Abstract
Like other cellular modules, the secretory pathway and the Golgi complex are likely to be supervised by control systems that support homeostasis and optimal functionality under all conditions, including external and internal perturbations. Moreover, the secretory apparatus must be functionally connected with other cellular modules, such as energy metabolism and protein degradation, via specific rules of interaction, or “coordination protocols”. These regulatory devices are of fundamental importance for optimal function; however, they are generally “hidden” at steady state. The molecular components and the architecture of the control systems and coordination protocols of the secretory pathway are beginning to emerge through studies based on the use of controlled transport-specific perturbations aimed specifically at the detection and analysis of these internal regulatory devices.
Modularity, control systems and coordination protocols in manmade and biological machines
Recent findings on the regulation of the secretory pathway [1,2] can be best understood in the context of the theory of control [3]. It is generally accepted that complex cellular behaviors are achieved through an internal organization based on modularity [4–6]. According to this concept, cells are composed of modules, or subsystems, often embodied in physical cellular structures, or organelles, that execute functions, such as protein synthesis or folding, metabolism, membrane transport, autophagy, and apoptosis [4–7]. Modules are partially independent of each other, but their activities must be coordinated to achieve harmonic global responses [5–7]. Moreover, modules and the related organelles must be able to preserve their homeostasis and to operate in a complex, sensitive, and robust fashion [8]. These features are essential for cells and cellular subsystems to survive and compete. But in what way and through which sophisticated regulatory devices is all this achieved?
Robustness, complexity, sensitivity and so on are also features that modern engineers tend to incorporate into manmade machines [4–7]. The theory of control has been developed over the last several decades to build these features into machines such as, airplanes, production plants, and cars. Later, the theory was applied to the study of cells and organisms. A central concept in control theory is that of the control system. Control systems are devices that regulate basic processes executed by a machine. They usually consist of a sensor to measure the output variables of the process of interest (and their possible deviations from a desired set-point), a computer to calculate the proper response (generally based on the negative feedback principle), and an actuator to bring about the desired response (see [9] for a simple introduction to control theory). The main goal of control systems is to optimize the function of complex machines, often by maintaining their homeostasis despite internal or external perturbations that otherwise might have disruptive effects [5,6,10]. Another important concept, in modular systems, such as cells, electronic circuits, or production plants, is that the activities of different modules must be coordinated by specific protocols (see above). The “coordination protocols” are the rules that manage the interface between modules (that is, in physical terms, the operation of the molecular pathways that coordinate separate modules) [5,6].
Do control systems that are similar to those designed by engineers exist in biological organisms? In the first approximation, this seems a surprising notion since biological evolution has not followed an engineering blueprint. Scientists, mostly those with a background in engineering or physics, have examined whether in biology there exist control systems that resemble the synthetic ones [5–7]. To this end, they have analyzed and modeled biological responses that previously had been extensively characterized at the molecular level, frequently in bacteria, to identify their (potential) control devices [3,5–7]. In most, if not all, of the cases studied, it has emerged that “classical” control systems do exist in biology and that they resemble the engineered ones. Although the physical implementations of control in the two areas (biology and engineering) are very different (most of the biological control systems are mediated by signaling or transcriptional pathways or both), the designs and the roles of control systems can be quite similar in, for instance, human cells and airplanes. Thus, the evolution of biological and engineered machines appears to have resulted in similar regulatory schemes, despite the different nature of the two sets of objects (however, the principle of survival of the fittest probably applies to the evolution of both). To reflect this notion, the concept of convergent evolution between biological and manmade systems has been coined [5,6]. Notably, whereas the idea of modularity is now commonplace in the biological community, the concepts of control systems and coordination protocols are used mostly by control engineers interested in comparing the properties of biological control systems with those of manmade devices but are used very rarely, if at all, by cell biologists.
Uncovering the control systems and coordination protocols of membrane transport
With the above considerations in mind, we have designed a research plan toward the identification of the control systems and coordination protocols of the membrane transport apparatus, and, more specifically, of the major traffic station called the Golgi complex. Membrane traffic is a fundamental housekeeping process by which roughly a third of the mammalian proteins are transported from their site of synthesis, the endoplasmic reticulum (ER), through a series of separated membranous compartments until they reach their cellular destinations in their final processed forms. Transport involves membrane fluxes across the transport compartments and is exposed to internal and external perturbations [1,11,12]. Such perturbations have the potential to disrupt the transport apparatus, particularly if they occur at the interface between the ER and the Golgi complex. The ER is up to 10-fold larger than the Golgi [13], and changes in its membrane output can profoundly alter Golgi morphology and composition if not compensated for by corresponding adaptive changes in the retrograde or anterograde traffic out of the Golgi [1,13–17]. However, the transport apparatus always maintains or rapidly recovers its homeostasis [1,11,12]. We examined whether such efficient adaptive responses [11,12] might be brought about by suitable control systems.
To uncover the transport control systems, we used controlled transport-specific perturbations and a two-stage strategy. The first stage was to apply a perturbation to the secretory pathway to identify the resulting molecular and functional responses, with a focus on signaling and transcriptional changes (see above). At least some of these changes are expected to be components of the reaction of the control system(s) to the perturbation. Second, the molecular components that were changed in the first stage were analyzed, and their roles in the adaptive response to the perturbation were verified and characterized [1,2]. Through this approach, the molecular nature and the design of the Golgi control system and coordination protocols have begun to emerge and are summarized below in the form of a working model.
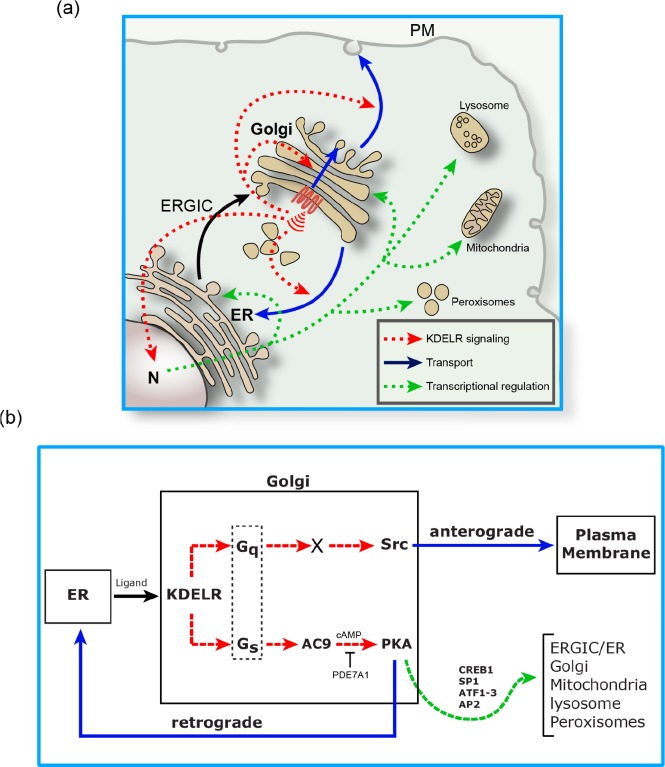
Membrane fluxes leaving the ER carry ER chaperones to the Golgi in quantities that presumably are proportional to the intensity of the flux. At the Golgi, these chaperones, which carry a KDEL signal at their C-terminus, encounter and bind to the KDEL receptor (KDELR), a seven-transmembrane domain protein belonging to the PQ-loop protein family [18] that is distantly related to the G protein-coupled receptor (GPCR) superfamily [19,20] and resembles the GPCRs in the topology and fold of the transmembrane helices [21]. The KDELR has long been considered a transport machinery protein involved in the retrieval of escaped chaperones to the ER. However, it now appears to be able to act also as a sensor of incoming traffic and as a signaling protein that activates the G proteins Gq and Gs and the relative transduction pathways (Figure 1A and 1B). Gs, when activated by the KDELR, activates adenylyl cyclase 9 and cyclic adenosine monophosphate (cAMP) formation at the Golgi, which then is partially degraded and spatially restricted by phosphodiesterase 7A1, resulting in the controlled activation of a Golgi pool of protein kinase A (PKA). PKA then phosphorylates a large number of Golgi and cytosolic proteins. Among these, the proteins relevant for retrograde traffic appear to be some coat protein complex I (COPI) subunits and actin-binding proteins [2]. This results in the activation of retrograde transport [2]. Gq, instead, appears to act by inducing a local release of calcium [22] (probably via stimulation of a phospholipase C and inositol trisphosphate production) and the activation of the kinase Src [21], which phosphorylates a large number of proteins, only a few of which are identified but some of which are involved in anterograde traffic. Indirect evidence indicates that Src regulates COPI-dependent trafficking [23,24]. Thus, a device based on the KDELR and two signaling pathways appears to sense incoming traffic into the Golgi and activate anterograde and retrograde traffic steps [25], helping to maintain Golgi homeostasis [2]. The architecture of this device is typical of a control system. The incoming chaperones (in fact, a specific class of chaperones that is currently being defined) represent a signal reflecting the transport input into the Golgi; the KDELR acts as a sensor of such a signal, Gq and Gs and the ensuing signaling pathways act as a “computer”, and the phosphorylation/activation of the traffic machinery represents the actuating device that aims to balance the transport fluxes (Figure 1) [2]. Figure 2 shows the Golgi control system(s) schematized as a block diagram (a tool for the synthesis and analysis of control systems), and its legend describes the equivalences between the elements in the diagram and the molecular pathways shown in Figure 1.
Figure 1. A controller composed of the KDEL receptor (KDELR) and the Gq and Gs signaling pathways maintains the homeostasis of membrane transport at the Golgi complex.
(A) Transport fluxes that reach the Golgi (black arrow) activate signaling responses (red dotted arrows) to accelerate anterograde traffic through the Golgi to the plasma membrane (PM) as well as retrograde traffic to the endoplasmic reticulum (ER) (blue arrows). A prolonged activation of this Golgi signaling results in transcriptional regulation of many transport machinery genes and other genes (green dotted arrows) presumably to support long-term adaptations of the transport apparatus to sustained overloads. (B) Molecular components of the Golgi control system. Transport from the ER to Golgi (black arrow) carries ER chaperones to the Golgi, where they bind to, and activate, the KDELR that promotes the activation of Golgi Gq and Gs (red dotted arrows). Gq activation induces the acceleration of anterograde traffic through the Golgi and transport from Golgi to PM by the activation of Src and a phosphorylation cascade (blue arrow). Gs leads to the activation of the Golgi-based adenylyl cyclase AC9 to increase cAMP levels (spatially restricted within the Golgi area by PDE71A). Cyclic adenosine monophosphate (cAMP) then activates protein kinase A (PKA), which activates retrograde transport via the phosphorylation of specific components to regulate retrograde traffic (blue arrow). The prolonged activation of the KDELR and of PKA results in the phosphorylation and activation of CAMP-responsive element binding protein 1 (CREB1) and possibly of other cAMP/PKA-regulated transcription factors (SP1, AP2, and ATF1/3). This results in the upregulation of many transport machinery genes as well as of a large number of genes involved in other functions, such as lipid and energy metabolism (green dotted arrows). The original observations on which these schemes are based upon are reported in [1,2,21]. ERGIC, endoplasmic reticulum-Golgi intermediate compartment; N, nucleus.
Figure 2. The Golgi control system as represented by a block diagram.
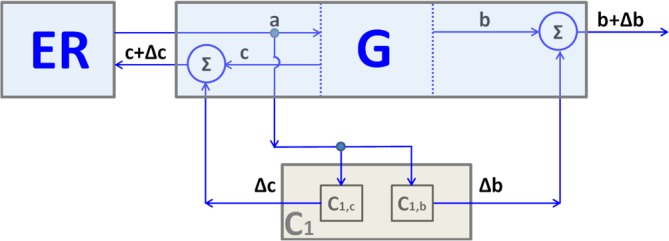
A flux of transport membranes leaves the endoplasmic reticulum (ER, light blue box), providing a membrane input (input a) into the Golgi (G). At the same time, membrane fluxes leave the Golgi in the anterograde direction toward the plasma membrane (PM) (G output b) and in the retrograde direction to the ER (G output c). The membrane flux (a) is sensed by the Golgi control system (grey box C1). C1 is composed of two subsystems: C1,b and C1,c. The subsystem C1,b controls anterograde flux through a sensor that measures the a value, a computer that calculates the response, and an actuator that activates the flux b by the factor Δb= kb×a; kb: constant value, kb≥0. In molecular terms (Figure 1), the sensor here is the KDEL receptor (KDELR), the a value is the transport flux that reaches the Golgi and carries chaperones that bind to, and are sensed by, the KDELR, the “computer” is the Gq-X-Src pathway, and the actuator is the Src kinase that phosphorylates and activates components of the anterograde transport machinery. The subsystem C1,c controls retrograde flux by sensing flux a and activating flux c by the factor Δc= kc×a; kc: constant value, kc≥0. Here, the signal and the sensor are the same as above (chaperones and the KDELR), the “computer” is the AC7-PDE7-PKA pathway, and the actuator is PKA, which phosphorylates/activates components of the retrograde transport machinery (Figure 1).
In addition to regulating the Golgi in an acute fashion, the system exerts a long-term control on the transport machinery components. Thus, the KDELR upregulates the expression of many genes that code for proteins of biosynthetic and endolysosomal organelles, such as SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors), adaptor complexes, GTPases, chaperones, Golgi, and lysosomal enzymes. Other upregulated genes, however, belong to different functional modules, such as energy metabolism, peroxisomal and lipid metabolism, and protein degradation, including autophagy, indicating that membrane transport is functionally interconnected with these modules. Most of the transcription factors involved are cAMP-dependent. Thus, these findings begin to identify cellular modules that operate in coordination with membrane traffic as well as the molecular components of the involved coordination protocols (the above transcription factors) [2].
In terms of design, the control device in Figures 1 and 2 appears to be based on the functional connections between successive traffic steps. Thus, traffic from the ER to the Golgi activates anterograde traffic through the Golgi and retrograde traffic from the Golgi to the ER. This decreases the load of membrane and proteins that accumulate in the Golgi and hence favors homeostasis. The diagram in Figure 2 also stimulates hypotheses on the existence and the kind of other possible control systems that might operate in the secretory pathway. For instance, it is reasonable to suspect that a control system might exist and function at the ER, where the accumulation of folded proteins ready for export might activate the next step, ER export, or at the trans-Golgi network, where the arrival of apical or basolateral cargo might activate the relevant Golgi export pathways. This diagram also raises the question of whether the controls described so far are sufficient to maintain homeostasis and optimal functionality of transport. For instance, it seems logical to hypothesize a size sensor for the main transport compartments to regulate the overall morphology of the transport organelles. Moreover, screening experiments have shown that a large number of kinases are capable of affecting the morphology, function, and biochemical properties (glycosylation) of the Golgi and ER-Golgi interface [26,27], suggesting that the potential for the regulation of the transport control system is far higher than we can currently demonstrate. It remains to be clarified, however, whether these kinases belong to cell-autonomous control systems, such as those discussed in this article or to “traditional” signaling pathways originated by exogenous regulatory inputs.
An important question is how broadly the Golgi control systems are conserved across species. It could be argued that, in yeast, some form of control is embedded in the basic transport effector machinery [28]. However, the yeast secretion apparatus appears to be potently controlled by signaling. Thus, glucose controls yeast secretion via signaling pathways that include GPCRs and PKA [29–31], osmolarity affects the secretory machinery via the HOG1 pathway [32,33], and the KDELR is essential for secretory traffic in yeast via a mechanism that is not understood but might well be mediated by signaling [34]. All of this is consistent with the possibility that homeostatic mechanisms based on signaling pathways, such as those described in mammals may also exist in yeast.
Another question arises from the fact that mammalian cells are wired to function within a multicellular context and hence that internal and external regulatory signals are probably deeply interconnected. This is an exceedingly complicated subject; however, some insight might come from a simple example, such as the mechanisms of temperature control (in both manmade and biological systems). Temperature control devices comprise a heater and a control tool (a thermostat) that acts to maintain the temperature at a desired set-point of the temperature generated by the heater. This system is stable, but it can be modified by an external regulatory signal designed to change the set-point by acting on the control system itself (the thermostat). The control system will then operate to maintain the new set-point. Similarly, we can imagine that in a multi-modular biological machine, such as a cell, various control systems act to maintain the cellular modules at the desired set-points of activity (with coordinating protocols operating to harmonize the functions of the modules). External regulatory signals may act to alter the set-points of the internal control subsystems and to switch on/off, or change the intensity of, the connectivity across different modules, resulting in overall changes of the cell’s functional organization. Thus, within this scenario, control systems act to maintain a given optimal functional configuration of a cell, whereas external regulatory inputs act to induce configuration changes that are suitable to respond to new needs.
Perspectives
The use of module-specific perturbations to discover hidden internal regulatory devices (Pulvirenti et al. [1], 2008; Cancino et al. [2], 2014) might be of general interest. Over the last 20 years, the basic cellular machineries underlying most if not all of the basic cellular processes, such as protein synthesis, membrane transport, and energy metabolisms, have been extensively investigated and elucidated. By contrast, only a small fraction of the control systems and coordination protocols that regulate such processes have even been recognized, and no systematic effort has been put into the identification of their molecular components and design [35]. A reason for this knowledge gap is probably that these internal regulatory devices are normally “hidden” by the smooth working of the cellular apparatuses unless specific perturbations designed to reveal their existence are applied. The apparently enormous functional complexity of the cell, much of which relates to regulatory complexity, is one of the most daunting difficulties in modern cell biology. It is conceivable that some of the concepts offered by the theory of control, coupled with perturbation-based strategies similar to those used for the analysis of transport (Pulvirenti et al. [1], 2008; Cancino et al. [2], 2014), might provide the investigator with ways to detect the control systems and coordination protocols operating within individual modules and hence to break down the regulatory complexity into manageable units. Once these units are recognized, classical molecular approaches can be used to dissect at the molecular and design level, and the individual devices, when assembled, generate the complexity. A description of the control systems that regulate the cellular modules, and of the coordination protocols that interconnect them, has the potential to provide at least a “coarse grain” level of understanding of complex cellular behaviors.
Finally, the study of the cellular control systems is likely to be relevant for studying pathological conditions and for identifying pharmacological targets. For instance, the analysis of the control systems of protein folding, and of the cell cycle, has already led to major advances in translational areas [36]. In the case of transport, the KDELR has been implicated in the clearance of neurodegeneration-inducing proteins, such as superoxide dismutase 1, α-synuclein, and huntingtin, possibly through the modulation of autophagy [37], and has been proposed to have pro-survival properties during the unfolded protein response [38]. Some genetic and proliferative diseases appear to originate from transport defects [39,40]. Understanding the control systems of the defective transport processes might lead to treatments of the related diseases.
Acknowledgments
The authors acknowledge the financial support of the AIRC (Italian Association for Cancer Research, IG 10593), the MIUR Project “FaReBio di Qualità”, the PON projects 01/00117 and 01-00862, PONa3-00025 (BIOforIU), PNR-CNR Aging Program 2012-2014, and Progetto Bandiera “Epigen”.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- COPI
coat protein complex I
- ER
endoplasmic reticulum
- GPCR
G protein-coupled receptor
- Gs/Gq
heterotrimeric G proteins
- KDELR
KDEL receptor
- PKA
protein kinase A
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/88
References
- 1.Pulvirenti T, Giannotta M, Capestrano M, Capitani M, Pisanu A, Polishchuk RS, San Pietro E, Beznoussenko GV, Mironov AA, Turacchio G, Hsu VW, Sallese M, Luini A. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008;10:912–22. doi: 10.1038/ncb1751. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1119969
- 2.Cancino J, Capalbo A, Di Campli A, Giannotta M, Rizzo R, Jung JE, Di Martino R, Persico M, Heinklein P, Sallese M, Luini A. Control systems of membrane transport at the interface between the endoplasmic reticulum and the Golgi. Dev Cell. 2014;30:280–294. doi: 10.1016/j.devcel.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Iglesias PA, Ingalls BP. Control theory and systems biology. Cambridge: MIT Press; 2009. [Google Scholar]
- 4.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718581364
- 5.Stelling J, Sauer U, Szallasi Z, Doyle FJ, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–85. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718688752
- 6.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–9. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1004690
- 7.Sontag ED. Some new directions in control theory inspired by systems biology. SystBiol (Stevenage) 2004;1:9–18. doi: 10.1049/sb:20045006. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877834
- 8.Waldherr S, Eissing T, Allgöwer F. World Congress. Elsevier; 2008. Analysis of Feedback Mechanisms in Cell-Biological Systems; pp. 15861–6. [Google Scholar]
- 9.Control system. http://en.wikipedia.org/wiki/Control_system
- 10.Kitano H. Towards a theory of biological robustness. Mol Syst Biol. 2007;3:137. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1091011
- 11.Mironov AA, Beznoussenko GV, Nicoziani P, Martella O, Trucco A, Kweon HS, Di Giandomenico D, Polishchuk RS, Fusella A, Lupetti P, Berger EG, Geerts WJ, Koster AJ, Burger KN, Luini A. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol. 2001;155:1225–38. doi: 10.1083/jcb.200108073. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1003081
- 12.Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts , Willie J C, Koster AJ, Burger , Koert N J, Mironov AA, Luini A. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–81. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1022128
- 13.Griffiths G, Warren G, Quinn P, Mathieu-Costello O, Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984;98:2133–41. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klumperman J. Transport between ER and Golgi. CurrOpin Cell Biol. 2000;12:445–9. doi: 10.1016/S0955-0674(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Menárguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- 16.Thor F, Gautschi M, Geiger R, Helenius A. Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic. 2009;10:1819–30. doi: 10.1111/j.1600-0854.2009.00989.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1168077
- 17.Wieland FT, Gleason ML, Serafini TA, Rothman JE. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- 18.Saudek V. Cystinosin, MPDU1, SWEETs and KDELR belong to a well-defined protein family with putative function of cargo receptors involved in vesicle trafficking. PLoS ONE. 2012;7:e30876. doi: 10.1371/journal.pone.0030876. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877823
- 19.Yee DC, Shlykov MA, Västermark A, Reddy VS, Arora S, Sun EI, Saier MH. The transporter-opsin-G protein-coupled receptor (TOG) superfamily. FEBS J. 2013;280:5780–800. doi: 10.1111/febs.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718088376
- 20.Zhai Y, Heijne WH, Smith DW, Saier MH. Homologues of archaeal rhodopsins in plants, animals and fungi: structural and functional predications for a putative fungal chaperone protein. Biochim Biophys Acta. 2001;1511:206–23. doi: 10.1016/S0005-2736(00)00389-8. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877824
- 21.Giannotta M, Ruggiero C, Grossi M, Cancino J, Capitani M, Pulvirenti T, Consoli Grazia Maria Letizia, Geraci C, Fanelli F, Luini A, Sallese M. The KDEL receptor couples to Gαq/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 2012;31:2869–81. doi: 10.1038/emboj.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717952359
- 22.Micaroni M, Mironov AA. Roles of Ca and secretory pathway Ca-ATPase pump type 1 (SPCA1) in intra-Golgi transport. Commun Integr Biol. 2010;3:504–7. doi: 10.4161/cib.3.6.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877825
- 23.Tisdale EJ, Artalejo CR. Src-dependent aprotein kinase C iota/lambda (aPKCiota/lambda) tyrosine phosphorylation is required for aPKCiota/lambda association with Rab2 and glyceraldehyde-3-phosphate dehydrogenase on pre-golgi intermediates. J Biol Chem. 2006;281:8436–42. doi: 10.1074/jbc.M513031200. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877826
- 24.Bard F, Mazelin L, Péchoux-Longin C, Malhotra V, Jurdic P. Src regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum. J Biol Chem. 2003;278:46601–6. doi: 10.1074/jbc.M302221200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877827
- 25.Cancino J, Jung JE, Luini A. Regulation of Golgi signaling and trafficking by the KDEL receptor. Histochem Cell Biol. 2013;140:395–405. doi: 10.1007/s00418-013-1130-9. [DOI] [PubMed] [Google Scholar]
- 26.Farhan H, Wendeler MW, Mitrovic S, Fava E, Silberberg Y, Sharan R, Zerial M, Hauri H. MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J Cell Biol. 2010;189:997–1011. doi: 10.1083/jcb.200912082. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/5374958
- 27.Chia J, Goh G, Racine V, Ng S, Kumar P, Bard F. RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells. Mol Syst Biol. 2012;8:629. doi: 10.1038/msb.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717967470
- 28.Heinrich R, Rapoport TA. Generation of nonidentical compartments in vesicular transport systems. J Cell Biol. 2005;168:271–80. doi: 10.1083/jcb.200409087. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1023752
- 29.Aoh QL, Graves LM, Duncan MC. Glucose regulates clathrin adaptors at the trans-Golgi network and endosomes. Mol Biol Cell. 2011;22:3671–83. doi: 10.1091/mbc.E11-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963234
- 30.Levi SK, Bhattacharyya D, Strack RL, Austin JR, Glick BS. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11:1168–79. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877828
- 31.Versele M, Lemaire K, Thevelein JM. Sex and sugar in yeast: two distinct GPCR systems. EMBO Rep. 2001;2:574–9. doi: 10.1093/embo-reports/kve132. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877835
- 32.Piao H, MacLean Freed J, Mayinger P. Metabolic activation of the HOG MAP kinase pathway by Snf1/AMPK regulates lipid signaling at the Golgi. Traffic. 2012;13:1522–31. doi: 10.1111/j.1600-0854.2012.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877829
- 33.Reynolds TB, Hopkins BD, Lyons MR, Graham TR. The high osmolarity glycerol response (HOG) MAP kinase pathway controls localization of a yeast golgi glycosyltransferase. J Cell Biol. 1998;143:935–46. doi: 10.1083/jcb.143.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877830
- 34.Semenza JC, Hardwick KG, Dean N, Pelham HR. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990;61:1349–57. doi: 10.1016/0092-8674(90)90698-E. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718699129
- 35.Cancino J, Luini A. Signaling circuits on the Golgi complex. Traffic. 2013;14:121–34. doi: 10.1111/tra.12022. [DOI] [PubMed] [Google Scholar]
- 36.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877831
- 37.Wang P, Li B, Zhou L, Fei E, Wang G. The KDEL receptor induces autophagy to promote the clearance of neurodegenerative disease-related proteins. Neuroscience. 2011;190:43–55. doi: 10.1016/j.neuroscience.2011.06.008. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877832
- 38.Yamamoto K, Hamada H, Shinkai H, Kohno Y, Koseki H, Aoe T. The KDEL receptor modulates the endoplasmic reticulum stress response through mitogen-activated protein kinase signaling cascades. J Biol Chem. 2003;278:34525–32. doi: 10.1074/jbc.M304188200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877833
- 39.De Matteis MA, Luini A. Mendelian disorders of membrane trafficking. N Engl J Med. 2011;365:927–38. doi: 10.1056/NEJMra0910494. [DOI] [PubMed] [Google Scholar]
- 40.Scott KL, Kabbarah O, Liang M, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, Huang J, Saci A, Widlund HR, Fisher DE, Xiao Y, Rimm DL, Protopopov A, Wong K, Chin L. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–90. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1163201