Abstract
Natural killer (NK) cells are lymphocytes that are important for early and effective immune responses against infections and cancer. In the last 40 years, many receptors, their corresponding ligands and signaling pathways that regulate NK cell functions have been identified. However, we now know that additional processes, such as NK cell education, differentiation and also the formation of NK cell memory, have a great impact on the reactivity of these cells. Here, we summarize the current knowledge about these modulatory processes.
Introduction
In the mid-1970s, a novel immune cell type was described based on its ability to lyse allogeneic tumor cells without the need for prior sensitization. The term “natural cytotoxicity” was introduced to describe this feature and the cells mediating this effect were named NK cells [1–5]. In the last 40 years, much progress has been made in the understanding of the function and regulation of NK cells. We now know that NK cells contribute to effective innate immune responses and provide the first important line of defense against parasites, viruses and cancer [6–10]. NK cells derive from the common lymphocyte progenitor, but they are independent of a functional thymus and rely on germ-line-encoded surface receptors that do not undergo somatic recombination. One important step for the understanding of NK cell regulation was the realization that NK cells preferentially kill cells with low or no major histocompatibility complex (MHC) class I expression that led to the formulation of the “missing-self hypothesis” [11,12]. This concept was later supported through the identification of MHC class I-specific inhibitory receptors, such as Ly49 receptors in mice and killer cell immunoglobulin-like receptors (KIRs) in humans [13–19]. These inhibitory receptors possess immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail that are phosphorylated upon binding to MHC class I. This leads to binding and activation of phosphatases, such as SHP1/2 and SH2 domain-containing inositol 5-phosphatase (SHIP), which in turn interfere with activating signaling pathways by dephosphorylation [20], effectively preventing NK cell activation.
NK cells are stimulated by a number of different activating receptors that can recognize a variety of ligands on potential target cells [21]. Engagement of these activating receptors can trigger NK cell functions via different signaling pathways [22–24]. Despite the diversity of these early signaling pathways, inhibitory receptors can effectively control NK cell activation [9,25]. It is, therefore, now generally accepted that NK cell activity is tightly regulated by an interplay between activating and inhibitory cell surface receptors. However, in recent years, it has become clear that this is not the only level at which the activity of NK cells is regulated. The fact that the triggering of the same receptor in individual NK cells does not necessarily lead to the same outcome already implies the presence of additional mechanisms for the regulation of NK cell functions. In the following article, we will describe three additional levels of NK cell regulation.
NK cell education
In accordance with the missing-self hypothesis, the “at least one” model was proposed [26]. This model assumed that NK cells need to express at least one inhibitory receptor that is specific for self-MHC class I in order to prevent autoreactivity. This hypothesis was supported by data from human NK clones that were all found to express at least one self-specific inhibitory receptor [27]. However, it was also known that NK cells from MHC class I-deficient hosts were not autoreactive despite the lack of ligands for the inhibitory receptors [28,29]. This already suggested that additional mechanisms must exist to ensure that NK cells are not autoreactive in the absence of inhibitory signaling. Indeed, it was later discovered that a significant subset of NK cells present in healthy mice and humans lack self-specific inhibitory receptors [30–32]. These NK cells were not autoreactive and were found to be hyporesponsive when triggered through activating receptor stimulation. This adaptation of the reactivity of NK cells depending on the inhibitory receptor ligand matches is generally referred to as NK cell education [26] (Figure 1) and assures the self-tolerance of NK cells.
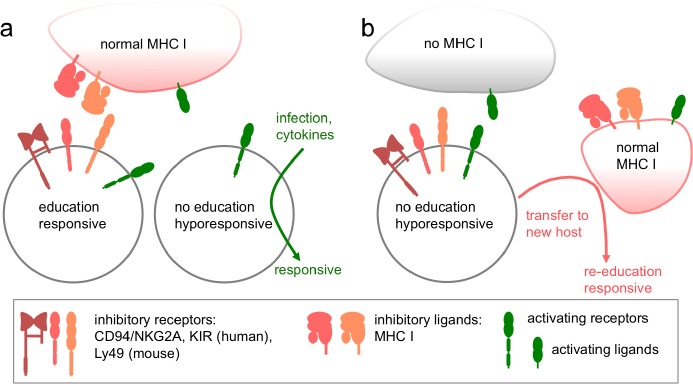
Figure 1. NK cell education: adaption of the responsiveness depending on inhibitory receptor - ligand interactions.
(a) In normal major histocompatibility complex (MHC) class I-sufficient individuals (humans and mice), NK cells expressing inhibitory receptors recognizing those MHC class I molecules become educated. Those cells are responsive to activating receptor stimulation. The subset of NK cells that lacks inhibitory receptors for self MHC class I are non-educated and hyporesponsive when triggered through activating receptor stimulation. Under certain conditions, such as infections or cytokine stimulation, this subset can become responsive.
(b) In MHC class I-deficient individuals, NK cells are non-educated and hyporesponsive due to the lack of inhibitory ligands. After transfer to a new MHC class I-sufficient host, NK cells can become “re-educated” and responsive if they express the matching inhibitory receptors.
KIR, killer cell immunoglobulin-like receptor.
Initially, two opposing mechanisms were discussed on how NK cells can become educated. In the “arming” or “licensing” model, NK cells are assumed to be inactive by default and only acquire their full functionality through the engagement and the signaling of an inhibitory receptor [33,34]. In the “disarming” model, NK cells are active by default but are rendered hyporesponsive or anergic through the continuous stimulation via activating receptors recognizing endogenous ligands. They can only maintain their functionality if this chronic stimulation is counteracted by signals of inhibitory receptors [34]. While inhibitory receptors have opposite functions in both models, the outcome would be comparable – only NK cells with an inhibitory receptor for self-MHC class I can become functionally active. Additionally, the education of NK cells is not an all or nothing decision, but can be tuned in a quantitative way. The stronger the inhibitory interaction(s) of an NK cell is, the stronger it responds to activating receptor signals [35,36]. Therefore, the rheostat model has been proposed [37]. This model does not replace but rather supplements the arming or the disarming model, and describes NK cell education as a dynamic process, rather than an on/off state.
With the data available today, both the arming and the disarming models are able to explain the observed phenotypes, but the mechanism remains unclear. The pros and cons of the different models have been extensively discussed [38,39]. It is not only inhibitory receptors that can dictate the reactivity of NK cells. The constitutive expression of a ligand for an activating NK cell receptor in mice results in the hyporesponsiveness of NK cells expressing the matching activating receptor [40,41]. This finding that chronic stimulation can render NK cells hyporesponsive would support the “disarming” model. Since many activating NK cell receptors are capable of recognizing endogenous ligands, NK cells probably have an individual activation threshold that is adjusted to their receptor expression and the available ligands [42]. Most importantly, these mechanisms must avoid autoreactivity while retaining maximal responsiveness of the NK cells toward infected or transformed cells.
While the functional mechanism of education is still unknown, we know from various studies that education is not final. The changes caused in educated and in hyporesponsive, uneducated cells are reversible, suggesting that there is plasticity in the education process. After transfer to an MHC-I0-sufficient environment, previously uneducated NK cells gained functional competence, whereas previously educated NK cells were rendered hyporesponsive in an MHC-I-deficient environment [43,44] (Figure 1). Similarly, non-educated human NK cells were shown to acquire functional inhibitory receptor expression upon stimulation with pro-inflammatory cytokines, which resulted in an educated phenotype [45]. Since this “re-education” is possible with mature cells and happens within a few days, it is likely uncoupled from the process of NK cell development in the bone marrow. However, findings from hematopoietic stem cell transplantations in humans suggest that the education of the donor NK cells remains even if the host MHC class I environment is different [46]. This would argue that at least in this setting, the cell responsible for the education of NK cells is of hematopoietic origin.
To complicate things even further, some inhibitory Ly49 receptors can also interact with their MHC class I ligand on the same cells in cis [47]. The importance of this cis binding for NK cell education is controversial. At least for some inhibitory receptors, it has been demonstrated that binding in cis contributes to this education [48,49], and the strength of the binding correlates with the potency of education [50]. However, in another experimental setup, only the interaction in trans was effective for the education of NK cells [51]. Additionally, the fact that NK cells can adjust their reactivity through a change in their MHC class I environment supports the importance of the trans interactions for the re-education [43,44].
Why are NK cells that lack sufficient inhibitory receptors for self-MHC class I only rendered hyporesponsive and why are they not deleted as in the case of autoreactive T cells? The answer to this question could be that under certain circumstances, non-educated NK cells can be beneficial to the host. During an acute virus infection, the non-educated NK cells can become functional under the influence of pro-inflammatory cytokines and can even be more efficient than educated NK cells [52] (Figure 1). Similarly, non-educated NK cells can be more effective in mediating antibody-dependent cellular cytotoxicity in neuroblastoma patients treated with an anti-GD2 antibody [53]. Under these circumstances, the lack of inhibition may make the non-educated NK cells the better effector cells.
What is the molecular mechanism that determines the reactivity of educated NK cells? Interestingly, there are only a few transcriptional changes when comparing educated with non-educated NK cells [54]. One possible mechanism that could cause these functional differences without the need for changes in gene expression is the organization of receptors in the membrane. Nanoscopic analysis revealed that in educated NK cells, activating receptors were localized in nanodomains, whereas they were confined to an actin meshwork in non-educated cells [54]. In those nanodomains, the activating receptors could have the proper environment of signaling molecules needed for efficient NK cell activation [55]. This would be consistent with the finding that the triggering of activating receptors in educated NK cells results in an efficient activation of the integrin lymphocyte function-associated antigen 1 via inside-out signaling, thereby promoting the adhesion of educated NK cells to target cells [56].
NK cell differentiation and subsets
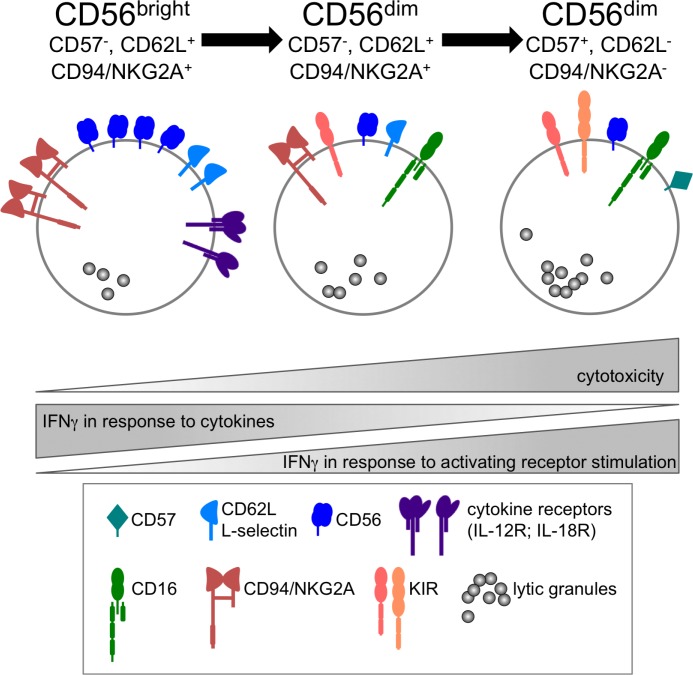
After the initial process of NK cell education, functionally competent NK cells can be found in the periphery. However, not all educated NK cells have the same functionality. Traditionally, human peripheral blood NK cells are divided in two functionally distinct subsets: CD56dim and CD56bright NK cells [57–59]. In recent years, it became clear that CD56dim NK cells can be further subdivided based on the expression of CD62L, CD57, or CD94/NKG2A [60–64]. Additionally, there is a developmental relationship between the different subpopulations, suggesting a differentiation of mature NK cells starting from CD56bright via CD56dim, CD57-, CD62L+, CD94/NKG2A+ to the more differentiated CD56dim, CD57+, CD62L-, CD94/NKG2A- NK cells [60–67] (Figure 2). Along this differentiation pathway, the functionality of NK cells change [68,69]. While CD56bright cells are not very cytotoxic, they are especially good at producing IFNγ after stimulation with pro-inflammatory cytokines, such as interleukin (IL)-12 and IL-18. This activity is gradually lost during the differentiation towards the more cytotoxic CD56dim, CD57+ NK cells. In contrast, these most differentiated NK cells can produce more interferon gamma (IFNγ) when triggered via activating surface receptors [70] and this IFNγ competence has recently been linked to the epigenetic remodeling of the IFNG promoter [71]. Therefore, the functionality of NK cells changes during their differentiation (Figure 2), which may be important for the orchestration of successful NK-mediated immune responses. Recently, a study identified several thousand distinct subpopulations of NK cells in the peripheral blood of humans [72]. If this is reflected in additional differences in functionality, this will have to be addressed.
Figure 2. Adaption of NK cell reactivity during differentiation.
Functionally distinct subsets of human NK cells differ in their surface receptor expression and their reactivity towards activating receptors triggering or cytokine stimulation. See text for details.
IFN, interferon; IL, interleukin; KIR, killer cell immunoglobulin-like receptor.
Memories of an NK cell
Recent studies have shown that NK cells can also acquire memory or memory-like functions, thereby challenging the classical distinction between innate and adaptive immunity [73]. As there are already excellent reviews about NK cell memory [73–76], we just want to briefly summarize the current knowledge about the different forms of NK cell memory that have been described so far (Table 1).
Table 1. Characteristics of NK cell memory in different models Comparison of the four major types of NK cell memory. Lymphopenia-induced memory NK cells are not shown. See text for details.
| Memory type | Liver | CMV | FcεRγ Deficiency | Cytokine-induced |
|---|---|---|---|---|
| Subpopulations | Thy1+CD11b+CD27- Thy1+Ly49C/I+ [78,116] CXCR6+ [78] CD49a+DX5-[79] |
Mouse: Ly49H+ [80] Human: NKG2C+ [86] CD57+ [92] |
CD57dimFcεRγ- [100] | CD25+ [107] |
| Antigen | Haptens or viral antigens [78,117] | m157 [80] | (m157?) | - |
| Proliferation | No [77] | Yes [80] | Unknown | Yes [96] |
| Involved cytokines | IL-12 [106] CXCL16 [78] |
IL-12, IL-15 [82] | IL-12? | IL-12, IL-15, IL-18 [94] |
| Signaling | IL-12R [106] CXCR6 [78] NKG2D [77] |
IL-12R /STAT4 [82] IL-15R CD25 [108] Ly49H/DAP12 [80] DNAM-1 via Fyn, PKC [81] Zbtb32 [84] Bim [85] miR-155 [110] |
IL-12R? | IL-12R /STAT4 IL-15R IL-18R CD25 [107] miR-155 [112] |
| Memory effect | DTH | Enhanced cytotoxicity and IFNγ production | Enhanced IFNγ production and ADCC | Enhanced IFNγ production |
ADCC, antibody-dependent cellular cytotoxicity; DTH, delayed-type-hypersensitivity; IL, IFNΦΦinterferon; interleukin; PKC, protein kinase C; STAT4, signal transducer and activator of transcription 4.
Liver-restricted memory NK cells
NK cells that exhibit a more potent secondary response were first described in a mouse model of delayed-type hypersensitivity (DTH) using hapten or viral antigens [77,78]. Recombination-activating gene (RAG)-deficient mice, lacking T and B cells, were sensitized with a hapten or a viral antigen and showed an NK cell-specific DTH response when challenged later with the same antigen. This antigen-specific type of NK cell memory is confined to CXCR6-positive liver NK cells [78,79]. However, it is currently unclear which receptors are responsible for the antigen-specific response and how liver NK cells can mediate specific and localized immune reactions at the site of antigen re-challenge.
CMV-specific memory NK cells
In another form of antigen-specific NK cell memory, the receptor responsible for the effect is known. Cytomegalovirus (CMV) infections in mice have been shown to induce a rapid and clonal-like expansion of a NK cell subset expressing Ly49H, which recognizes the CMV-encoded protein m157 [80]. These NK cell memory subsets show enhanced immune responses upon secondary challenge with CMV. The activating receptor DNAX accessory molecule-1 (CD226) cooperates with Ly49H for the expansion of these memory NK cells by signaling through Fyn and protein kinase Cη (PKCη [81]. Additionally, the expansion of CMV-specific memory NK cells is dependent on IL-12 and IL-15 and the subsequent signaling via signal transducer and activator of transcription 4 (STAT4) [82,83]. This induces the transcription factor zinc finger and BTB domain containing 32 (Zbtb32), which was shown to be essential for the proliferation and the protective capacity of the virus-specific NK cells [84]. Finally, the pro-apoptotic factor Bim is responsible for the contraction of the expanded Ly49H+ NK cells population, resulting in mature, murine CMV-specific memory NK cells [85].
CMV infection is also associated with the generation of memory NK cells in humans, where the expansion and long-term persistence of NKG2C+ NK cells can be observed [86,87]. However, NKG2C does not seem to be involved in the direct recognition of CMV [88]. Interestingly, similar expansions of NKG2C+ NK cells have been observed during and after other virus infections, such as hantavirus, HIV and hepatitis B [89–91], but they were always restricted to human CMV-seropositive individuals. Additionally, these NKG2C+ NK cells are also positive for CD57 [92,93], demonstrating a terminal differentiation of these human CMV-dependent memory NK cells.
Cytokine-induced memory-like NK cells
In vitro exposure of NK cells to a combination of IL-12, IL-15 and IL-18 generates memory-like cells that show enhanced effector functions [94–96]. In vivo, inflammation or other immune responses could result in the exposure of NK cells to these cytokines. Dendritic cells are the main producers of IL-12 and IL-18, thereby regulating NK cells [97]. In a recent study, adoptive co-transfer of NK cells with dendritic cells without exogenous cytokines showed increased tumor infiltration relative to NK cell transfer alone [98]. Additionally, the improved effector functions of cytokine-exposed NK cells might be a valuable tool in enhancing the effectiveness of NK cell-based therapies against tumors [94,96,99].
Finally, there have been other reports describing NK cells with certain memory-like phenotypes. While they may be related to the types of memory described above, we list them here as separate examples of NK cell memory.
FcεRγ-deficient memory NK cells
A subpopulation of human NK cells has been described that is deficient for the FcεRγ signaling adaptor. In NK cells, FcεRγ is a signaling partner chain for CD16, an activating Fc receptor responsible for the recognition of antibody-coated cells. NK cells lacking FcεRγ display poor cytotoxicity but significantly enhanced IFNγ production upon CD16 stimulation [100]. FcεRγ− NK χελλσ have a CD56dim phenotype and the existence of this subset is also associated with prior human CMV infection. However, these NK cells also demonstrate enhanced responses against other viruses [101].
Lymphopenia-induced long-lived NK cells
In a lymphopenic environment (e.g., Rag2-/- IL2rγ-/- mice), NK cells undergo a rapid but non-specific proliferation after adoptive transfer and, similar to memory T cells, show self-renewal at a steady state. These NK cells are able to respond robustly to viral infection more than 6 months after transfer [102–105].
How does NK cell memory work?
It is likely that the different forms of NK cell memory described above are not mutually exclusive and independent phenomena. Rather, there are some common denominators that suggest that they are connected and possibly represent different forms of common “memory NK cells”. The signaling via pro-inflammatory cytokines seems to be important for the generation of NK cell memory. IL-12 in particular is essential for the generation of CMV-specific memory NK cells [82], for cytokine-induced memory NK cells, likely also for the generation of liver-restricted memory NK cells [106] and is possibly important for FcεRγ− memory NK cells. IL-12 induces the expression of a high-affinity IL-2 receptor via the up-regulation of the IL-2Rα chain (CD25) [107,108], which might serve as an early marker for memory NK cells. However, the molecular basis for the increased IFNγ production and the (in some cases) enhanced cytotoxicity of memory NK cells is still unclear. Analysis of gene expression data show specific differences between resting, activated and CMV-induced memory NK cells and suggests a common transcriptional program that is conserved in the memory differentiation of NK cells and CD8+ T cells in response to infection [109]. Additionally, microRNAs (miRNA) have been shown to play a role in the regulation of NK cell functions, and the upregulation of miRNA-155 has been observed in CMV and cytokine-induced memory NK cells [110–112]. Finally, memory NK cells often display a more differentiated phenotype with the expression of CD57 and KLRG1 [92,93]. As described above, NK cells gain IFNγ-competence in response to activating receptor triggering when they mature, which is connected to a partial epigenetic remodeling of the IFNG promoter [71]. Extending these findings, recent data suggest that a broader epigenetic remodeling of the IFNG locus may be the basis for the enhanced IFNγ production of memory NK cells (C Romagnani, personal communication). Therefore, similar to what has been found for memory T cells [113,114], a global epigenetic reprogramming may also be responsible for the generation of memory NK cells [115]. However, in the current situation, it is very difficult to judge how much memory NK cells can contribute to immune responses against secondary infections with the same pathogen.
Concluding remarks
Some 45 years after the first description of “natural cytotoxicity”, we already know a lot about NK cells, their important contribution to early and effective immune responses and how their effector functions are regulated through different surface receptors and cytokines. However, we now know that processes, such as education, differentiation and finally also the formation of a memory pool, additionally impacts on the activity of NK cells. Uncovering the molecular details of these processes will greatly enhance our understanding of these important immune cells and will pave the way for more effective NK cell-based therapies.
Acknowledgments
The authors thank all the members of the Watzl lab for their support and for the helpful discussions. Our work is generously supported by the Deutsche Forschungsgemeinschaft DFG (WA 1552/5-1) and the SAW program of the Leibniz Association.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- CMV
cytomegalovirus
- DTH
delayed-type-hypersensitivity
- IFN
interferon
- IL
interleukin
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- KIR
killer cell immunoglobulin-like receptor
- MHC
major histocompatibility complex
- NK
natural killer
- RAG
recombination activating gene
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/87
References
- 1.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–9. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 3.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 4.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 5.Peter HH, Kalden JR, Seeland P, Diehl V, Eckert G. Humoral and cellular immune reactions ‘in vitro’ against allogeneic and autologous human melanoma cells. Clin Exp Immunol. 1975;20:193–207. [PMC free article] [PubMed] [Google Scholar]
- 6.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 7.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 9.Watzl C, Urlaub D. Molecular mechanisms of natural killer cell regulation. Front Biosci (Landmark Ed) 2012;17:1418–32. doi: 10.2741/3995. [DOI] [PubMed] [Google Scholar]
- 10.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–25;. doi: 10.1016/j.jaci.2013.07.020. quiz 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 12.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–59. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–35. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 15.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati MS, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–49. doi: 10.1016/1074-7613(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 16.Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3:801–9. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 17.Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, Angman L, Cella M, López-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–18. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Andrea A, Chang C, Franz-Bacon K, McClanahan T, Phillips JH, Lanier LL. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J Immunol. 1995;155:2306–10. [PubMed] [Google Scholar]
- 19.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–8. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 20.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 21.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 24.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–42. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 25.Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol. 2010;Chapter 11:Unit 11.9B. doi: 10.1002/0471142735.im1109bs90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raulet DH, Held W, Correa I, Dorfman JR, Wu MF, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065X.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 27.Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, Phillips JH, Lanier LL, Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–51. doi: 10.1016/S1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 28.Höglund P, Ohlén C, Carbone E, Franksson L, Ljunggren HG, Latour A, Koller B, Kärre K. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci USA. 1991;88:10332–6. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmer J, Donato L, Hanau D, Cazenave JP, Tongio MM, Moretta A, de la Salle H. Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (type I bare lymphocyte syndrome) J Exp Med. 1998;187:117–22. doi: 10.1084/jem.187.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877809
- 31.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1028002
- 32.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1082907
- 33.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 34.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 35.Brodin P, Lakshmikanth T, Johansson S, Kärre K, Höglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–41. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877810
- 36.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1158782
- 37.Brodin P, Kärre K, Höglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–72. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–34. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 40.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–28. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1118780
- 41.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–41. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1118781
- 42.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–56. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–9. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877811
- 44.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877812
- 45.Juelke K, Killig M, Thiel A, Dong J, Romagnani C. Education of hyporesponsive NK cells by cytokines. Eur J Immunol. 2009;39:2548–55. doi: 10.1002/eji.200939307. [DOI] [PubMed] [Google Scholar]
- 46.Haas P, Loiseau P, Tamouza R, Cayuela J, Moins-Teisserenc H, Busson M, Henry G, Falk CS, Charron D, Socié G, Toubert A, Dulphy N. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117:1021–9. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877813
- 47.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–78. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bessoles S, Angelov GS, Back J, Leclercq G, Vivier E, Held W. Education of murine NK cells requires both cis and trans recognition of MHC class I molecules. J Immunol. 2013;191: 5044–51. doi: 10.4049/jimmunol.1301971. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718133632
- 49.Chalifour A, Scarpellino L, Back J, Brodin P, Devèvre E, Gros F, Lévy F, Leclercq G, Höglund P, Beermann F, Held W. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–47. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877814
- 50.Jonsson AH, Yang L, Kim S, Taffner SM, Yokoyama WM. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184:3424–32. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebihara T, Jonsson AH, Yokoyama WM. Natural killer cell licensing in mice with inducible expression of MHC class I. Proc Natl Acad Sci USA. 2013;110:E4232–7. doi: 10.1073/pnas.1318255110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718151513
- 52.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–7. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2736956
- 53.Tarek N, Le Luduec J, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung NV, Hsu KC. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122:3260–70. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963796
- 54.Guia S, Jaeger BN, Piatek S, Mailfert S, Trombik T, Fenis A, Chevrier N, Walzer T, Kerdiles YM, Marguet D, Vivier E, Ugolini S. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal. 2011;4:ra21. doi: 10.1126/scisignal.2001608. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718877815
- 55.Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med. 2003;197:77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas LM, Peterson ME, Long EO. Cutting edge: NK cell licensing modulates adhesion to target cells. J Immunol. 2013;191:3981–5. doi: 10.4049/jimmunol.1301159. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718109926
- 57.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 58.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10%3C3121::AID-IMMU3121%3E3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park I, Liu S, McClory S, Marcucci G, Trotta R, Caligiuri MA. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–81. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1168506
- 61.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116: 3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, Guzmán CA, Ljunggren H, Malmberg K. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–64. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 64.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE. 2010;5:e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan A, Hong D, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 66.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo James P. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Münz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 68.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–91. [PubMed] [Google Scholar]
- 69.Ellis TM, Fisher RI. Functional heterogeneity of Leu 19“bright”+ and Leu 19“dim”+ lymphokine-activated killer cells. J Immunol. 1989;142:2949–54. [PubMed] [Google Scholar]
- 70.Fauriat C, Long EO, Ljunggren H, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877816
- 71.Luetke-Eversloh M, Cicek BB, Siracusa F, Thom JT, Hamann A, Frischbutter S, Baumgrass R, Chang H, Thiel A, Dong J, Romagnani C. NK cells gain higher IFN-γ competence during terminal differentiation. Eur J Immunol. 2014;44:2074–84. doi: 10.1002/eji.201344072. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718358858
- 72.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718153908
- 73.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–8. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rölle A, Pollmann J, Cerwenka A. Memory of infections: an emerging role for natural killer cells. PLoS Pathog. 2013;9:e1003548. doi: 10.1371/journal.ppat.1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun JC, Ugolini S, Vivier E. Immunological memory within the innate immune system. EMBO J. 2014;33:1295–303. doi: 10.1002/embj.201387651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Leary JG, Goodarzi M, Drayton DL, von Andrian Ulrich H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1031572
- 78.Paust S, Gill HS, Wang B, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian Ulrich H. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/5808956
- 79.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–56. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1144990
- 81.Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne, Nicholas RJ, Lanier LL. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–34. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718243207
- 82.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–54. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717147910
- 83.Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med. 2013;210:2981–90. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718188253
- 84.Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15:546–53. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718356624
- 85.Min-Oo G, Bezman NA, Madera S, Sun JC, Lanier LL. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med. 2014;211:1289–96. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718465508
- 86.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 87.Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–31. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 88.Della Chiesa M, Falco M, Bertaina A, Muccio L, Alicata C, Frassoni F, Locatelli F, Moretta L, Moretta A. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C-/- umbilical cord blood. J Immunol. 2014;192:1471–9. doi: 10.4049/jimmunol.1302053. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718243132
- 89.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg K, Klingström J, Ahlm C, Ljunggren H. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/7906956
- 90.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 91.Béziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debré P, Björkström NK, Malmberg K, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–57. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 92.Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang S, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108:14725–32. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/12631956
- 93.Hendricks DW, Balfour HH, Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol. 2014;192:4492–6. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877819
- 95.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–60. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718877820
- 96.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–65. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718412509
- 97.Chijioke O, Münz C. Dendritic cell derived cytokines in human natural killer cell differentiation and activation. Front Immunol. 2013;4:365. doi: 10.3389/fimmu.2013.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cui Y, Yang X, Zhu W, Li J, Wu X, Pang Y. Immune response, clinical outcome and safety of dendritic cell vaccine in combination with cytokine-induced killer cell therapy in cancer patients. Oncol Lett. 2013;6:537–41. doi: 10.3892/ol.2013.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehmann D, Spanholtz J, Sturtzel C, Tordoir M, Schlechta B, Groenewegen D, Hofer E. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PLoS ONE. 2014;9:e87131. doi: 10.1371/journal.pone.0087131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int Immunol. 2012;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963795
- 101.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013;190:1402–6. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717978947
- 102.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–68. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 105.Ranson T, Vosshenrich Christian AJ, Corcuff E, Richard O, Müller W, Di Santo James P. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 106.Majewska-Szczepanik M, Paust S, von Andrian Ulrich H, Askenase PW, Szczepanik M. Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-α, interferon-γ and interleukin-12. Immunology. 2013;140:98–110. doi: 10.1111/imm.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20:463–73. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee S, Fragoso MF, Biron CA. Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J Immunol. 2012;189:2712–6. doi: 10.4049/jimmunol.1201528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–9. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963788
- 110.Zawislak CL, Beaulieu AM, Loeb GB, Karo J, Canner D, Bezman NA, Lanier LL, Rudensky AY, Sun JC. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc Natl Acad Sci USA. 2013;110:6967–72. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sullivan RP, Leong JW, Fehniger TA. MicroRNA regulation of natural killer cells. Front Immunol. 2013;4:44. doi: 10.3389/fimmu.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-γ production in natural killer cells. Blood. 2012;119:3478–85. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zediak VP, Wherry EJ, Berger SL. The contribution of epigenetic memory to immunologic memory. Curr Opin Genet Dev. 2011;21:154–9. doi: 10.1016/j.gde.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 114.Weng N, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–15. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cichocki F, Miller JS, Anderson SK, Bryceson YT. Epigenetic regulation of NK cell differentiation and effector functions. Front Immunol. 2013;4:55. doi: 10.3389/fimmu.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gillard GO, Bivas-Benita M, Hovav A, Grandpre LE, Panas MW, Seaman MS, Haynes BF, Letvin NL. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7:e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tong L, Assenmacher M, Zänker KS, Jähn P. Virus-specific peptide dependent NK cell cytotoxicity. Inflamm Allergy Drug Targets. 2014;13:128–33. doi: 10.2174/1871528113666140211100616. [DOI] [PubMed] [Google Scholar]