Abstract
Glucocorticoids (Gc) are potent anti-inflammatory agents with wide clinical application. We have previously shown that increased serum concentration significantly attenuates regulation of a simple Gc-responsive reporter. We now find that glucocorticoid receptor (GR) regulation of some endogenous transactivated but not transrepressed genes is impaired, suggesting template specificity. Serum did not directly affect GR expression, activity or trafficking, implicating GR crosstalk with other signalling pathways. Indeed, a JNK inhibitor completely abolished the serum effect. We identified the Gc modulating serum component as cholesterol. Cholesterol loading mimicked the serum effect, which was readily reversed by JNK inhibition. Chelation of serum cholesterol with methyl-β-cyclodextrin or inhibition of cellular cholesterol synthesis with simvastatin potentiated the Gc response. To explore the effect in vivo we used ApoE −/− mice, a model of hypercholesterolaemia. Consistent with our in vitro studies, we find no impact of elevated cholesterol on the expression of GR, or on the hypothalamic–pituitary–adrenal axis, measured by dexamethasone suppression test. Instead we find selective Gc resistance on some hepatic target genes in ApoE −/− mice. Therefore, we have discovered an unexpected role for cholesterol as a selective modulator of Gc action in vivo. Taken together these findings reveal a new environmental constraint on Gc action with relevance to both inflammation and cancer.
Keywords: glucocorticoid receptor, inflammatory disease, cholesterol, transcription factors, signal transduction
Introduction
Glucocorticoids (Gc) are potent anti-inflammatory agents and are widely prescribed for the treatment of a range of inflammatory and immune diseases including rheumatoid arthritis and asthma (McMaster & Ray 2007, Barnes & Adcock 2009, De et al. 2011). In humans cortisol is the principal circulating Gc, but additional modulatory molecules are present including cortisone and the 5α-reduced cortisol metabolites (McMaster et al. 2008, Nixon et al. 2012).
Natural and synthetic Gc molecules modulate the activity of the near ubiquitously expressed glucocorticoid receptor (GR). The GR is a member of the nuclear hormone receptor superfamily and acts as a ligand-inducible transcription factor by interacting with chromatin to regulate gene transcription (Ito et al. 2006, Schacke et al. 2007, Sommer & Ray 2008). Selection of GR binding sites across the genome is dependent on cell-type specific chromatin structure and activity of comodulator proteins which regulate the accessibility of GR to target DNA (Herrera et al. 1989, Biddie et al. 2011, John et al. 2011).
Patients' responses to Gc treatment may vary, which is a major factor that limits the therapeutic utility of Gc. Many human disorders are affected by Gc signalling; therefore, there is great interest in defining mechanisms underlying how local tissue Gc sensitivity is being regulated (Yang et al. 2012). Amongst the best-characterised alterations in local Gc sensitivity is the acquired resistance that occurs at foci of inflammation, which is attributed to multiple pathways including cytokine action and activation of transcription factors including nuclear factor kappa B (NFκB) and activator protein 1 (AP1) (Yang et al. 2012).
While developing a sensitive Gc bioassay, we identified a striking and unexplained effect of serum which reduced the ability of GR to transactivate a simple reporter gene (Perogamvros et al. 2011, 2012). Serum contains a complex mixture of nutritional and signalling components, as well as classical hormones. The powerful effect of serum components on transcriptional responses have been known for a number of years, primarily through the activation of serum response factor (SRF) acting at AP1 elements (Treisman 1986, Shaw et al. 1989). More recently, a blood-borne signal with powerful activity to reset cellular circadian oscillators was discovered. The circulating factor in this case was thought to be a polypeptide, as yet unidentified, but one capable of acting through serum-response factor (Gerber et al. 2013).
Herein, we define the role of serum components on regulating Gc sensitivity. We identify cholesterol as a critical Gc regulatory serum component, and also that activation of JNKs and AP1 is essential for mediating the cholesterol effect. We further show that modulating cellular cholesterol metabolism using statins increases cellular sensitivity to Gc action. Given that membrane cholesterol release in response to cell necrosis is found both in cancers and at sites of inflammation, this provides a new mechanism to explain how a local environmental signal can selectively modulate the Gc response.
Materials and methods
Dexamethasone (Dex), water soluble cholesterol and methyl-β-cyclodextrin were purchased from Sigma. The following antibodies were used: anti-GR (mouse clone 41, BD Biosciences, San Jose, CA, USA); anti-(s211)phospho-GR, anti-JNK and anti-phospho JNK, from Cell Signalling Technology (Beverly, MA, USA); anti-caveolin-1 from Santa Cruz; anti-α-tubulin from Sigma; anti-β-actin from Abcam (Cambridge, UK); and HRP-conjugated anti-mouse and anti-rabbit from GE Healthcare (Little Chalfont, UK). Fluorophore-conjugated (Alexa Fluor 546 and 488) anti-mouse and anti-rabbit antibodies were Invitrogen.
Human WT GRα expression vector and pMMTV-Luc have been described before (Ray et al. 1996). TAT3-Luc (Iniguez-Lluhi et al. 1997) was a kind gift from K Yamamoto (University of California San Francisco, CA, USA). The pSG5–steroid receptor coactivator 1 (SRC1) expression vector (Chen et al. 1999) was the kind gift of M Stallcup (University of Southern California, CA, USA). interleukin 6 (IL6)-Luc reporters were obtained from the BCCM plasmid collection (Zwijnaarde, Belgium). Expression vectors of MKK7 activated JNK and ERK (Tsuruta et al. 2004) were kind gifts from A Sharrocks (University of Manchester). pcDNA5 (Invitrogen) was used as an empty control. CMV-Renilla (Promega) was used to correct for transfection efficiency.
Animals
ApoE −/− mice have been described previously (Plump et al. 1992). Transgenic and WT (C57BL/6) mice were routinely housed in 12 h light:12 h darkness (L:D) cycles with ad libitum access to food and water in a pathogen-free animal facility at the University of Manchester. All experiments were carried out in strict accordance with the Animals (Scientific Procedures) act 1986.
Serum samples were collected from age matched (12–18 weeks) female WT (C57BL/6) and ApoE −/− mice at morning (0700 h) and evening (1900 h) from the sequential tail vein bleeds. The following day (1500 h), animals were weighed and injected with either Dex (1 mg/kg i.p.) or vehicle control. Four hours later (to coincide with peak corticosterone levels), mice were killed (pentobarbital i.p.) and trunk blood was collected for the assessment of serum lipid and corticosterone levels. The livers were snap frozen for protein and RNA analysis.
Cell culture
Human epithelial carcinoma (HeLa) cells were obtained from the European Collection of Cell Cultures (Salisbury, UK) and maintained in DMEM supplemented with GlutaMAX I and 10% fetal bovine serum (FBS, Invitrogen), 10% charcoal stripped FBS (CSS, Gibco), or lipoprotein deficient FBS (LDS, Sigma) in a humidified atmosphere of 5% CO2 at 37 °C.
Reporter gene assay
1×106 cells were transfected with 2 μg of firefly luciferase reporter and 0.5 μg of CMV-Renilla luciferase using FuGENE 6 (Roche Diagnostics). After 24 h, the cells were pooled and transferred to 24 well plates, then treated before lysis, and assay for dual luciferase activity according to the manufacturer's instructions (Promega). Some experiments also required cotransfection with an expression plasmid. For these studies, 1 μg expression plasmid (SRC1, ERK, or JNK) was included in the reporter gene transfection mix. Where appropriate, total transfected DNA was maintained by including an empty vector control, pcDNA5. To control for transfection efficiency, firefly luciferase counts were normalised with CMV-driven Renilla luciferase. Dose–response curves were generated using GraphPad Prism (La Jolla, CA, USA).
Quantitative RT (RT-PCR)
Total RNA was prepared from HeLa cells using the RNeasy Mini Kit with on column DNase I digestion (Qiagen), and from mouse livers using SV Total RNA Isolation System (Promega). cDNA was synthesised using a High Capacity RNA-to-cDNA Kit and analysed using Power SYBR Green PCR Master Mix (Applied Biosystems). A group of Gc-target genes were selected from our previous microarray expression studies (Donn et al. 2007). Quantitative RT-PCR primer sequences are provided as supplementary information (Supplementary Tables 1 and 2, see section on supplementary data given at the end of this article). Expression levels were calculated using the comparative C t method, normalising with the GAPDH or β-actin control, as indicated in the results.
Immunoblot analysis
HeLa cells and murine liver tissue (in FastPrep-24 lysing matrix tubes; MP Biomedicals, Santa Ana, CA, USA) were lysed using radio-immunoprecipitation assay buffer (50 mM Tris–HCl, pH 7.4, 1% NP40, 0.25% Na-deoxycholate, 150 mM NaCl, and 1 mM EDTA) containing protease (Calbiochem, San Diego, CA, USA) and phosphatase inhibitors (Sigma–Aldrich Corp.). The proteins were separated by SDS gel electrophoresis and transferred to 0.2 μM nitrocellulose membranes (Bio-Rad Laboratories) overnight at 4 °C. The membranes were blocked for 6 h (0.15 M NaCl, 1% milk, and 0.1% Tween 20) and incubated with primary antibodies (diluted in blocking buffer) overnight. Following three washes (88 mM Tris, pH 7.8, 0.25% dried milk, and 0.1% Tween 20), membranes were incubated with a species-specific HRP-conjugated secondary antibody (in wash buffer) for 1 h at room temperature and washed a further three times, each for 10 min. Immunoreactive proteins were visualised using ECL Advance (GE Healthcare).
Immunofluorescence
The cells were fixed with 4% paraformaldehyde for 20 min at room temperature and permeabilised (0.25% Triton X-100 in PBS) for 5 min at room temperature. The cells were blocked (3% goat serum and 0.1% Triton X-100 in PBS) for 30 min and then in primary antibody (diluted in blocking buffer) overnight at 4 °C. After three 5 min washes in PBS, cells were incubated in the secondary antibody for 2 h. Following three further 5 min washes, coverslips were mounted using Vectasheild hard-set mounting compound containing the nuclear DAPI stain (Vector Laboratories, Burlingame, MA, USA). The images were acquired on a DeltaVision RT (Applied Precision, Issauah, WA, USA) restoration microscope using a ×60/1.42 Plan Apo objective and the Sedat filter set (Chroma 89000; Chroma Technology Corp., Rockingham, VT, USA). The images were collected using a CoolSNAP HQ (Photometrics, Tuscon, AZ, USA) camera with a Z optical spacing of 0.5 μm. The images were deconvolved using Softworx Software (GE Healthcare, Issaquah, WA, USA) and maximum intensity projections of images processed using Image J (http://imagej.nih.gov/ij/; National Institute of Health, Bethesda, MD, USA).
Live cell imaging
Transfection was performed by seeding 1×106 HeLa cells (Fugene 6) with 5 μg hGR–GFP. After 24 h, the cells were transferred to glass bottomed 24 well plates. They were maintained at 37 °C and 5% CO2 for the duration of data collection. The images were acquired on a Nikon TE2000 PFS microscope using a 60×/1.40 Plan Apo objective and the Sedat filter set (Chroma 89000) and images collected using a Cascade II EMCCD camera (Photometrics). Raw time series images were processed using Image J.
Fluorescent recovery after photobleaching
Transfection was performed by seeding 1×106 HeLa cells (Fugene 6) with 5 μg hGR–GFP overnight then transferred to glass bottomed 24 well plates. They were maintained at 37 °C and 5% CO2 for the duration of data collection. The images were collected on a Leica TCS SP5 AOBS inverted confocal using a 63×/0.50 Plan Fluotar objective and 7× confocal zoom. The confocal settings were as follows, pinhole 1 airy unit, scan speed 1000 Hz unidirectional and format 1024×1024. The images were collected using the following detection mirror settings; FITC 494–530 nm using the 488 nm (13%). Raw time series images were processed using Image J.
Proliferation assay
The levels of proliferation were measured using Cell Titer 96 AQueous One Solution Cell Proliferation Assay (MTS, Promega) as per instructions provided in the kit.
Corticosterone measurements
Corticosterone in serum samples was measured using enzyme immunoassay (ENZO Life Sciences Ltd, Farmingdale, NY, USA) as per manufacturer's instructions. All serum samples were diluted 1:40 and assayed individually due to the low volumes obtained. A standard (five-point, 20 000 pg/ml max) curve comprising fivefold serial dilutions was generated alongside test samples.
Statistical analysis
Data were expressed as average±s.e.m. (of at least three independent triplicate experiments) and compared using SPSS Software (version 16, SPSS, Inc.). For more than two groups, means were compared by one-way ANOVA followed by Bonferroni's post hoc test, and for comparison of two groups a Student's t-test for independent samples or Mann–Whitney was used. P<0.05 was considered statistically significant.
Results
Serum impairs Gc action through a post-receptor mechanism
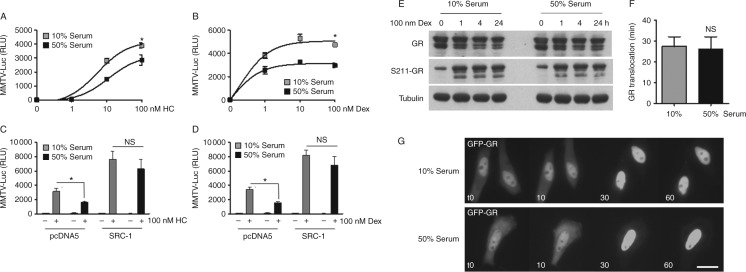
GR is a ligand-activated transcription factor. After nuclear translocation, ligand-bound GR dimerises and binds directly to DNA sequences to activate target gene expression. To study serum regulation of Gc action, synthetic reporter assays were performed using the GR-responsive reporter gene MMTV-Luc. Higher serum concentration (50%) impaired GR transactivation of MMTV-Luc in response to treatment with either Dex or hydrocortisone, demonstrable by a markedly decreased maximum response (reduced efficacy) (Fig. 1A and B). Overexpression of SRC1, which binds the GR to potentiate GR transactivation, rescued GR transactivation, suggesting a reversible model of impaired Gc action (Fig. 1C and D).
Figure 1.
Serum factors induce Gc resistance. HeLa cells were transiently transfected with MMTV-Luc, Renilla-Luc and GR (A and B) together with pcDNA5 or SRC1 expression vectors (C and D). Following overnight culture in either 10 or 50% serum, cells were treated with hydrocortisone (HC) (A and C) or dexamethasone (Dex, 0–100 nM) (B and D) for 16 h before luciferase assay. Cells were cultured in either 10 or 50% serum, treated with 100 nM Dex for up to 24 h before immunoblot for GR and PS211-GR (E). Tubulin was used as a loading control. Cells were transfected with GR–GFP and then cultured in either 10 or 50% serum overnight. Cells were treated with 100 nM Dex and imaged in real time. Images were acquired every 5 min. Average time for GR nuclear translocation in either 10 or 50% serum was calculated and presented in (F). Representative images are shown (G). Scale bar, 50 μM. Experiments were performed in triplicate and repeated three times. Graphs show mean±s.e.m. *P<0.05.
To determine whether the serum effect was a consequence of modulating GR expression or phosphorylation, immunoblot analysis was performed using global and phospho state-specific antibodies. In response to Dex treatment, steady state GR expression decreases, together with an increase in GRS211 phosphorylation, evident by 1 h (Fig. 1E). Culture in 50% serum did not affect either the expression or phosphorylation of the GR. Serum concentration also did not alter cell morphology, steady-state GR localisation or Dex-induced nuclear translocation (Supplementary Fig. 1, see section on supplementary data given at the end of this article). To investigate the rate of GR subcellular trafficking with better temporal resolution, live cell imaging was also completed. Serum had no effect on the rate of Dex-induced GR nuclear translocation (Fig. 1F and G). FRAP studies also identified no serum effects on GR intranuclear mobility either unliganded or after Dex treatment (Supplementary Fig. 2). Collectively, these studies suggest that the serum effect is not due to a direct regulation of GR expression, modification, or subcellular trafficking.
Serum selectively and reversibly impairs GR transactivation of endogenous gene expression
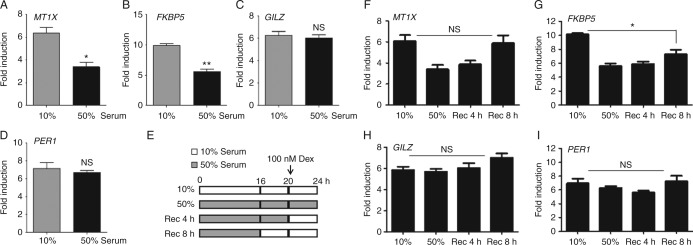
QRT-PCR was used to explore serum regulation of four well-characterised GR transactivated genes, metallothionein 1X (MT1X), FK506 binding protein 5 (FKBP5), Gc-induced leucine zipper (GILZ, TSC22D3), and period circadian protein homolog 1 (PER1). Following 4 h Dex treatment, all four genes were robustly induced: 50% serum impaired transactivation of both FKBP5 and MT1X (Fig. 2A and B) whereas expression of GILZ and PER1 was comparable in 10% and 50% serum (Fig. 2C and D). There were no serum effects on baseline gene expression or cell proliferation (Supplementary Fig. 3, see section on supplementary data given at the end of this article), suggesting that serum selectively impairs Gc-induced GR activity.
Figure 2.
Serum impairs GR transactivation in a reversible manner. HeLa cells were cultured in either 10 or 50% serum overnight, then treated with 100 nM Dex for 4 h before RNA purification. Transcripts of four well-characterised GR transactivated endogenous genes FK506 binding protein 5 (FKBP5), metallothionein 1X (MT1X), glucocorticoid-induced leucine zipper (GILZ), and period circadian protein homolog 1 (PER1) were measured by qRT-PCR (all normalised to GAPDH) (A, B, C and D). Cells were either continually cultured in either 10 or 50% serum, or cultured in 50% serum then returned to 10% serum as shown in (E) before treatment with 100 nM Dex for 4 h and transcript analysis for MT1X (F), FKBP5 (G), GILZ (H), and PER1 (I). Experiments were performed in triplicate and repeated three times. Graphs show mean±s.e.m. *P<0.01 and **P<0.001. NS, not significant.
Further qRT-PCR assays were completed to investigate whether the impairment of GR transactivation was recovered following return to culture in 10% serum (Fig. 2E). Impairment of MT1X transactivation was rapidly reversed (Fig. 2F), whereas FKBP5 regulation was not recovered even after 8 h (Fig. 2G). This suggests the existence of different mechanisms regulating variation in Gc response, acting in a template-dependent manner.
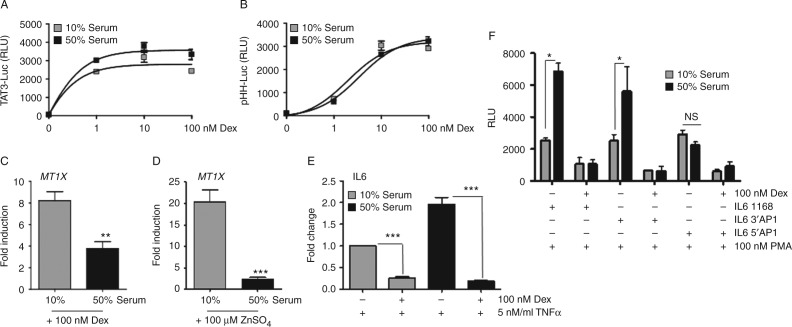
To define further response element specificity of serum action, two simple Gc response element (GRE)-reporter genes were examined. Serum had no effect on either the pTAT3-Luc, which contains tandem repeats of a simple, consensus GRE (Fig. 3A) or pHH-Luc, which is a truncated MMTV promoter containing just the GR binding elements (Fig. 3B). These findings suggest that target elements may be less important than the flanking, contextual DNA around the GR binding site for mediating the serum effect.
Figure 3.
Serum increases GR transrepression of IL6 through AP1 elements. HeLa cells were transiently transfected with TAT3-Luc (A) or pHH-Luc (B), together with Renilla-Luc and GR. 24 h later, cells were transferred to culture in either 10 or 50% serum then treated with Dex for 16 h before luciferase assay. HeLa cells were cultured in either 10 or 50% serum overnight, and then treated with either 100 nM Dex (C) or 100 μM ZnSO4 (D) for 4 h before RNA purification and MT1X transcript measured by qRT-PCR (normalised to GAPDH). Cells were cultured in 10 or 50% serum overnight then treated with 5 ng/ml TNF for 1 h and Dex for a further 4 h. RNA was extracted and IL6 transcript measured by qRT-PCR (normalised to GAPDH) (E). HeLa cells were transiently transfected with WT or AP1 deletant IL6-Luc plasmids then cultured in 10 or 50% serum. Cells were treated with 100 nM PMA and 100 nM Dex for 16 h and then lysed for luciferase assay (F). Graphs show mean±s.e.m. Experiments were carried out in triplicate and repeated three times. *P<0.05, **P<0.01, and ***P<0.001. NS, not significant.
As expression of MT1X is transactivated by Zn ions as well as Gc, the serum effect of Zn transactivation on MT1X was also determined. The cells cultured in 10% serum had an eightfold induction of MT1X after treatment with Dex, and a 17-fold induction in response to ZnSO4 treatment. However, both of these robust transcriptional effects were dramatically decreased following culture with 50% serum, with only three- and twofold induction of MT1X respectively (Fig. 3C and D). This further suggests that the response element contextual DNA is more important for the serum effect than the GR, or the GR binding site itself, a conclusion strengthened by observing that GR repression of IL6 (Fig. 3E), MIF-Luc and IL8 (Supplementary Fig. 4, see section on supplementary data given at the end of this article) were in fact augmented by culture in 50% serum. To investigate Gc repression of the IL6 gene in more depth, additional reporter assays were completed using a series of human IL6 luciferase constructs. Consistent with regulation of the endogenous gene, Gc repression of IL6-Luc was increased in 50% serum. Interestingly, this augmented Gc repression was lost when the 5′AP1-binding site was deleted (Fig. 3F), implicating AP1 action as part of the mechanism.
Serum regulates Gc action through MKK7-activated JNK
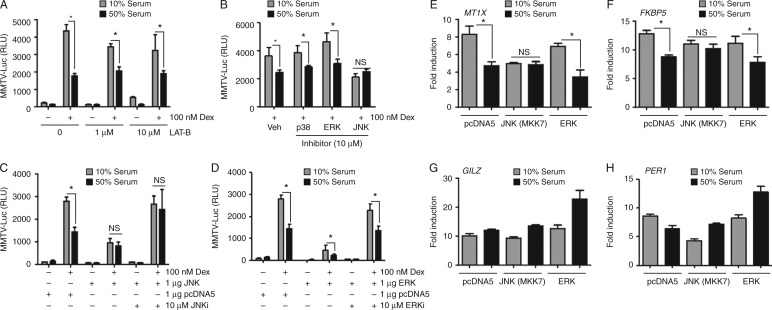
To delineate the signalling mechanism responsible for modulating the Gc response, a series of reporter gene studies were performed in conjunction with small molecule inhibitors. First, Gc-activated expression of MMTV reporter gene was measured following treatment with Latrunculin-B, a drug that sequesters G-actin and inhibits activation of SRF (Gineitis & Treisman 2001, Gerber et al. 2013). There was no effect on the serum induction of Gc resistance, suggesting a mechanism independent of SRF activation (Fig. 4A). To explore the role of MAP kinases, which activate AP1, in mediating repression of GR transactivation, cells were treated with three MAPK inhibitors. Inhibition of JNK reversed the inhibitory effect serum, whereas inhibition of either ERKs or p38 had no impact (Fig. 4B). To further specify the role of JNK, cells were transfected with either JNK or ERK expression vectors. Hyperactivation of JNK signalling attenuated GR transactivation and mimicked the serum effect, such that culture in 50% serum had no additional effect. Sensitivity to Gc could be restored by co-treatment with JNK inhibitor (Fig. 4C). Although overexpression of ERK alone also impaired Gc action, an inhibitory effect of serum was still observed suggesting coincident activation of parallel unrelated pathways (Fig. 4D).
Figure 4.
Serum impairs Gc action through activation of JNK. HeLa cells were transiently transfected with MMTV-Luc and GR overnight and then cultured in 10 or 50% serum. The cells were treated with Latrunculin B (A) or MAP kinase inhibitors (B) (ERK PD98059, p38 SB203580, and JNK SP600125) together with 100 nM Dex for 6 h before luciferase assay. The cells were transiently transfected with MMTV-Luc and GR, together with JNK (C) or ERK (D) expression vectors (or pcDNA5 as a control) overnight. The cells were transferred to 10 or 50% serum, treated with 100 nM Dex and 10 μM JNK inhibitor (SP600125) or ERK inhibitor (PD98059) for 6 h and then assayed for luciferase activity. HeLa cells were transfected with JNK or ERK expression vectors (or pcDNA5 as a control), cultured in 10 or 50% serum, and then treated with 100 nM Dex for 4 h before RNA extraction. Expression of MT1X (E), FKBP5 (F), GILZ (G), and PER1 (H) transcripts were measured by qRT-PCR (all normalised to GAPDH). The experiments were carried out in triplicate and repeated three times. Graphs show mean±s.e.m. *P<0.05. NS, not significant.
To define the role of JNK on endogenous gene regulation, again cells were transfected with activated JNK and then analysed by qRT-PCR. As expected both MT1X and FKBP5 were affected by serum concentration, in a JNK-dependent manner (Fig. 4E and F) and, induction of PER1 or GILZ expression by Dex was not affected by serum or activation of JNK (Fig. 4G and H).
Lipid components within serum impair Gc action
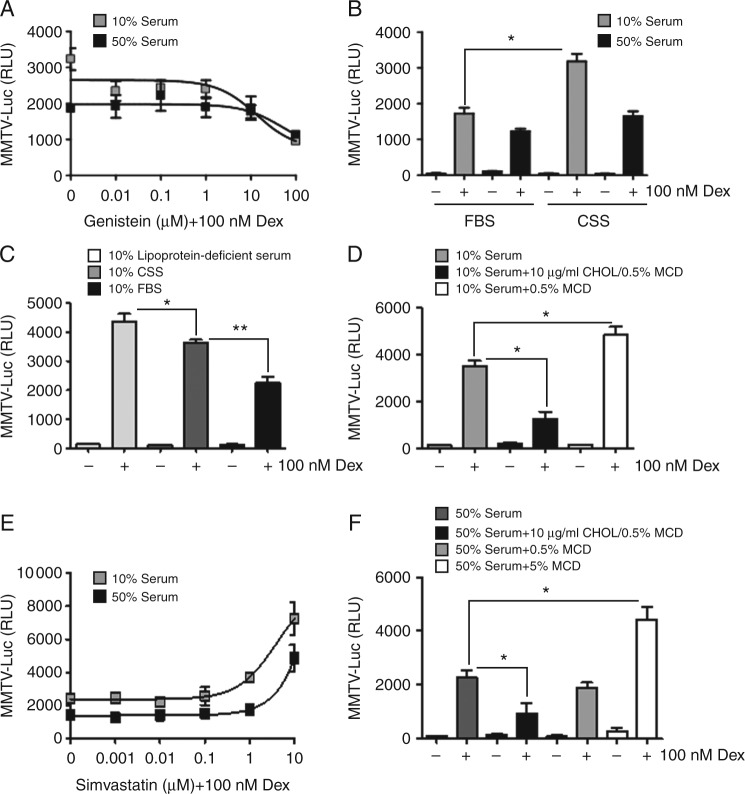
We show that culture in 50% serum selectively impairs GR transactivation through modulation of JNK, and sought to identify the component in serum responsible for mediating the effect. Serum contains many growth factors that signal through tyrosine kinase pathways. Treatment with genistein, a general inhibitor of tyrosine kinases, had no effect on serum induction of Gc resistance (Fig. 5A). In addition neither heat treatment, nor trypsin digestion of serum impaired the inhibitory effect of serum on GR action (Supplementary Fig. 5, see section on supplementary data given at the end of this article). In search of serum metabolites capable of regulating Gc function, activated charcoal was used to ‘strip’ small molecules. Culture in 10% charcoal stripped (delipidated) serum-permitted enhanced GR transactivation at 10%, and had no effect at 50% compared with full serum (Fig. 5B).
Figure 5.
Modulation of cellular cholesterol regulates Gc effects. HeLa cells were transiently transfected with MMTV-Luc reporter gene and GR then transferred to 10 or 50% serum overnight. Cells were treated with Genistein (as indicated) for 4 h, and then treated with 100 nM Dex for a further 4 h before lysis and luciferase assay (A). Cells were also cultured in either normal serum, charcoal stripped serum or lipoprotein-deficient serum (B and C), and then treated with 100 nM Dex for 16 h before luciferase assay. Cells were cultured in 10% serum with 10 μg/ml cholesterol (CHOL) complexed with 0.5% methyl-β-cyclodextrin (MCD) or with 0.5% MCD alone (D). Cells transfected with MMTV-Luc and GR, cultured in 10 or 50% serum were treated with Simvastatin (as indicated) together with 100 nM Dex for 16 h before luciferase assay (E). Cells were cultured in 50% serum, together with cholesterol complexed with 0.5% MCD, 0.5%, or 5% MCD alone, then treated with 100 nM Dex for 16 h before luciferase assay (F). Experiments were carried out in triplicate and repeated three times. Graphs show mean±s.e.m. *P<0.05 and **P<0.01.
To further analyse the possible role of serum lipids in the regulation of Gc action, lipoprotein-deficient serum (containing <5% of normal lipoprotein) was also used. Culture in the reduced lipoprotein serum further increased the Gc response, implicating serum lipids in negatively regulating Gc responses (Fig. 5C). To pursue a possible role for cholesterol, cells were loaded with exogenous cholesterol (25 μmol/l, in a water-soluble complex with 0.5% methyl-β-cyclodextrin), or with 0.5% methyl-β-cyclodextrin alone (Fig. 5D). Cholesterol loading decreased GR transactivation, and treatment with methyl-β-cyclodextrin increased Gc action. Treatment with Simvastatin, which inhibits cholesterol synthesis, also potentiated Gc transactivation in either 10 or 50% serum (Fig. 5E). Importantly, methyl-β-cyclodextrin increased GR action in a concentration-dependent manner, where a higher concentration of methyl-β-cyclodextrin (5%) was necessary to quench the higher concentration of cholesterol present in 50% serum (Fig. 5F). The same high concentration of methyl-β-cyclodextrin was toxic to cells cultured in 10% serum overnight, presumably a consequence of quenching available serum cholesterol and also extracting cholesterol from cell membranes and inducing apoptosis. Importantly, we found only modest effects of Simvastatin, cholesterol or methyl-β-cyclodextrin treatment on cell proliferation or viability, excluding a possible cell cycle-dependent effect (Supplementary Fig. 6A, see section on supplementary data given at the end of this article). We also demonstrate that treatment with 5% methyl-β-cyclodextrin, which is able to bind Dex, does not alter the onset of GR translocation thereby excluding any direct effect of methyl-β-cyclodextrin on uptake of Dex by the cells or on the timing of GR activation (Supplementary Fig. 6B).
Cholesterol selectively impairs GR transactivation through activation of JNK
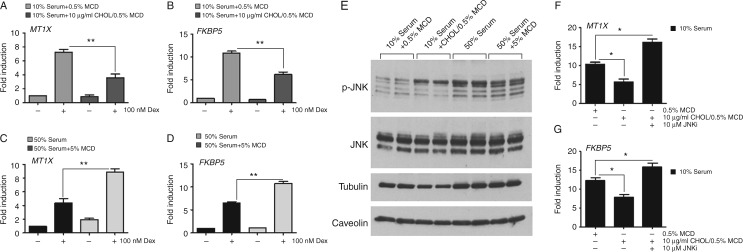
To investigate the cholesterol effect on GR transactivation of endogenous genes, the previous group of GR-transactivated genes MT1X, FKBP5, GILZ, and PER1 were measured by qRT-PCR. After 4 h treatment with Dex, incubation with exogenous cholesterol inhibited induction of MT1X and FKBP5 (Fig. 6A and B), but had no effect on GILZ or PER1 in cells cultured in 10% serum (Supplementary Fig. 7A, see section on supplementary data given at the end of this article). Treatment with 5% methyl-β-cyclodextrin abolished the impact of 50% serum on GR transactivation of MT1X and FKBP5 (Fig. 6C and D), again with no effect on GILZ or PER1 (Supplementary Fig. 7B). There was no cholesterol effect on the baseline expression of these endogenous genes (Supplementary Fig. 7C). The liver X receptor (LXR, NR1H3) antagonist GSK1440233A did not reverse the effect of cholesterol, excluding the action of oxidised cholesterol through the LXR.
Figure 6.
Cholesterol regulates GR transactivation via activation of JNK. HeLa cells were cultured in 10% serum or 10% serum supplemented with 10 μg/ml cholesterol overnight (A and B), or with 50% serum or 50% serum supplemented with 5% MCD (C and D). Cells were harvested after 4 h treatment with either 100 nM Dex or DMSO and analysed by qRT-PCR for expression of MT1X and FKBP5 (normalised to GAPDH). HeLa cells cultured in 10% serum supplemented with 10 μg/ml cholesterol (CHOL) or 50% serum supplemented with 5% MCD overnight, and then protein lysates analysed by immunoblot for JNK and phosphorylated JNK (E). Tubulin and caveolin expression was used as loading controls. Cells were cultured in 10% serum supplemented with 10 μg/ml cholesterol, together with 10 μM JNK inhibitor (SP600125) for 6 h. Following treatment with 100 nM Dex for an additional 4 h, expression of GR transactivation of either MT1X (F) or FKBP5 (G) was measured by qRT-PCR (normalised to GAPDH). Graphs show average±s.e.m. Experiments were carried out in triplicate and repeated three times. *P<0.05 and **P<0.001.
To determine whether the mechanism of cholesterol action was the same as that seen for culture in high-serum alone, through activation of JNK, immunoblot analysis was performed. Indeed, both high serum and cholesterol loading increased phosphorylation of JNK, a marker of increased activity. The serum-induced activation of JNK was attenuated by the addition of 5% methyl-β-cyclodextrin (Fig. 6E). The impaired GR transactivation of both MT1X and FKBP5 after treatment with cholesterol was rescued by cotreatment with a JNK inhibitor (Fig. 6F and G), which confirms the requirement of JNK signalling in mediating the regulatory effect of cholesterol.
Cholesterol selectively impairs GR transactivation in vivo
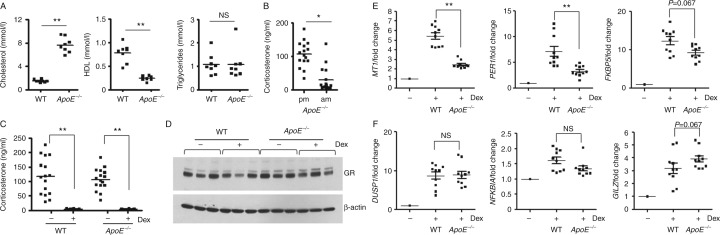
To investigate the effect of high cholesterol on GR transactivation in vivo, we studied Gc sensitivity in ApoE −/− mice (Plump et al. 1992). These mice have high serum cholesterol (Fig. 7A) but have rhythmic corticosterone levels within the normal range (Fig. 7B). We performed a Dex suppression test to measure ‘systemic’ Gc sensitivity and found no difference in response between WT and ApoE −/− mice (Fig. 7C). Analysis of protein from the livers of WT and ApoE −/− mice identifies no differences in GR expression (Fig. 7D). We next examined the impact of high serum cholesterol on GR transactivation in vivo. Consistent with our findings in cell lines, we find target-specific resistance to Dex treatment in ApoE −/− mice. Induction of MT1 and Per1 following i.p. Dex (1 mg/kg, 4 h) was attenuated in ApoE −/− mice, and although induction of FKBP5 was reduced, this did not reach statistical significance (Fig. 7E). We found no effect of cholesterol on Dex-dependent induction of dual specificity phosphatase 1 (DUSP1), NFκB inhibitor alpha (NFKBIA) or GILZ (Fig. 7F). High circulating cholesterol therefore selectively modulates GR function in vivo.
Figure 7.
Cholesterol selectively regulates GR transactivation in vivo. Serum samples from WT and ApoE −/− mice (eight animals per group) were analysed for levels of cholesterol, HDL and triglycerides (A). Morning (0700 h) and evening (1900 h) serum samples from ApoE −/− mice (16 animals per group) were assayed for corticosterone (B). WT and ApoE −/− mice were subjected to a dexamethasone (Dex) suppression test. Mice (eight animals per group) were given i.p. injection of Dex (1 mg/kg) and then serum collected 4 h later (1900 h). Serum samples were assayed for corticosterone (C). WT and ApoE −/− mice (five animals per group) were given i.p. injection of Dex (1 mg/kg) and then livers collected 4 h later. Livers were analysed for GR expression by immunoblot (D). Blots show samples from three different animals. Livers were also analysed for induction of GR target genes MT1, PER1, and FKBP5 (E), DUSP1, NFKBIA, and GILZ (F) by qRT-PCR (all normalised to GAPDH). Graphs show mean±s.e.m. *P<0.05 and **P<0.01. NS, not significant.
Discussion
Optimising Gc treatment is a challenge, a consequence of wide variation in host sensitivity, localised resistance at sites of inflammation, and undesired effects attributed to long-term use. The GR is ubiquitously expressed, and therefore obtaining selectivity of action is difficult. Developing selective GR modulators to improve the therapeutic index of Gc represents an essential step to improve existing Gc therapies (Beck et al. 2009). However, as the precise mechanism by which Gcs mediate their range of anti-inflammatory effects remains unclear, attempts to identify new molecules with selective action has proven difficult (Sommer & Ray 2008). Given the widespread therapeutic application of synthetic Gc in healthcare, understanding how target cell sensitivity to Gc is regulated is of major interest. While developing a Gc bioassay we discovered a potent effect of human serum on target cell response to Gc (Perogamvros et al. 2011). Serum is a complex mixture of nutrients and signalling molecules (hormones, cytokines, and growth factors) and new discoveries are still being made identifying novel physiological functions for serum in signalling to target tissues, such as providing time-of-day information (Gerber et al. 2013).
Herein, we show that serum impairs Gc action in a template-specific manner, where high serum impairs GR transactivation on some targets, but paradoxically augments GR transrespression of others. Our studies did not reveal any differences in GR expression, phosphorylation, trafficking, or intranuclear mobility either at steady state or following addition of Gc. These results suggest that the gene-selective mechanism of action of serum results from parallel activation of other signalling cascades and convergence on target genes based on the presence of transcription factor binding sites for GR, and other factors lying in such a way as to permit cross-talk. We investigated several endogenous GR-transactivated genes. Gc-induced expression of MT1X and FKBP5 was attenuated by serum, while GILZ and PER1 remains unaffected. Interestingly, sequence analysis of the MT1X gene sequence showed both AP1 and GRE-binding sites in close proximity (Viarengo et al. 2000, Velazquez & Gariglio 2002). In response to heat shock, which induces activation of AP1, there was also reduced Gc transactivation of MT1X (Andrews et al. 1987). The transcription factor AP1 is dimeric, consisting of JUN and FOS family members in a variety of homodimeric and heterodimeric forms. Studies have previously defined a mutually antagonistic mode of interaction (Lucibello et al. 1990, Schule et al. 1990), but more recently, AP1 has emerged as a necessary pioneer factor for GR access to target sites in chromatin, suggesting a far more complex mechanism of interaction than previously thought (Biddie et al. 2011, Uhlenhaut et al. 2013).
AP1 is activated downstream of MAP kinases, and using JNK inhibitors and constitutive JNK expression studies we were able to show that the serum effect required activation of JNK, and, indeed, serum shock induced JNK activation. The action of JNK on GR activity and subcellular shuttling has been previously reported in other contexts (Rogatsky et al. 1998, Loke et al. 2006).
Having identified a serum effect activating JNK/AP1 as the mechanism acting on GR signalling, we started by considering serum components likely capable of activating JNK. In contrast to other recent studies that identified a protein factor as the modulatory serum component (Gerber et al. 2013), we were able to exclude a significant effect mediated by serum protein components, using heat-inactivation, trypsin digestion, and growth factor signalling tyrosine kinase inhibition approaches. Instead, we did discover a key role for serum lipid components. We show that the response to Gc was augmented in cells cultured in charcoal dextran-treated (delipidated) serum, and lipoprotein-deficient serum increased Gc action to an even greater extent. Adding cholesterol alone was sufficient to modify the Gc response, which is consistent with an early report that found high cholesterol blocked the antiproliferative actions of Gc in leukaemia cells (Madden et al. 1986). We also used the cholesterol-binding drug methyl-β-cyclodextrin to sequester serum cholesterol (Brown & London 2000, Simons & Toomre 2000), which not only increased Gc transactivation in 10% serum culture, but also rescued the impairment of Gc action by high serum. We found that loading with exogenous cholesterol alone was sufficient to activate JNK, and that loss of GR transactivation with cholesterol was rescued by cotreatment with a JNK inhibitor. Treatment with Gc is reported to inhibit cholesterol synthesis, and so this may provide an indirect mechanism to modify JNK signalling, and also modify cellular responses to subsequent Gc treatments (Johnston et al. 1980).
In humans, circulating cholesterol (∼100–200 mg/dl or 2.5–5 mmol/l) is typically higher than that present in FBS (∼30–50 mg/dl or 0.8–1.3 mmol/l) and there are also differences in lipoprotein content between species. To assess the role of cholesterol rather than lipoproteins, we delivered cholesterol directly to target cells in complex with methyl-β-cyclodextrin and also regulated local cholesterol production using statin treatment. These studies reveal a specific effect of cellular cholesterol content on Gc sensitivity. Given the widespread application of cholesterol regulating drugs, and their reported impact on parameters of inflammation (Krane & Wanner 2011, Rodriguez et al. 2012), this is likely a clinically relevant interaction.
To explore the effects of high cholesterol on Gc sensitivity in vivo, we analysed GR function in ApoE null, hypercholesterolaemic mice. Although globally sensitive to Gc treatment, we again find target-selective resistance to Dex in livers from ApoE −/− mice, although the specific genes affected were slightly different between human and murine models. Our in vitro and in vivo data provide strong evidence for a novel and important regulatory role for cholesterol in the regulation of Gc responses in vivo. Gc are key components of the stress response, and are necessary for regulation of immune and metabolic function. Our findings therefore have important implications for patients with dyslipidaemia, treated with statins, or indeed at foci of inflammation, or injury in healthy individuals where cholesterol is found at high concentration.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/JOE-14-0456.
Author contribution statement
N Y, C M H, D W R, and L C M designed the research; N Y, G C, L M I, P J T, T M P, and L C M performed the research and analysed the data; N Y, G C, L M I, D W R, and L C M wrote the paper.
Supplementary Material
Acknowledgements
The authors acknowledge gifts of plasmids from K Yamamoto, R Evans, M Stallcup, and A Sharrocks. They acknowledge Handrean Soran and Yifen Liu (Cardiovascular Sciences, University of Manchester) for lipid profiling and also thank Roger Meadows and Peter March (University of Manchester Bioimaging) for technical support.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
G C was supported by MRC and GSK. P J T and L M I were supported by BBSRC and GSK. L C M was supported by University of Manchester Stepping Stones Fellowship. T M P and D W R were supported by MRC and Wellcome Trust respectively. University of Manchester Bioimaging Facility microscopes were purchased with grants from BBSRC, Wellcome Trust and the University of Manchester Strategic Fund.
References
- Andrews GK, Harding MA, Calvet JP, Adamson ED. The heat shock response in HeLa cells is accompanied by elevated expression of the c-fos proto-oncogene. Molecular and Cellular Biology. 1987;7:3452–3458. doi: 10.1128/mcb.7.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Beck IM, Vanden BW, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocrine Reviews. 2009;30:830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddie SC, John S, Sabo PJ, Thurman RE, Johnson TA, Schiltz RL, Miranda TB, Sung MH, Trump S, Lightman SL, et al. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Molecular Cell. 2011;43:145–155. doi: 10.1016/j.molcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. Journal of Biological Chemistry. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- De IS, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World Journal of Gastroenterology. 2011;17:1095–1108. doi: 10.3748/wjg.v17.i9.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donn R, Berry A, Stevens A, Farrow S, Betts J, Stevens R, Clayton C, Wang J, Warnock L, Worthington J, et al. Use of gene expression profiling to identify a novel glucocorticoid sensitivity determining gene, BMPRII. FASEB Journal. 2007;21:402–414. doi: 10.1096/fj.06-7236com. [DOI] [PubMed] [Google Scholar]
- Gerber A, Esnault C, Aubert G, Treisman R, Pralong F, Schibler U. Blood-borne circadian signal stimulates daily oscillations in actin dynamics and SRF activity. Cell. 2013;152:492–503. doi: 10.1016/j.cell.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Gineitis D, Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. Journal of Biological Chemistry. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- Herrera RE, Shaw PE, Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989;340:68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Lou DY, Yamamoto KR. Three amino acid substitutions selectively disrupt the activation but not the repression function of the glucocorticoid receptor N terminus. Journal of Biological Chemistry. 1997;272:4149–4156. doi: 10.1074/jbc.272.7.4149. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. Journal of Experimental Medicine. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nature Genetics. 2011;43:264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Matthews ER, Melnykovych G. Glucocorticoid effects on lipid metabolism in HeLa cells: inhibition of cholesterol synthesis and increased sphingomyelin synthesis. Endocrinology. 1980;107:1482. doi: 10.1210/endo-107-5-1482. [DOI] [PubMed] [Google Scholar]
- Krane V, Wanner C. Statins, inflammation and kidney disease. Nature Reviews. Nephrology. 2011;7:385–397. doi: 10.1038/nrneph.2011.62. [DOI] [PubMed] [Google Scholar]
- Loke TK, Mallett KH, Ratoff J, O'Connor BJ, Ying S, Meng Q, Soh C, Lee TH, Corrigan CJ. Systemic glucocorticoid reduces bronchial mucosal activation of activator protein 1 components in glucocorticoid-sensitive but not glucocorticoid-resistant asthmatic patients. Journal of Allergy and Clinical Immunology. 2006;118:368–375. doi: 10.1016/j.jaci.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Lucibello FC, Slater EP, Jooss KU, Beato M, Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO Journal. 1990;9:2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden EA, Bishop EJ, Fiskin AM, Melnykovych G. Possible role of cholesterol in the susceptibility of a human acute lymphoblastic leukemia cell line to dexamethasone. Cancer Research. 1986;46:617–622. [PubMed] [Google Scholar]
- McMaster A, Ray DW. Modelling the glucocorticoid receptor and producing therapeutic agents with anti-inflammatory effects but reduced side-effects. Experimental Physiology. 2007;92:299–309. doi: 10.1113/expphysiol.2006.036194. [DOI] [PubMed] [Google Scholar]
- McMaster A, Chambers T, Meng QJ, Grundy S, Loudon AS, Donn R, Ray DW. Real-time analysis of gene regulation by glucocorticoid hormones. Journal of Endocrinology. 2008;197:205–211. doi: 10.1677/JOE-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon M, Upreti R, Andrew R. 5α-Reduced glucocorticoids: a story of natural selection. Journal of Endocrinology. 2012;212:111–127. doi: 10.1530/JOE-11-0318. [DOI] [PubMed] [Google Scholar]
- Perogamvros I, Kayahara M, Trainer PJ, Ray DW. Serum regulates cortisol bioactivity by corticosteroid-binding globulin dependent and independent mechanisms, as revealed by combined bioassay and physicochemical assay approaches. Clinical Endocrinology. 2011;75:31–38. doi: 10.1111/j.1365-2265.2011.04003.x. [DOI] [PubMed] [Google Scholar]
- Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability-effects on hormone measurement and action. Nature Reviews. Endocrinology. 2012;8:717–727. doi: 10.1038/nrendo.2012.134. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-G. [DOI] [PubMed] [Google Scholar]
- Ray DW, Davis JR, White A, Clark AJ. Glucocorticoid receptor structure and function in glucocorticoid-resistant small cell lung carcinoma cells. Cancer Research. 1996;56:3276–3280. [PubMed] [Google Scholar]
- Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD, Jr, Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. Journal of Thrombosis and Thrombolysis. 2012;33:371–382. doi: 10.1007/s11239-012-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Logan SK, Garabedian MJ. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. PNAS. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacke H, Berger M, Rehwinkel H, Asadullah K. Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index. Molecular and Cellular Endocrinology. 2007;275:109–117. doi: 10.1016/j.mce.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Schule R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-W. [DOI] [PubMed] [Google Scholar]
- Shaw PE, Schröter H, Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989;56:563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews. Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sommer P, Ray DW. Novel therapeutic agents targeting the glucocorticoid receptor for inflammation and cancer. Current Opinion in Investigational Drugs. 2008;9:1070–1077. [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO Journal. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Barish GD, Yu RT, Downes M, Karunasiri M, Liddle C, Schwalie P, Hubner N, Evans RM. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Molecular Cell. 2013;49:158–171. doi: 10.1016/j.molcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez TA, Gariglio VP. Possible role of transcription factor AP1 in the tissue-specific regulation of human papillomavirus. Revista de Investigación Clínica. 2002;54:231–242. [PubMed] [Google Scholar]
- Viarengo A, Burlando B, Ceratto N, Panfoli I. Antioxidant role of metallothioneins: a comparative overview. Cellular and Molecular Biology. 2000;46:407–417. [PubMed] [Google Scholar]
- Yang N, Ray DW, Matthews LC. Current concepts in glucocorticoid resistance. Steroids. 2012;77:1041–1049. doi: 10.1016/j.steroids.2012.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a