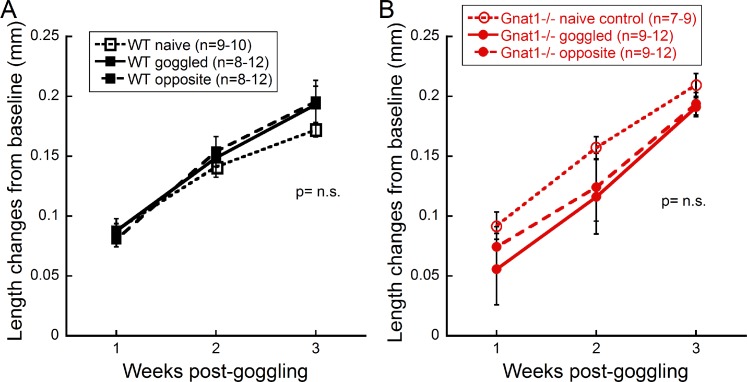
Axial length differences were not detected after form deprivation with the instruments used. In WT mice, goggled and opposite eyes had longer axial lengths after 3 weeks of FD compared to naïve controls; however, this was not statistically significant (n = 8, Fig. 3A). In Gnat1−/− mice, similar axial lengths were measured between goggled and opposite eyes, with naïve control eyes showing a trend for longer axial lengths (n = 9, Fig. 3B). No statistically significant differences were found between the body weights of goggled and control mice (data not shown). Schematic models of the mouse eye predict that 5 to 6 μm change in axial length is needed for 1 D change in optical power.42 Thus, it is possible that the interuser measurement variability of the SD-OCT instruments (21 μm) used here58 is not sufficient to detect differences in axial length, as the measured axial shifts of goggled mice were less than the resolution limit.
Figure 3.
Axial length changes with FD. Axial length measurements in WT (A) or Gnat1−/− (B) mice showed no significant differences between naïve, goggled, or opposite eyes. Data are mean ± SEM.
Dopamine Metabolism Altered in Gnat1−/− Mice
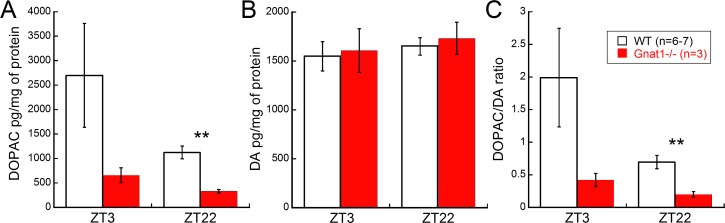
The retinal dopaminergic systems in WT and Gnat1−/− mice responded differently to light and dark cycles. The DOPAC levels were lower in Gnat1−/− mice compared to WT at night (ZT22; Student's t-test, t = 4.11, P = 0.004; Fig. 4A). Comparing the response between light (ZT3) and dark (ZT22) cycles within each genotype, Gnat1−/− mice had a diminished response to light (658 ± 153 to 331 ± 36, respectively, 98% difference) compared to WT mice (2699 ± 1062 to 1123 ± 130, respectively, 140% difference). The DOPAC/DA ratio also was significantly decreased in Gnat1−/− mice compared to WT in the dark cycle, as reported previously (Fig. 4C; Student's t-test, t = 3.24, P = 0.01).33 However, the DA levels in WT and Gnat1−/− mice were similar between the genotypes and different light phases (Fig. 4B).
Figure 4.
The DA and DOPAC analyses between day (ZT3) and night (ZT22) in WT and Gnat1−/− mice at 8 weeks of age. (A, C) The DOPAC levels and DOPAC/DA ratios were lower in Gnat1−/− compared to WT retinas at ZT22 (Students t-test, P < 0.01). The WT retinas showed a greater increase in DOPAC levels and DOPAC/DA ratio in the light phase compared to Gnat1−/− retinas. (B) DA levels were similar between day and night in WT and Gnat1−/− mice. Error bars: mean ± SEM.
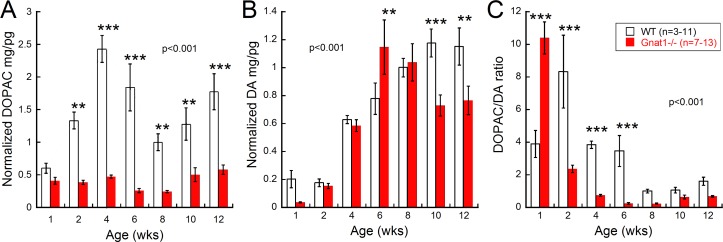
Next, we examined DOPAC and DA across postnatal development. We found that DOPAC levels in WT mice (Fig. 5A) significantly increased from 1 to 4 weeks, then decreased until week 8 before rising again (2-way ANOVA, F(6,123) = 5.499, P < 0.001). In contrast, DOPAC levels remained consistent in Gnat1−/− mice, with no statistically significant differences across age. The pattern of DA in the retina was fairly similar between the two genotypes, with low levels at 1 and 2 weeks of age that increased and then became stable until 12 weeks of age (Fig. 5B; 2-way ANOVA, F(6,123) = 4.56, P < 0.001). These differences between genotypes in the pattern of DOPAC and DA indicated significant differences in dopamine metabolism, as illustrated by the DOPAC/DA ratio. The DOPAC/DA ratio was significantly higher in Gnat1−/− mice at 1 week of age, due to very low levels of DA at this age, and then decreased rapidly at 2 weeks and beyond (Fig. 5C; 2-way ANOVA, F(6,123) = 20.17, P < 0.001). In contrast, the DOPAC/DA ratio in WT retinas increased from 1 to 2 weeks of age, decreased at 4 to 6 weeks, and then further diminished at 8 to 12 weeks of age.
Figure 5.
Dopamine analysis across postnatal development. (A) DOPAC levels were significantly altered across age in WT mice, while levels remained significantly lower and stable in the Gnat1−/− mice (2-way ANOVA, F(6,123) = 5.499, P < 0.001). (B) The overall patterns in DA levels across development were similar between the two genotypes. However, the Gnat1−/− mice appeared to reach peak levels of retinal DA earlier than WT mice (2-way ANOVA, F(6,123) = 4.56, P < 0.001). (C) The DOPAC/DA ratios showed different development patterns between WT and Gnat1−/− mice. The Gnat1−/− mice appeared to have an earlier DOPAC/DA ratio peak than WT mice and then dropped rapidly to become significantly lower (2-way ANOVA, F(6,123) = 20.17, P < 0.001). Holm-Sidak post hoc comparisons **P < 0.01, ***P < 0.001. Error bars: mean ± SEM.
Lastly, we examined the levels of DOPAC and DA and DOPAC/DA ratios (data not shown) after FD and found no significant differences due to genotype or goggling.
Discussion
Functional Rod Photoreceptors Needed for Visually-Guided Refractive Development
In most animals, refractive development follows a predictable pattern. In mammals, this typically starts with hyperopic refractions and then shifts to emmetropia during early development to adolescence.63,64 In WT mice used here and reported previously,42,49,51,57,65–69 the normal refractive development curve starts with hyperopic refractions, then shifts to greater levels of hyperopia (Fig. 1). Surprisingly, the Gnat1−/− mice did not have this same refractive development curve, but instead maintained the same range of refractions across the entire experimental period (4–12 weeks), not deviating more than 1.03 D (maximum, 7.88 D; minimum, 6.85 D). We hypothesized that the initial shift toward more hyperopic refractions in WT mice is due to visual input that drives signaling pathways controlling refractive development. Thus, in the Gnat1−/− mice the critical signaling pathways may not be activated, since there was no change in refractive error across age. Another explanation is that Gnat1−/− mice have reached the refractive error plateau more quickly than WT, perhaps due to a change in dopamine metabolism, as indicated by the high initial DOPAC levels in the retina (Fig. 5).
Functional Rods Needed to Respond to FD Myopia
The use of FD has become a standard method to induce experimental myopia.70 In our WT mice, application of the diffuser goggle induced a myopic shift within 1 week (Fig. 2). However, Gnat1−/− mice did not respond to FD, even in mice that were followed for up to 8 weeks of goggling. One interpretation of this result is that functional rod photoreceptors are needed to signal myopic eye growth. Without functional rods, Gnat1−/− mice may be unable to detect rod-mediated aspects of the disrupted form-deprived image and, therefore, lack the typical FD myopic shift. Alternatively, rod and cone pathways may produce a balance in controlling refractive eye growth, such that cone-mediated signaling may became stronger without functional rods and prevent excessive eye growth signaling. This scenario may explain the more hyperopic refractions in the Gnat1−/− mice at younger ages with normal visual input. Another possibility is that an alternative, transducing-α1-independent form of signaling, may have a role in refractive development.71 We also cannot rule out the possibility that the deletion of Gnat1 may alter normal retinal development, and disrupt the retinal pathways that drive refractive development and myopia. Reduced retinal dopamine levels may increase gap junction conductance between rod and cone photoreceptors, horizontal cells, and amacrine cells, and AII amacrine and cone bipolar cells.72 This could have the effect of decreasing the cone pathway signal and altering the response to normal and form-deprived visual input, consistent with other data showing that photopic conditions are required for FD myopia. Regardless of the underlying mechanisms, it would be interesting to examine how Gnat1−/− mice respond to plus or minus lens defocus.
Role of DA in Refractive Eye Growth
There is some evidence that DA synthesis and signaling takes several postnatal weeks to fully develop and mature. Some dopamine receptors are expressed in the vertebrate retina,73 along with L-DOPA74 before detectable immunohistochemical expression of tyrosine hydroxylase, a key enzyme in DA synthesis. The DA receptor expression continues to increase after birth, with dopamine D4 receptor expression reaching a peak at postnatal day 12.75 The retinal dopaminergic neurons do not reach maturation with typical ring-like axonal processes until the third postnatal week.76 Finally, L-DOPA content increases through development until the fourth postnatal week and then sharply declines.77 These observations correspond well with our data (Fig. 5) that show DOPAC levels increased until 4 weeks of age in WT mice and then decreased as the animals aged. This pattern may be indicative of a reduction in the amount of diffusible or nonvesicular dopamine with age concomitant with an increase in vesicular DA within the cell, which would increase the overall steady-state DA content (see continued increased in Fig. 5B).
Relating DA development to refractive state, it is not clear from these data that DA is directly signaling visually-driven eye growth in the mouse. If DA directly modulates eye growth, it would be expected that refractive values would follow closely the changes in DOPAC or DA levels. However, the rapid hyperopic shift from 4 to 6 weeks in WT mice is not mirrored in the DA data, although steady DOPAC levels in Gnat1−/− mice are similar to the consistent refractive values across age. Furthermore, DOPAC and DA levels were not significantly different between strains after FD, although they had drastically different refractive responses to FD. It is important to point out that HPLC analysis of DA and DOPAC levels has limitations, as it only measures total retinal levels and does not provide information about whether DA is intra- or extracellular. Previous studies in form deprived chickens have shown that dopamine release by amacrine cells and diffusion through the retina, and not the total dopamine retinal content, is most relevant to the myopic response.78 Nevertheless, these data suggested that DA may not have a direct effect on refractive development in mice; instead, dopamine metabolism may predispose the retina to certain levels of myopia susceptibility. For instance, the loss of functional rods across development may alter dopamine release and the expression of several other associated molecules, leading to chronic changes in extracellular dopamine levels. Dopamine and DOPAC have been found to decrease with form deprivation in many species (see review26) and, thus, it would be expected that chronically low levels of retinal dopamine may increase susceptibility to FD myopia. We have found previously that low levels of DOPAC correlated with increased susceptibility to FD myopia in the rd1 and rd10 mouse models of retinitis pigmentosa.23 However, the role of DA in refractive development is likely very complex as this hypothesis did not seem to apply to Gnat1−/− mice, which are resistant to FD even though they had lower retinal DOPAC levels (Fig. 5). Furthermore, recent reports have suggested that dopamine may differentially act on dopamine receptor subtypes to influence eye growth in mice53 (Zhou X, et al. IOVS 2014;55:ARVO E-Abstract 3038), adding further complexity to the mechanisms involved.
Thus, more research is needed to elucidate how DA influences refractive development and susceptibility to FD, or myopia in general, so that it may serve as a potential therapeutic target for myopia in the future.
Effects of Gnat1 Deletion on Retinal Dopaminergic System
These studies showed that nonfunctionality of rod photoreceptors have relatively small effects on steady-state levels of retinal dopamine, as indicated by the nearly normal levels of DA in the Gnat1−/− retina in the light/dark phases and across age (Figs. 4, 5). This may indicate that DA is synthesized in Gnat1−/− retinas, but not properly metabolized. However, loss of rod function appears to disrupt DA metabolism, resulting in diminished DOPAC levels between 1 and 4 weeks postnatally and during the light phase in Gnat1−/− mice. This could be due to a variety of reasons, such as diminished neuronal activity to stimulate DA release, insufficient reuptake of DA to the presynaptic terminal, or defective metabolism of DA to DOPAC.38 Further research is needed to determine if these effects are due primarily to the loss of rod function or if the absence of rod signaling unmasks contributions from other sources, such as the RPE or choroidal innervation.
How Could Rods Contribute to Refractive Development?
Cone-mediated visual processing has been assumed to regulate refractive development based on the fact that emmetropia produces a focused image on the retina and FD myopia occurs under photopic illumination, with little emphasis placed on a potential role of rod-mediated processing. Using the Gnat1−/− model provided a unique opportunity to isolate the contributions of functional rod photoreceptors in refractive development. Our data showed that functional rods are important for normal refractive development and the response to FD. However, the exact mechanism of how rod signaling could contribute to refractive development and the detection of FD is not known.
While rod and cone photoreceptor sensitivity traditionally has been thought to occur in a binary fashion under scotopic and photopic conditions, there is increasing evidence that retinal circuitry is much more complicated. For instance, rods in the mouse retina in vivo are capable of providing detectable signals in the presence of steady lights that are 2 log units higher than the Weber line where rod saturation is behaviorally shown to occur; although the sensitivity is decreased compared to cones.79 Furthermore, rod photoreceptors can drive circadian photoentrainment under high light intensities80 and mediate vision under photopic conditions.81 Since the ocular growth response to defocus occurs over minutes,82 compared to the millisecond response needed to detect photons for functional vision, the role of rod-mediated signaling in refractive development may involve retinal pathways with reduced sensitivity and/or roles in circadian rhythms.
Thus, our results suggested that functional rods may be a critical component of retinal signaling for refractive development. Further experiments are needed to examine the contribution of cone photoreceptors in isolation as well as testing the effects of eliminating other elements of visual pathways for their potential role in refractive development.
Acknowledgments
The authors thank Moe H. Aung, PhD, for insightful comments and discussions on the manuscript.
Supported by the National Institutes of Health (NIH) Grants R01 EY016435 (MTP), P30 EY006360 (PMI), and R01 EY004864 (PMI); a Department of Veterans Affairs Rehabilitation R&D Service Research Career Scientist Award (MTP), and an unrestricted departmental grant and a Senior Scientific Investigator Award from the Research to Prevent Blindness (PMI).
Disclosure: H.N. Park, None; S.B. Jabbar, None; C.C. Tan, None; C.S. Sidhu, None; J. Abey, None; F. Aseem, None; G. Schmid, None; P.M. Iuvone, None; M.T. Pardue, None
References
- 1. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987; 237: 73–77 [DOI] [PubMed] [Google Scholar]
- 2. Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Invest Ophthalmol Vis Sci. 1984; 25: 652–659 [PubMed] [Google Scholar]
- 3. Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997; 37: 659–668 [DOI] [PubMed] [Google Scholar]
- 4. Smith EL III, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009; 50: 5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987; 6: 993–999 [DOI] [PubMed] [Google Scholar]
- 6. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35: 1175–1194 [DOI] [PubMed] [Google Scholar]
- 7. Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994; 11: 143–153 [DOI] [PubMed] [Google Scholar]
- 8. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468 [DOI] [PubMed] [Google Scholar]
- 9. McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res. 1995; 35: 1141–1152 [DOI] [PubMed] [Google Scholar]
- 10. Choh V, Lew MY, Nadel MW, Wildsoet CF. Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res. 2006; 46: 1070–1079 [DOI] [PubMed] [Google Scholar]
- 11. Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003; 27: 371–385 [DOI] [PubMed] [Google Scholar]
- 12. Nevin ST, Schmid KL, Wildsoet CF. Sharp vision: a prerequisite for compensation to myopic defocus in the chick? Curr Eye Res. 1998; 17: 322–331 [DOI] [PubMed] [Google Scholar]
- 13. Huang J, Hung LF, Smith EL., III. Effects of foveal ablation on the pattern of peripheral refractive errors in normal and form-deprived infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2011; 52: 6428–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith EL, III., Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3914–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EL, III., Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009; 49: 2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crewther DP. The role of photoreceptors in the control of refractive state. Prog Retin Eye Res. 2000; 19: 421–457 [DOI] [PubMed] [Google Scholar]
- 17. Schmucker C, Seeliger M, Humphries P, Biel M, Schaeffel F. Grating acuity at different luminances in wild-type mice and in mice lacking rod or cone function. Invest Ophthalmol Vis Sci. 2005; 46: 398–407 [DOI] [PubMed] [Google Scholar]
- 18. Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986; 104: 1013–1020 [DOI] [PubMed] [Google Scholar]
- 19. Pras E, Abu A, Rotenstreich Y, et al. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis. 2009; 15: 1709–1716 [PMC free article] [PubMed] [Google Scholar]
- 20. Smith M, Whittock N, Searle A, Croft M, Brewer C, Cole M. Phenotype of autosomal dominant cone-rod dystrophy due to the R838C mutation of the GUCY2D gene encoding retinal guanylate cyclase-1. Eye (Lond). 2007; 21: 1220–1225 [DOI] [PubMed] [Google Scholar]
- 21. Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. Br J Ophthalmol. 1978; 62: 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998; 39: 2443–2449 [PubMed] [Google Scholar]
- 23. Park H, Tan CC, Faulkner A, et al. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis. 2013; 19: 2068–2079 [PMC free article] [PubMed] [Google Scholar]
- 24. Chui TY, Bissig D, Berkowitz BA, Akula JD. Refractive development in the “ROP rat.” J Ophthalmol. 2012; 2012: 956705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007; 48: 4351–4359 [DOI] [PubMed] [Google Scholar]
- 26. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119 [DOI] [PubMed] [Google Scholar]
- 27. Voigt T, Wassle H. Dopaminergic innervation of A II amacrine cells in mammalian retina. J Neurosci. 1987; 7: 4115–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boatright JH, Gordon JR, Iuvone PM. Inhibition of endogenous dopamine release in amphibian retina by L-2-amino-4-phosphonobutyric acid (L-AP4) and trans-2-aminocyclopentane-1,3-dicarboxylate (ACPD). Brain Res. 1994; 649: 339–342 [DOI] [PubMed] [Google Scholar]
- 29. Boelen MK, Boelen MG, Marshak DW. Light-stimulated release of dopamine from the primate retina is blocked by 1-2-amino-4-phosphonobutyric acid (APB). Vis Neurosci. 1998; 15: 97–103 [DOI] [PubMed] [Google Scholar]
- 30. Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009; 517: 226–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000; 870: 118–125 [DOI] [PubMed] [Google Scholar]
- 32. Newkirk GS, Hoon M, Wong RO, Detwiler PB. Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. J Neurophysiol. 2013; 110: 536–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, Lucas RJ. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci. 2009; 29: 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008; 105: 14181–14186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res. 2011; 92: 40–46 [DOI] [PubMed] [Google Scholar]
- 36. Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103: 33–40 [DOI] [PubMed] [Google Scholar]
- 37. Megaw PL, Boelen MG, Morgan IG, Boelen MK. Diurnal patterns of dopamine release in chicken retina. Neurochem Int. 2006; 48: 17–23 [DOI] [PubMed] [Google Scholar]
- 38. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004; 108: 17–40 [DOI] [PubMed] [Google Scholar]
- 39. Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013; 114: 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou X, Ji F, An J, et al. Experimental murine myopia induces collagen type Ialpha1 (COL1A1) DNA methylation and altered COL1A1 messenger RNA expression in sclera. Mol Vis. 2012; 18: 1312–1324 [PMC free article] [PubMed] [Google Scholar]
- 41. Faulkner AE, Kim KK, Iuvone PM, Pardue MT. Head-mounted goggles for murine form deprivation myopia. J Neurosci Methods. 2007; 161: 96–100 [DOI] [PubMed] [Google Scholar]
- 42. Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004; 81: 99–110 [DOI] [PubMed] [Google Scholar]
- 43. Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008; 48: 904–916 [DOI] [PubMed] [Google Scholar]
- 44. Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003; 44: 32–36 [DOI] [PubMed] [Google Scholar]
- 45. Qian YS, Chu RY, Hu M, Hoffman MR. Sonic hedgehog expression and its role in form-deprivation myopia in mice. Curr Eye Res. 2009; 34: 623–635 [DOI] [PubMed] [Google Scholar]
- 46. Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010; 51: 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brand C, Burkhardt E, Schaeffel F, Choi JW, Feldkaemper MP. Regulation of Egr-1, VIP, and Shh mRNA and Egr-1 protein in the mouse retina by light and image quality. Mol Vis. 2005; 11: 309–320 [PubMed] [Google Scholar]
- 48. Brand C, Schaeffel F, Feldkaemper MP. A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Mol Vis. 2007; 13: 920–932 [PMC free article] [PubMed] [Google Scholar]
- 49. Schippert R, Burkhardt E, Feldkaemper M, Schaeffel F. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007; 48: 11–17 [DOI] [PubMed] [Google Scholar]
- 50. Barathi VA, Kwan JL, Tan QS, et al. Muscarinic cholinergic receptor (M2) plays a crucial role in the development of myopia in mice. Dis Model Mech. 2013; 6: 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou X, Huang Q, An J, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010; 51: 4362–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou G, Strom RC, Giguere V, Williams RW. Modulation of retinal cell populations and eye size in retinoic acid receptor knockout mice. Mol Vis. 2001; 7: 253–260 [PubMed] [Google Scholar]
- 53. Huang F, Yan T, Shi F, et al. Activation of dopamine D2 receptor is critical for the development of form deprivation myopia in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2014; 55: 5537–5544 [DOI] [PubMed] [Google Scholar]
- 54. Calvert PD, Krasnoperova NV, Lyubarsky AL, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci U S A. 2000; 97: 13913–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mattapallil MJ, Wawrousek EF, Chan CC, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aleman TS, Cideciyan AV, Aguirre GK, et al. Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model. Invest Ophthalmol Vis Sci. 2011; 52: 6898–6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pardue MT, Faulkner AE, Fernandes A, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008; 49: 706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park H, Qazi Y, Tan C, et al. Assessment of axial length measurements in mouse eyes. Optom Vis Sci. 2012; 89: 296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmid GF, Papastergiou GI, Nickla DL, et al. Validation of laser Doppler interferometric measurements in vivo of axial eye length and thickness of fundus layers in chicks. Curr Eye Res. 1996; 15: 691–696 [DOI] [PubMed] [Google Scholar]
- 60. Turner PV, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005; 55: 175–182 [PubMed] [Google Scholar]
- 61. Ritchey ER, Zelinka C, Tang J, et al. Vision-guided ocular growth in a mutant chicken model with diminished visual acuity. Exp Eye Res. 2012; 102: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stats Soc Series B Stat Methodol. 1995; 57: 289–300 [Google Scholar]
- 63. Siegwart JT Jr, Norton TT. Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optom Vis Sci. 2011; 88: E365–E372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Norton TT, Siegwart JT Jr. Animal models of emmetropization: matching axial length to the focal plane. J Am Optom Assoc. 1995; 66: 405–414 [PubMed] [Google Scholar]
- 65. Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004; 44: 1857–1867 [DOI] [PubMed] [Google Scholar]
- 66. Wisard J, Faulkner A, Chrenek MA, et al. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011; 52: 5804–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou X, Shen M, Xie J, et al. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008; 49: 5208–5214 [DOI] [PubMed] [Google Scholar]
- 68. Yu Y, Chen H, Tuo J, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011; 46: 80–87 [DOI] [PubMed] [Google Scholar]
- 69. Zhou X, An J, Wu X, et al. Relative axial myopia induced by prolonged light exposure in C57BL/6 mice. Photochem Photobiol. 2010; 86: 131–137 [DOI] [PubMed] [Google Scholar]
- 70. Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013; 114: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Allen AE, Cameron MA, Brown TM, Vugler AA, Lucas RJ. Visual responses in mice lacking critical components of all known retinal phototransduction cascades. PLoS One. 2010; 5: e15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009; 10: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reis RA, Ventura AL, Kubrusly RC, de Mello MC, de Mello FG. Dopaminergic signaling in the developing retina. Brain Res Rev. 2007; 54: 181–188 [DOI] [PubMed] [Google Scholar]
- 74. Kubrusly RC, Guimaraes MZ, Vieira AP, et al. L-DOPA supply to the neuro retina activates dopaminergic communication at the early stages of embryonic development. J Neurochem. 2003; 86: 45–54 [DOI] [PubMed] [Google Scholar]
- 75. Klitten LL, Rath MF, Coon SL, Kim JS, Klein DC, Moller M. Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Exp Eye Res. 2008; 87: 471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Witkovsky P, Arango-Gonzalez B, Haycock JW, Kohler K. Rat retinal dopaminergic neurons: differential maturation of somatodendritic and axonal compartments. J Comp Neurol. 2005; 481: 352–362 [DOI] [PubMed] [Google Scholar]
- 77. Roffler-Tarlov S, Liu JH, Naumova EN, Bernal-Ayala MM, Mason CA. L-Dopa and the albino riddle: content of L-Dopa in the developing retina of pigmented and albino mice. PLoS One. 2013; 8: e57184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci. 1997; 14: 493–505 [DOI] [PubMed] [Google Scholar]
- 79. Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP, Pugh EN Jr. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010; 30: 12495–12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Altimus CM, Guler AD, Alam NM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010; 13: 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cachafeiro M, Bemelmans AP, Canola K, et al. Remaining rod activity mediates visual behavior in adult Rpe65-/- mice. Invest Ophthalmol Vis Sci. 2010; 51: 6835–6842 [DOI] [PubMed] [Google Scholar]
- 82. Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005; 46: 2238–2241 [DOI] [PubMed] [Google Scholar]