Abstract
We compared 223 consecutive intensive care unit (ICU) admissions to a community hospital (CH) with 613 such admissions at a university hospital (UH) using a new clinical scale aimed at quantifying severity of illness.
Both ICU's had similar technical resources and treatment capabilities. At the CH, however, patients were more often admitted for monitoring rather than for treatment of UH admissions had a substantially greater acute severity of illness (p<.001) than CH patients in most diagnostic categories.
These findings suggest that use of the ICU was substantially different in the two hospitals, with the CH admitting many more stable patients. This study also suggests that evaluation of ICU use is improved by quantitative measurement of severity of illness.
Introduction
Two decades ago intensive care units (ICU's) were found only in a few large U.S. medical center hospitals. Today, almost every American hospital with more than 200 beds has an ICU. In 1979 this nation's 55,000 ICU beds accounted for 5 percent of all acute care beds and 15 percent of all hospital expenditures (Knaus and Thibault, 1981). Each year the number of ICU beds continues to increase by 4 percent, with the largest growth taking place in community hospitals (American Hospital Association, 1980).
To some observers ICU's are examples of physicians' enthusiasm for new medical technology combined with an increased number of complex surgical procedures which depend on intense postoperative care. A similar view holds that the demand for ICU's stems from the increased age of our population and our progressively more aggressive approach to serve illness (Russell, 1979). Recently, attention has been directed toward the monitoring or close observation function of ICU's highlighted by the decline in the percentage of coronary care unit admissions who actually have heart attacks, and, for surgical ICU's the high percentage of postoperative monitoring (Thibault, Mulley, et al., 1980; Knaus, Wagner, et al., 1981).
These tentative observations and the issues raised by them are important. As the number and cost of ICU's increase, State and local planning officials are beginning to restrict the growth of ICU's ((Dept. of Public Health, State of Massachusetts, 1978). But are such regulations necessary? If so, how should they be developed? Recent evidence suggests that restrictions on regular hospital beds by certificate of need regulations may have led to some of the increases in ICU beds (Salkever and Bice, 1976). But ICU beds are costly, with charges approximately three times the cost of regular hospital care and, under current reimbursement procedures, incentives exist to keep ICU occupancy rates high.
This case study describes the patient mix of two ICU's using a new severity of illness measurement technique. This new index would be useful in examining reimbursement issues and regional distribution of ICU beds by providing a way to study use in existing units.
Definitions
Intensive care refers to two distinct clinical activities: coronary care units (CCU's), and general medical-surgical or multi-disciplinary ICU's. The latter are commonly called MICU's (medical intensive care units), SICU's (surgical intensive care units) or simply ICU's.1
CCU's contain a narrow range of diagnoses, mainly patients with suspected or actual heart attacks and those with related cardiac problems. CCU patients are generally not as critically ill as ICU patients, although individual admissions can be similar. The therapeutic services provided within a CCU are fewer than those available in a multi-disciplinary ICU, and these services emphasize diagnosis, particularly confirmation of acute myocardial infarction, which is not found in ICU's.
Most multi-disciplinary ICU's treat patients with a wide variety of diagnoses. In a medical ICU there are patients recovering from acute drug overdoses, or suffering from respiratory failure, gastrointestinal bleeding or diabetic coma. A surgical ICU treats patients recovering from open heart surgery, neurosurgery and other major operations. This study involved two such multi-disciplinary or medical-surgical ICU's.
Hospitals Studied
We collected information on 223 consecutive admissions to the medical and surgical ICU's of a community hospital and compared them with 613 consecutive ICU admissions at the George Washington University Medical Center reported in Knaus, Zimmerman, et al., 1981.
The George Washington University Medical Center (the UH) is a 500-bed medical school affiliated teaching hospital within a large metropolitan area. GW's ICU is a 16-bed medical-surgical unit admitting patients with a wide range of diagnoses, (except acute myocardial infarctions and burns). Patients' attending physicians request admission. One of the three full-time ICU directors reviews all requests. Usually this review is informal, becoming intense only during occasional periods of peak use. During the eight-month study period (April-November 1979) only 23 admissions were denied or delayed, primarily because of a shortage of ICU nurses.
The community hospital (CH) used in this study is a 300 bed facility with a limited teaching service. It is located in a suburb of a large metropolitan area in a mid-Atlantic State. It has a 10-bed medical ICU and a 10-bed surgical ICU, for a total of 20 medical-surgical ICU beds. As with the UH, suspected or acute myocardial infarction patients are admitted to a separate CCU. Both the CH MICU and SICU have part-time directors, but they do not routinely review admissions. No patient whose admission had been requested by his attending physician was denied ICU treatment during the CH study period (April-July 1980).
With the exception of the full-time versus part-time directors, both hospitals' ICU's have the same technical and personal resources. Both can do pulmonary artery or right heart catheterization (Swan-Ganz) at the bedside, both use ventilators to treat respiratory failure, and both have similar nurse-to-patient ratios which vary from 1:1 to 1:3.
Methods
In both hospitals information collected consisted of diagnosis, sex, age, race, and the specific indication for ICU admission. In addition, we recorded the patient's prior health status, a physiologic score measuring the severity of acute illness, and the type and amount of therapy received during the initial 24 hours of the patient's ICU stay.
The Therapeutic Intervention Scoring System (TISS) was used to measure therapeutic effort (Cullen, et al., 1974). The TISS system assigns a score of 1 to 4 to a listing of 75 therapeutic tasks routinely performed on ICU patients. The higher the number of TISS points, the greater the nursing time and effort involved. Although the inter-hospital reliability of TISS has never been formally tested, there is a good deal of experience with TISS in other hospital settings (Byrick, et al., 1980). A critically ill patient usually requires about 30 or more TISS points per shift. Though variations in TISS points have been associated with hospital outcome for some groups of ICU patients, this association is not consistent, and TISS is not a direct measure of severity of illness because it reflects the physician's therapeutic response only (Cullen, et al., 1976).
Because total TISS points reflect only the intensity rather than the actual type of service provided, we reclassified the 75 original TISS tasks into three distinct categories: those tasks which, in the clinical judgment of two authors, (Elizabeth Draper, R.N., and William A. Knaus, M.D.) reflect: 1.) active treatment; 2.) monitoring; or 3.) standard floor care services.
Active treatment refers to 33 tasks that involve direct therapy using techniques unique to or best performed in an ICU, such as maintaining a patient on a ventilator or assisting his heart's contraction with a balloon pump. ICU monitoring includes eight tasks usually requiring either the facilities or personnel of a special care unit but which are observational, as opposed to therapeutic, in nature. The eight monitoring tasks consist of two labor-intensive services, hourly vital signs and neurologic checks, and six other activities requiring technology not normally available on hospital floors, such as EKG monitoring or pulmonary arterial lines. Standard floor care refers to those remaining nursing services performed on ICU patients but also commonly done in other hospital settings.
In this study patients receiving one or more of the 33 active treatment tasks during the initial 24 hours of their ICU stay are classified as “active treatment” patients. All others are classified as “monitoring,” since all admissions received at least one of the ICU monitoring tasks. In a separate study this approach proved useful in identifying neurosurgical admissions who had a low risk of subsequently requiring active treatment during their ICU stay (Knaus, Draper, et al., 1981).
The reason for an ICU admission was designated as failure of, insufficiency of, or monitoring of one or more of the seven major vital organ systems: neurologic, cardiovascular, respiratory, gastrointestinal, renal, hematologic, and metabolic. These seven categories are a more concise description of ICU patients because frequently diagnoses (cancer, infection, trauma) are not the primary reason for ICU admission.2 It is rather the need for life support or monitoring of one of the seven organ systems that directly leads to ICU admission. Failure of one of these organ systems is also the major reason ICU patients die (Cullen, et al., 1976).
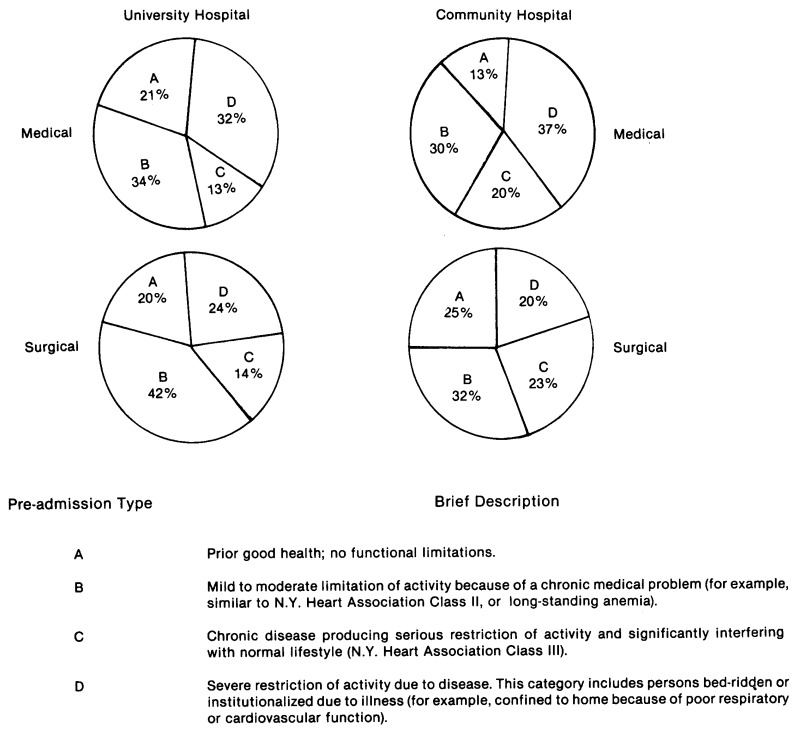
Pre-admission health status designation was a system of four patient types or categories. (See Figure 1). After reviewing a patient's medical record within 24 hours of admission, the patient is placed in one of the four health types. These categories are designed to obtain a general assessment of the patient's chronic health status six months prior to ICU admission. The categories are directly related to hospital survival with approximately a four-fold increase in probability of death for Class D patients when compared to Class A patients (Knaus, Zimmerman, et al., 1981).
Figure 1. Comparison of Pre-Admission Health Status of ICU Admissions to a University and Community Hospital.
Severity of Illness
The most important descriptor of the ICU case mix is the Acute Physiology Score (APS). It is designed to be a measure of the severity of illness of acutely ill hospitalized patients, regardless of diagnosis or disease state.
The APS consists of a weighted sum of each of 34 potential physiologic measurements obtained from the patient's clinical record within the first 24 to 32 hours of ICU admission. A weight ranging from 0 to 4 is assigned for each recorded measurement to reflect “how sick” the patient is. For example, a heart rate (pulse) between 70 and 110 is assigned a weight of 0, but a heart rate over 180 or under 40 is assigned a weight of 4.
The 34 potential measurements that determine the acute physiology score reflect the degree of derangement of one or more of the body's seven major vital physiologic systems: neurologic, cardiovascular, respiratory, gastrointestinal, renal, metabolic and hematologic. We developed this list by reviewing the literature for measurements that had demonstrated promise in estimating severity of illness and that were generally tested and recorded in most ICU's. We presented an initial list to a panel of 7 experienced ICU physicians from major medical centers around the country. They rejected some of the initial measures and added others.
This panel of experts also decided where to divide each physiologic parameter into ranges and what weight to assign to the ranges. We chose the 0 to 4 weighting system to convert the 34 physiologic measurements into a reproducible score that could easily be used in multiple institutions. Another important advantage of a simple weighting system is that it closely duplicates the clinical evaluation of a critically ill patient. Depending upon the patient's medical history, the more abnormal each measurement becomes, the more concern or anxiety the clinician has, and by inference, the more severely ill the patient.
The panel of ICU experts represented all the major specialties. The weights they assigned particular values reflect the level of anxiety they would feel for a particular patient because of the physiologic measurements. The panel sought internal agreement among individual weights and, as a result not every physiologic measurement has a range of weights from 0 to 4. The scale does exhaust the sample space, with each possible value of each physiologic measurement represented.
When multiple measurements are available, the assigned weight is determined by the value furthest removed from normal, that is, the lowest blood pressure for a patient in shock or the highest respiratory rate for a patient in respiratory distress. Clinical judgment is used to insure that the lowest or highest reading is a legitimate value and not a measurement error or some other outlying number. In practice this decision is easily adhered to and minimizes the opportunity for recording error. All patients do not have every physiologic variable measured. Unmeasured variables are assumed to be unnecessary for the patient's care in the ICU and are considered normal. We selected the initial 24 to 32 hours following ICU admission as the time most likely to reflect the greatest degree of abnormality of all potential measurements. This time period also helps insure that all pertinent laboratory values will have been determined and returned to the patient's record.
In Appendix A, we provide further information on the statistical validation of the APS. Within the mid-range of the APS (15-20) there is approximately a 2 percent increase in the probability of hospital death with each one point increase in physiology score.
Two former ICU nurses collected all data. They developed objective operational definitions of many of the data items on a pilot data set. Inter-observer reliability testing revealed 96 percent accuracy on 30 patients. Variations between observers did not produce significant changes in clinical classifications.
Results
Table 1 compares the UH and CH populations in terms of general characteristics such as origin of admission. Demographically, the two hospitals were similar except the UH ICU admits more black patients. A greater percentage of CH patients are Medicare beneficiaries over the age of 65.
Table 1. Description and Origin of ICU Admissions to a University (UH) and Community Hospital (CH).
| University Hospital | Community Hospital | |
|---|---|---|
|
|
||
| Total Admissions | 613 | 223 |
| Mean Age (Years) | 53 | 57 |
| Percent over 65 | 26* | 44 |
| Percent Female | 46 | 47 |
| Percent Black | 40* | 17 |
| Origin of Admission (in Percent) | ||
| Postoperative | 55 | 51 |
| Emergency Room | 25 | 30 |
| Other Hospitals | 2 | 4 |
| Transfer from Floor | 18 | 15 |
(P<.05) t - test
The indications for admission (Table 2), arranged according to organ system, show that the largest differences are in the surgical patients, with a greater percentage of neurologic and cardiovascular surgical patients at the UH. A larger portion of the CH surgical patients was admitted to the ICU following gastrointestinal operations, an infrequent indication at the UH. The medical patients were quite similar in their distribution.
Table 2. Percent Distribution of Admissions by Medical-Surgical Status and Major Indication for ICU Treatment.
| Medical Patients | Surgical Patients | |||
|---|---|---|---|---|
| University Hospital | Community Hospital | University Hospital | Community Hospital | |
|
| ||||
| Cardiovascular | 32 | 22 | 43 | 31 |
| Neurologic | 28 | 35 | 34 | 13 |
| Respiratory | 21 | 17 | 7 | 13 |
| Gastrointestinal | 9 | 14 | 9 | 35 |
| Renal, Metabolic and Hematologic | 10 | 12 | 7 | 8 |
In Figure 1 the distributions of admissions are grouped according to health status six months prior to their ICU stay. CH admissions tended to be in poor or failing health more often than those in the UH, although the difference was small (p<.07).
We next compared the two populations according to their acute severity of illness and the amount of therapy they received. We did this in three ways: first, we tabulated the average acute physiology and TISS scores across all major organ systems as well as for selected diagnostic groups (Tables 3 and 4); second, we determined the frequency distribution of physiology and TISS scores for both populations (Figure 2); and third, we classified the two populations according to whether or not they required active treatment or monitoring during their initial 24 hours of ICU care.
Table 3. Comparison of Severity of Illness and Therapeutic Effort According to Principal Indication for ICU Admission.
| Acute Physiology Score | TISS | Points | ||
|---|---|---|---|---|
|
|
|
|||
| Diagnostic Group | UH | CH | UH | CH |
| Medical | ||||
| Cardiovascular | 24.0 *** | 11.0 | 30.4 * | 23.1 |
| Neurologic | 13.3*** | 8.9 | 18.4 | 17.8 |
| Respiratory | 19.4 ** | 12.1 | 25.9 | 20.7 |
| Gastrointestinal | 22.3 *** | 7.5 | 31.5 ** | 22.8 |
| Renal, Hematologic and Metabolic | 24.4 | 17.3 | 23.1 | 14.7 |
| All Medical | 19.8*** | 10.7 | 25.4*** | 19.8 |
|
| ||||
| Surgical | ||||
| Cardiovascular | 18.3*** | 4.4 | 36.9*** | 15.7 |
| Neurologic | 8.8 | 5.8 | 19.4 | 16.7 |
| Respiratory | 11.0 *** | 4.4 | 22.7 *** | 14.8 |
| Gastrointestinal | 14.2*** | 4.2 | 27.2 *** | 15.4 |
| Renal, Hematologic and Metabolic | 15.8*** | 7.3 | 24.9 *** | 12.8 |
| All Surgical | 14.1 *** | 4.7 | 28.2 *** | 15.4 |
| All Patients | 16.6*** | 7.0 | 27.0 *** | 17.1 |
Note: Case counts are at least 10 in all categories.
denotes significantly different (p<.05) from corresponding mean at CH on a t-test.
denotes significantly different (p<.01) from corresponding mean at CH on a t-test.
denotes significantly different (p<.001) from corresponding mean at CH on a t-test.
Table 4. Comparison of Severity of Illness and Therapeutic Effort for Selected Diagnostic Groups.
| Diagnostic Group | ICDA Diagnosis | Acute Physiology Score1 | TISS Points | ||
|---|---|---|---|---|---|
| Medical | UH | CH | UH | CH | |
| Overdose | 960-979 | 10.2 (30) | 7.6 (15) | 13.3 | 13.5 |
| Cerebrovascular | 430-439 | 17.2 (18) | 11.4 (10) | 23.3 | 24.2 |
| Congestive Heart Failure | 427.9 | 19.0* (10) | 9.4 (11) | 24.9 | 22.5 |
| Surgical | |||||
| Gastrointestinal Neoplasm | 150-159 | 8.4 (8) | 4.1 (23) | 23.2 | 15.4 |
| Obstruction, Perforation or Infection | 530-537 | 14.6**(10) | 3.5 (17) | 24.8** | 13.4 |
| Peripheral Vascular Disease | 440-448 | 14.1**(23) | 4.1 (14) | 25.6* | 19.1 |
| Cerebrovascular Disease | 430-439 | 12.2* (32) | 6.7 (6) | 22.8* | 17.7 |
p<.05 on 2-tailed t-test.
p<.01 on 2-tailed t-test.
Figures in parentheses are the number of patients.
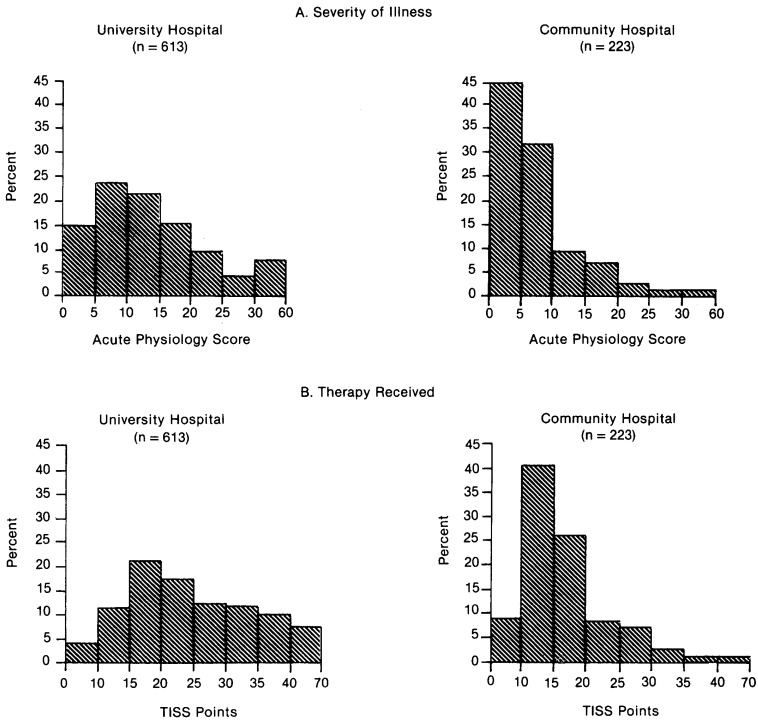
Figure 2. Percentage Distribution of ICU Admissions by Severity of Illness and Therapy Received in a University and Community Hospital.
These results showed that for most indications for admission and for most of the narrower, diagnostic categories analyzed, acute physiology scores were generally two to three times higher at the UH (Figure 2, Tables 3 and 4).
Analysis of the APS in the UH revealed a nonlinear relationship between the score and the probability of death (Appendix Figure A-1). Applying this relationship to CH admissions implies that, on admission, UH ICU admissions were almost two times as likely to die during their hospitalization as CH patients. The actual in-hospital death rate was 19 percent for UH patients and 11 percent for the CH.
In addition to significant differences in severity of illness and projected and observed death rates, there were also differences in the therapy received. This was especially true with CH surgical admissions who received significantly fewer TISS points (p<.001). Likewise, 86 percent of the CH surgical patients received only monitoring or routine floor care during their initial 24 hours in the ICU, compared to 48 percent of the UH surgical patients.
Less difference in TISS scores occurred between medical admissions despite the fact the medical patients had significantly higher physiology scores at the UH. There was also little difference in the percentage of monitoring admissions of medical patients (CH, 42 percent; UH, 44 percent). For both medical and surgical admissions, however, we found that the CH patients more often received one of the two labor-intensive, as opposed to six technology-intensive, monitoring tasks.
Discussion
What is an intensive care unit? This limited comparison suggests that the definition varies substantially among hospitals.
The UH ICU treated patients recovering from complicated cardiovascular and neurologic surgical procedures. The CH ICU admitted an equal percentage of post-operative patients, but they were more frequently admitted following routine surgical procedures, such as gastrointestinal operations, than were UH patients. There was also less complicated neurosurgery at the CH (Table 2).
For both medical and surgical patients, there were substantial differences in acute severity of illness. The APS's were consistently higher in the UH across all classes of patients (Table 3). Even when categories were narrowed to more homogeneous diagnostic categories, we found that CH patients were less severely ill than UH patients (Table 4).
One might speculate that the difference in severity of illness between the two patient groups is due entirely to the larger number of patients in failing health at the CH. However, the APS is designed to capture both the chronically abnormal physiology reading in failing health patients who are frequently monitored for potential problems and the acute derangement in the chronically ill patients who require active treatment. Furthermore, the increased number of CH admissions with serious chronic health problems is very small in comparison to the marked disparity in acute physiology scores between the two institutions.
We infer from this difference in severity of illness that the threshold for ICU admission, both medical and surgical, is lower in the CH than in the UH. This conclusion is supported when we examine the differences in treatment that the two ICU's provide. The overall difference in total TISS points and their distribution between the UH and CH is most prominent for surgical admission. One reason for this difference is that admission to both ICU's results in a minimum of 8 to 12 TISS points regardless of severity of illness. When we examine the type of treatment provided, however, we find that 86 percent of the CH surgical patients received only monitoring or routine floor care during their first 24 hours, compared to 48 percent of UH surgical admissions. These figures do not necessarily imply that the CH monitored patients should not have been admitted for monitoring; more extensive analysis would be required to determine whether the risks and costs of ICU treatment justify the expected benefits. Furthermore, though severity of illness and therapy differed, the similarity between projected and actual death rates strongly suggests that the CH achieved quality of care comparable to that of the UH.
It could be argued that these different ICU populations simply reflect differences in the two hospitals' primary roles. The UH is a tertiary center aimed at providing sophisticated surgical, diagnostic, and treatment services; the other is a community facility oriented toward more routine hospital care. Yet the ICU's in both institutions have similar diagnostic and treatment equipment, and they both have equivalent nurse-to-patient ratios. In fact, with the exception of three full-time physician directors at the UH (as opposed to two part-time directors at the CH), the units' technical and personnel capabilities were equivalent. At the CH, however, these extensive resources were seldom used.
Only one of the 223 CH patients used the pulmonary artery catheterization capabilities, compared to 25 percent of the UH population. Only 12 percent of CH patients received ventilatory treatment, compared to 50 percent at the UH. This usage probably occurred because, of the 223 CH patients studied, we could identify only 11 patients (5 percent) whose physiology score was within the range of what is clinically considered an unstable, critically ill patient (physiology score > 20). This figure compares to 135 UH admissions (22 percent) (Figure 2). These findings are compatible with recent surveys emphasizing the number of stable, noncritically ill patients admitted to a university ICU (Thibault, et al., 1980).
Although the results of this survey are limited and cannot be extrapolated nationwide, they support claims that the demand for more ICU beds is due to many factors. Within the UH, patients who had complicated surgery were frequent admissions; and within both hospitals elderly patients, many with chronic medical problems, contributed to demand.
The survey's results also support the suggestion made by Russell (1979), however, that the primary factor behind ICU growth may be that the medical staff is seeking the latest in monitoring and treatment technology. Although the vast majority of ICU admissions at the CH did not receive active treatment, there was a substantial investment in such equipment at the CH. These facts raise several questions, such as: Is this the best use of health care funds? Since so few critically ill patients are treated at the CH, much of the equipment and personnel are under-used. Moreover, in light of recent studies that have shown outcome from complex procedures varying directly with the number performed, (Luft, Bunker and Enthoven, 1979; Luft, 1980) should an ICU treat a minimum number of critically ill patients in order to maintain its competence?
These findings also raise questions about health planning. American Hospital Association statistics show that the number of ICU beds nationwide continues to grow each year and that the largest growth is in community hospitals. Are these new beds, as suggested by this case study, monitoring stable, older, chronically, but not critically, ill patients. Could these patients be treated on regular floors? If so, then efforts to restrict the growth of ICU beds could proceed without fear that such moves would limit access for critically ill patients.
This study did not address the issue of efficiency. Perhaps, considering current nursing staffing patterns and third party payment for semi-private rooms, concentrating high-risk patients in an ICU setting is the most efficient way of providing adequate nursing care. If this is true, however, future ICU's might be designed without the technological support that was largely unused in the CH.
This design could produce substantial cost savings. Using 1978 prices, it is estimated that it costs $44,000 to $75,000 to build or convert an ICU bed (U.S. Government Printing office, 1979). Also once a patient is in an ICU his use of ancillary services increases (Griner, 1972). The distribution of TISS points in this study suggests that almost all ICU admissions receive a minimum amount of therapy which is higher than they would receive elsewhere, regardless of severity of illness.
But, in order to improve ICU use, we may need to measure severity of illness when we examine diagnostic case mix. In this comparison, we were able to consistently discover substantial differences between UH and CH admissions only because we could measure their underlying severity of illness. If we were to rely on more traditional descriptions such as age, sex, or H-ICDA (International Classification of Diseases, Adapted for Hospitals) diagnostic codes, utilization differences would not have been as apparent, particularly with the medical patients. Likewise, an etreatment received, as measured by TISS points alone, would tion of overall not have disclosed the substantial variations in case-mix or their severity of illness.
We found other clues pointing out the differences in UH and CH patients. The difference in the type of operative procedures in the two hospitals suggested a greater intensity of services at the UH. The larger ICU death rate at the UH could have been interpreted as a reflection of increased severity. Neither of these descriptors, however, can be used in isolation. A variation in procedure codes does not necessarily imply any variation in severity of illness, and the lower death rate could have meant that treatment was better at the CH. Only by quantifying the underlying severity of illness did its relationship to use of services become clear.
We believe collection of similar severity of illness data at a number of ICU's nationwide would provide valuable unique insights into how the growing number of ICU beds are being used and how their use might be improved in the future.
There are several reasons to be optimistic about the results of such an external validation using the APS. First, the measure is simple, being based on objective numbers which can be easily and reliably recorded. Second, APS is relatively immune to manipulation. Over-measurement of the physiologic variables will not lead to higher APS scores unless the patient has deranged physiologic values. Under-measurement could lead to lower scores, but this is also likely to result in lower therapy and worse than expected outcome. The APS is not, however, entirely independent of therapy. Good clinical practice can normalize some physiologic measures and reduce the APS score. Third, the strength and stability of the APS coefficients in the estimated appendix equations are very promising. The explanatory power of the APS in these equations, while insufficient for clinical decision-making, is strong enough to yield relatively narrow predicted confidence bands for groups of patients. This use of the APS would make possible the analysis of such critical issues as quality of patient care and resource use.
Acknowledgments
This study was made possible by Grant 18-P-97079/3-03 from the Health Care Financing Administration.
Appendix A
Validity of Acute Physiology Score (APS) as a Severity of Illness Index
The initial question to ask about a new index number is how is it distributed? The upper left panel of Figure 2 tabulates the frequency distribution of the APS for 613 consecutive admissions to the UH ICU. The distribution is approximately normal with a slight skew to the right. Although a patient could theoretically score over 100 APS points, in practice the highest score was 54. The mean score for the 613 patients is 14.9.
We tested the validity and sensitivity of the APS by examining how well it predicts two subsequent events in each admission's hospital stay: 1) intensity of therapeutic effort in the ICU, and 2) whether the patient was discharged alive or dead. While neither of these is a perfect test of validity, both do capture important consequences of acute illness.
Therapeutic Intervention Scoring System (TISS) points are the most widely used index of therapeutic effort in ICU's, and they are a reasonable measure of the resource cost of treating an ICU patient. TISS points directly measure a substantial portion of the labor input in producing intensive care services and are a good proxy for other resources used in ICUs.1
Hospital survival is a particularly appropriate outcome measure for ICU's because of the relatively high probability of death. More sensitive measures of health status among the survivors would also be desirable. Future efforts will examine the relationship between the APS and chronic disability six months post-hospital discharge.
Table A-1 reports simple and multiple regression analyses of two different measures of TISS points. The first two equations (First Day TISS) use the APS to explain concurrent therapeutic effort during the first 24 hours of ICU care. The second two equations (Four Day TISS) explain variations in therapeutic effort accumulated over up to the first 4 days of ICU care. For patients who were discharged before the 4 days elapsed, this method measured total therapy in the unit. We truncated the remaining 20 percent at 4 days because that length of time represents a reasonable amount of time to capture the therapeutic response to the severity of illness measured during the first ICU day.2
Examination of the first two columns (First Day TISS) of Table A-1 reveals that concurrent therapeutic effort has a highly significant relationship with the APS. The most important result from the second column is that the coefficient on APS is virtually unchanged by the introduction of 17 other independent variables into the equation, 9 of which are highly significant and raise the overall explanatory power from an R2 of .29 to .52. The diagnostic groupings confirm that patients admitted to ICU primarily for cardiovascular, respiratory or gastrointestinal reasons receive more therapeutic effort in their initial day than do neurologic patients, the reference group. The substantial intercept conforms with expectations, because standard operating procedures insure that an ICU patient receives a minimum of 10 to 15 TISS points per day, or one-third of a nurse's time.
Table A-1. Regression Analysis of TISS: First Day and First 4 Days.
| First Day TISS | Four Day TISS | |||
|---|---|---|---|---|
|
|
|
|||
| Acute Physiology Score | .578 ** (15.77) |
.593 ** (16.77) |
4.82 ** (13.67) |
4.94 ** (12.52) |
| Age over 40 (in years) | — | .019 ** (.83) |
— | .42 (1.62) |
| Sex (Female=1) | — | -1.94 ** (3.12) |
— | -4.86 (.70) |
| Smoking History (yes=1) | — | 2.18 ** (3.20) |
— | 12.96 (1.70) |
| Drinking History (yes=1) | — | -1.09 (1.17) |
— | 2.99 (.29) |
| Preadmission Health and Operative Status1 | ||||
| Type B Post-Operative | — | -1.10 (.99) |
— | -7.42 (.59) |
| Type C Post-Operative | — | .66 (.47) |
— | -.71 (.04) |
| Type D Post-Operative | — | -.87 (-.65) |
— | -13.92 (-.93) |
| Type A Non-Operative | — | -4.75 ** (3.40) |
— | -12.15 (.77) |
| Type B Non-Operative | — | -4.10 ** (3.34) |
— | -25.34 (1.85) |
| Type C Non-Operative | — | -4.35 ** (3.06) |
— | -28.37 (1.78) |
| Type D Non-Operative | — | -8.06 ** (5.79) |
— | -43.57 ** (2.80) |
| Principal Reason For Intensive Care2 | ||||
| Cardiovascular | — | 7.49 ** (9.12) |
— | 28.95 ** (3.16) |
| Respiratory | — | 3.33 ** (3.10) |
— | 27.88 * (2.33) |
| Gastrointestinal | — | 6.13 ** (5.17) |
— | 35.61 ** (2.69) |
| Renal | — | -.995 (.55) |
— | 7.21 (.36) |
| Hematologic | — | 1.90 (.43) |
— | 94.56 (1.93) |
| Metabolic | — | -1.57 ** (3.63) |
— | -20.68 (1.11) |
| Intercept | 17.05 ** (26.11) |
15.61 ** (14.86) |
63.98 ** (10.18) |
50.46 ** (4.30) |
| R2 | .29 | .51 | .23 | .30 |
| F | 248.7 ** | 34.9 ** | 186.9 ** | 13.82 ** |
| N | 613 | 613 | 613 | 613 |
t-ratios are in parentheses.
p<.05.
p<.01.
The reference group for preadmission health type is Type A Post-Operative.
The reference group for systems is Neurologic.
The second two equations in Table A-1 replicate the first two, though, as one would expect, the explanatory power is decreased because of the longer time period of subsequent therapy captured by the dependent variable. The difference in magnitude between the first two columns and the second pair of columns reflects scale differences in the measurement of the dependent variable. Again the strong significance and stability of the APS coefficient between the two equations is of particular importance. This stability implies that though patient age, chronic health status, surgical status, and diagnostic data influence subsequent therapeutic effort in an ICU, they do not bias the influence of the APS. More than 75 percent of the explanatory power is captured by the APS.
Table A-2 reports simple and multivariate probit equations predicting hospital survival. This nonlinear regression technique is chosen as an appropriate statistical technique because the dependent variable is dichotomous (Tobin, 1958, Pindyck and Rubinfeld, 1976). An alternate regression technique for dichotomous dependent variables, logistic regression, produces virtually identical results to those reported here. One important implication of using probit or logistic regression is that the functional form of the relationship between the independent and dependent variables is assumed to be bell-shaped, as opposed to the constant partial derivative assumed in ordinary least squares regression.
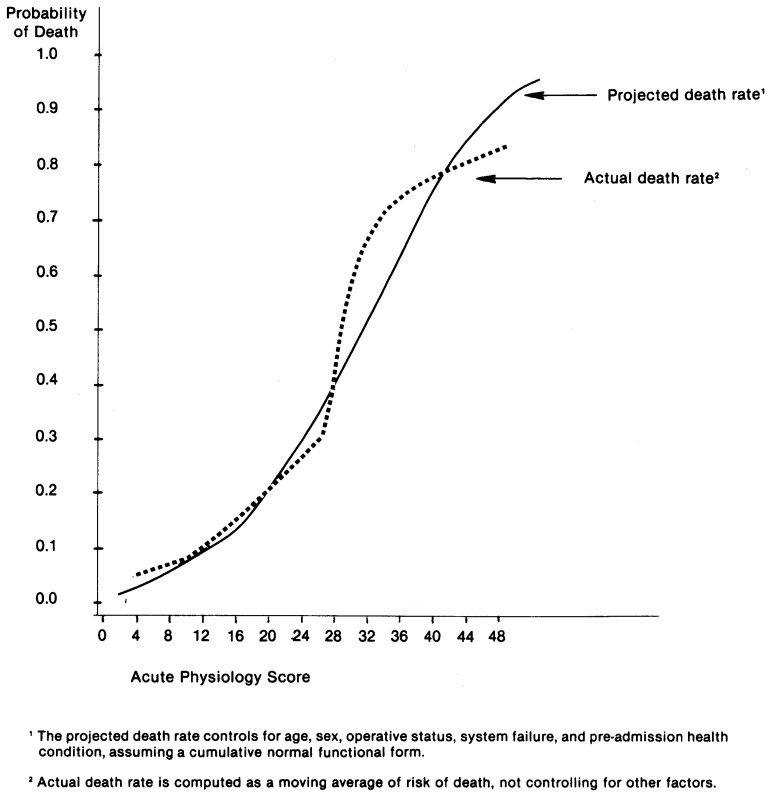
The interesting and important results in Table A-2 are that the regression coefficient on the APS is highly significant and quite stable in the two equations. The magnitude of the coefficients implies that in the vicinity of the sample mean, an extra point of the APS is associated with a 2 percent increase in the probability of death. A more detailed description of the nonlinear relationship between probability of death and the APS is plotted in Figure A-1. The solid line in Figure A-1 represents the estimated relationship between the APS and the probability of in-hospital death, controlling for the impact of age, sex, smoking and drinking history, pre-admission health status, operative status, and principal reason for intensive care. The dotted line represents a simpler statistic, a moving average of the death rate for these ICU admissions. The latter is an assumption-free relationship, but it does not control for the possible confounding influence of other variables. For both curves, the slope at any point represents the marginal probability of death per extra APS point.
Table A-2. Determinants of Hospital Survival: Probit Analysis.
| Simple | Multivariate | |
|---|---|---|
|
|
|
|
| Acute Physiology Score | .0729** (10.78) |
.0853 ** (9.64) |
| Age over 40 (in years) | — | .033 ** (5.54) |
| Sex (Female=1) | — | .237 (1.53) |
| Smoking History (yes=1) | — | .473 ** (2.93) |
| Drinking History (yes=1) | — | -.244 (.179) |
| Preadmission Health and Operative Status1 | ||
| Type B Post-Operative | — | -.404 (1.37) |
| Type C Post-Operative | — | -.369 (1.03) |
| Type D Post-Operative | — | -.427 (1.26) |
| Type A Non-Operative | — | .432 (1.28) |
| Type B Non-Operative | — | -.048 (.16) |
| Type C Non-Operative | — | -.369 (1.05) |
| Type D Non-Operative | — | .708 * (2.22) |
| Principal Reason for Intensive Care2 | ||
| Cardiovascular | — | -.329 (1.66) |
| Respiratory | — | -.851 ** (3.07) |
| Gastrointestinal | — | -.111 (.42) |
| Renal | — | -.830 (1.55) |
| Hematologic | — | 1.18 (1.07) |
| Metabolic | — | -1.83 ** (3.63) |
| Intercept | -2.13 ** (15.82) |
-2.82 ** (9.22) |
| Percent of Cases Correctly Classified | 85% | 88% |
| N | 613 | 613 |
Note: Asymptotic t-ratios are in parentheses: significance levels are approximate.
p<.05.
p<.01.
The reference group for preadmission health type is Type A Post-operative.
The reference group for systems is Neurologic.
Figure A-1. Relationship Between Acute Physiology Score and Death Rate.
Table A-3 summarizes the explanatory power of the second equation in Table A-2 by comparing predicted outcomes with actual outcomes.
Another statistical test of the robustness of the APS as a severity of illness score relates to the sensitivity of the score to diagnostic case mix. We have not used principal discharge diagnosis as an independent variable, principally because this group of 613 admissions has 209 different principal diagnoses at the 3-digit level. Within each of the seven indications for admission, however, there is a grouping of similar H-ICDA diagnostic codes. Table A-4 reports probit multiple regression equations for 4 of these mutually exclusive but non-exhaustive subgroups of the UH admissions. The results document that the APS is significant within each of the indications for admission as well as across all admissions. In addition, the magnitude of the coefficients is reasonably similar in each of the four equations, particularly given the small sample sizes and small number of deaths in 3 of the 4 groups.
Several other statistical tests of robustness of the APS score are not reported here. One simple question would be what happens to the APS coefficient in the mortality equation when TISS is introduced as an independent variable? Elsewhere we have reported that the predictive power of the APS is essentially unchanged, but the predictive power of total TISS points is changed substantially (Scheffler, et al., 1981).
A final question relates to the amount of time elapsing between the measurement of the APS and the death of a patient. It would be possible to obtain excellent predictive power in an equation merely by measuring physiological parameters shortly before death. However, the data analyzed here excludes values measured shortly before death and most deaths occurred substantially after the measurement of the APS. Elsewhere we reported essentially identioal results on a subset of 582 of the 613 admissions reported here (Knaus, et al., 1981). The difference in samples is that the subset of 582 admissions excluded all patients who were discharged from the ICU for any reason during their first two shifts in the unit. The exclusion of those 31 patients, about half of whom died, improved the explanatory power of both the TISS and death equations. We believe the improvement occurs because a few of the patients who were discharged quickly from the ICU were not in the ICU long enough to receive much therapy or have a reasonably full set of physiological parameters measured.
In summary, this appendix demonstrates that the APS is a strong and consistent predictor of two subsequent events—mortality and total therapeutic effort in the ICU—in 613 patients in one university hospital. The magnitude of the relationship appears to be insensitive to diagnostic mix, though more thorough testing of this phenomenon awaits a larger data base.
Before this index could be used for reimbursement, planning, or evaluation purposes it should be externally validated with a multi-institutional data base. This validation is necessary since any index related to outcome should not be the result of one institution's experience but should be validated against a group of reference hospitals chosen because of their high quality of care.
Table A-3. Classification Matrix of Acute Physiology Score of 613 Consecutive University Hospital ICU Admissions.
| Prediction1 | Confirmed Hospital Condition Discharge | |
|---|---|---|
| Alive | Dead | |
|
|
||
| Predicted to Live | 477 | 56 |
| Predicted to Die | 18 | 62 |
| Total Misclassification Rate = 12% | ||
| Sensitivity = 477/495 = 96% | ||
| Specificity = 62/118 = 53% | ||
| Positive Predictive Value = 477/533 =89% | ||
| Negative Predictive Value = 62/80 =78% | ||
| False Positive Rate =100% - 89% =11% | ||
| False Negative Rate =100% - 78% =22% | ||
A predicted probability of .50 is the criteria dividing the two predictions.
Table A-4. Multiple Probit Analysis of Hospital Survival Within Broad Diagnostic Groupings.
| Neurologic | Cardiovascular | Respiratory | Gastrointestinal | |
|---|---|---|---|---|
|
|
||||
| Acute Physiology Score | .102 ** (5.35) |
.113 ** (6.39) |
.084 ** (2.97) |
.054 * (2.21) |
| Age over 40 (in years) | .031 * (2.53) |
.041 ** (3.48) |
.058 ** (2.83) |
.022 (1.39) |
| Sex (Female=1) | -.29 (.93) |
.47 (1.67) |
-.377 (.77) |
-.124 (.70) |
| Smoking History (yes=1) | 1.09 ** (3.10) |
.358 (1.29) |
.058 (.125) |
.39 (.79) |
| Drinking History (yes=1) | -.082 (.98) |
.234 (.572) |
-.38 (.52) |
-.06 (.12) |
| Preadmission Health and Operative Status1 | ||||
| Type B Post-Operative | -.436 (.92) |
-.347 (.53) |
1.42 (.006) |
5.62 (.0005) |
| Type C Post-Operative | -1.18 (1.10) |
-.89 (1.18) |
6.42 (.01) |
6.81 (.0006) |
| Type D Post-Operative | -.466 (.69) |
-.399 (.57) |
6.75 (.01) |
5.55 (.005) |
| Type A Non-Operative | .827 1.82 |
-.412 (.386) |
.145 (.01) |
5.40 (.0005) |
| Type B Non-Operative | -.068 (.13) |
.421 (.62) |
5.60 (.008) |
-.68 (.0001) |
| Type C Non-Operative | .032 (.048) |
-.12 (.17) |
4.27 (.006) |
5.02 (.0004) |
| Type D Non-Operative | .832 (1.36) |
1.147 (1.67) |
6.58 (.01) |
5.77 (.53) |
| Intercept | -2.98 ** (5.81) |
-4.05 ** (5.13) |
-9.47 (.015) |
-1.33 (.0) |
| Percent of Cases Correctly Classified | 90% | 90% | 87% | 85% |
| Death Rate | 14% | 24% | 19% | 27% |
| N | 195 | 234 | 79 | 55 |
Note: Asymptotic t-ratios are in parentheses: significance levels are approximate.
p<.05.
p<.01.
The reference group for preadmission health type is Type A Post-operative.
Footnotes
On the basis of available data, we estimate that there are approximately 16,000 CCU beds and 39,000 multi-disciplinary ICU beds nationwide, excluding approximately 6,000 pediatric and neonatal ICU beds.
There were 209 different principal discharge diagnoses at the 3 digit level among the 613 UH patients.
In work in progress we have found a high correlation (r > .6) within narrow diagnostic groups between TISS points and total charges for ancillary services during the patient's ICU stay.
The following reported results are not sensitive to the truncation.
References
- American Hospital Association. Hospital Statistics, 1980 Edition. Chicago, Illinois: 1980. [Google Scholar]
- Byrick PJ, Mindorff C, McKee L, et al. Cost-effectiveness of Intensive Care for Respiratory Failure Patients. Critical Care Medicine. 1980;8:332. doi: 10.1097/00003246-198006000-00003. [DOI] [PubMed] [Google Scholar]
- Cullen DJ, Civetta JM, Briggs BA, et al. Therapeutic Intervention Scoring System: A Method for Quantitative Comparison of Patient Care. Critical Care Medicine. 1974;2:57. [PubMed] [Google Scholar]
- Cullen DJ, Ferrara LC, Briggs BA, et al. Survival, Hospitalization Charges and Follow-up Results in Critically III Patients. New England Journal of Medicine. 1976;294:982. doi: 10.1056/NEJM197604292941805. [DOI] [PubMed] [Google Scholar]
- Department of Public Health, State of Massachusetts. Determination of Need Guidelines for Intensive and Coronary Care Units. 1978 [Google Scholar]
- Griner PF. Treatment of Acute Pulmonary Edema: Conventional or Intensive Care. Annals of Internal Medicine. 1972;77:501–4. doi: 10.7326/0003-4819-77-4-501. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper E, Lawrence DE, Wagner DP, Zimmerman JE. Neurosurgical Admissions to the ICU: Intensive Monitoring Versus Intensive Therapy. Neurosurgery. 1981;8:438–442. doi: 10.1227/00006123-198104000-00006. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Thibault GE. Intensive Care Units Today. In: Cravalho EG, McNeilt BJ, editors. Critical Issues in Medical Technology. Auburn Press; Boston, Mass: 1981. in press. [Google Scholar]
- Knaus WA, Wagner DP, Draper EA, Lawrence DE, Zimmerman J. The Range of ICU Services Today. JAMA. 1981 in press. [PubMed] [Google Scholar]
- Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-Acute Physiology and Chronic Health Evaluation: A Physiologically Based Classification System. Critical Care Medicine. 1981;9:591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- Luft HS. The Relation Between Surgical Volume and Mortality: An Exploration of Causal Factors and Alternative Models. Medical Care. 1980;18:940. doi: 10.1097/00005650-198009000-00006. [DOI] [PubMed] [Google Scholar]
- Luft HS, Bunker JP, Enthoven AC. Should Operations be Regionalized? An Empirical Study of the Relation Between Surgical Volume and Mortality. New England Journal of Medicine. 1979;301:1364. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- Pindyck RS, Rubinfeld DL. Econometric Models and Economic Forecasts. New York: McGraw-Hill; 1976. [Google Scholar]
- Russell LB. Technology in Hospitals. The Brookings Institution; Washington, D.C.: 1979. [Google Scholar]
- Salkever DS, Bice TS. The Impact of Certificate-of-Need Controls on Hospital Investment. Milbank Memorial Fund Quarterly: Health and Society. 1976;54:185. [PubMed] [Google Scholar]
- Scheffler RM, Knaus WA, Wagner DP, Zimmerman JE. Severity of Illness and the Relationship Between Intense Treatment and Survival. George Washington University; 1981. submitted for publication. [Google Scholar]
- Thibault GE, Mulley AG, Barnett CO, et al. Medical Intensive Care: Indications, Interventions and Outcomes. The New England Journal of Medicine. 1980;302:938–42. doi: 10.1056/NEJM198004243021703. [DOI] [PubMed] [Google Scholar]
- Tobin J. Estimation of Relationships for Limited Dependent Variables. Econometrica. 1958;26:24–36. [Google Scholar]
- U.S. Government Printing Office. Planning for Intensive Care Units: A Technical Assistance Document for Planning Agencies. Arthur D Little, Inc.; Washington, D.C.: 1979. DHEW Publication No. (HHS) 79-14020. [Google Scholar]