Abstract
The dialysis treatment rate is more than 50 percent higher in the United States than it is in any West European nation. Relman and Rennie's analysis of this difference in rates raised the possibility that the extra care provided in the United States is unnecessary and is partially attributable to the existence of a private market for renal dialysis services. Their analysis ignores the effect of race on treatment needs in the United States. About 50 percent of the difference observed in rates between the American experience and the European maximum can be attributed to differences in the black/white composition of the populations. Most of the remaining difference in rates appears to be due to European policies that prohibit or severely limit access to dialysis by the elderly and those potential patients with significant medical complications.
Introduction
The End-Stage Renal Disease (ESRD) Program, which provides Medicare coverage for the victims of kidney failure, has been criticized because of its high cost. When the program was initiated in 1973, its anticipated cost was a few hundred million dollars per year; today the program costs exceed $1.2 billion and are still growing rapidly. Much of the increase in costs can be attributed to a greater than expected patient load (Rettig, 1980), but this increase in patients is in itself potentially worrisome. As Relman and Rennie have recently noted, the prevalence of dialysis treatment per million population in the United States greatly exceeds that of other nations (Relman, 1980; Relman and Rennie, 1980). In 1978, there were 46,568 people receiving renal dialysis (unless otherwise stated, “dialysis” includes all forms, home and facility, hemodialysis and peritoneal dialysis) treatments in the United States, a rate of 209 per million population.1 France, the nation with the highest per million prevalence rate in Europe, was dialyzing only 133 patients per million. Israel had the second highest rate in the world, registering 144 dialysis patients per million population (See Table 1.) Relman and Rennie (1980) reported similar differences in the treatment rates among American States. Hawaii's rate is 383 per million, while Idaho's is 67. An explanation of these variations, it is implied, may hold the key to the formulation of an effective cost control strategy for the ESRD program.
Table 1. Dialysis Prevalence Rates1.
| Nation | Dialysis Prevalence2 (per million) |
|---|---|
| Austria | 69 |
| Belgium | 123 |
| Denmark | 86 |
| Finland | 36 |
| France | 133 |
| West Germany | 117 |
| Greece | 61 |
| Ireland | 47 |
| Israel | 144 |
| Italy | 120 |
| Luxembourg | 80 |
| Netherlands | 92 |
| Norway | 31 |
| Spain | 78 |
| Sweden | 65 |
| Switzerland | 127 |
| United Kingdom | 53 |
| United States | 2093 |
Dialysis totals include both home and facility dialysis.
F. P. Brunner et al., Combined Report on Regular Dialysis and Transplantation in Europe, IX, 1978, Tables II, III, in B. H. B. Robinson et al., Proceedings of the European Dialysis and Transplant Association (Tumbridge Wells: Pittman Medical, 1979). Rates are for 1978.
See Footnote 3.
Variations in the prevalence of a treatment modality can be explained in three ways: 1) differences in treatment choices made by medical practitioners; 2) differences in public policy decisions affecting the availability of treatment options; and 3) differences in the incidence of the underlying illnesses, reflecting, among other things, demographic differences in the compared populations. These, of course, are not mutually exclusive explanations. Until now, most analysis has focused on differences in medical practice. Here we evaluate those arguments and explore alternative explanations, to determine whether differences between American and foreign dialysis rates imply the need for policy changes in the ESRD program.
Two recent articles have explored the possibility of attributing interstate differences in dialysis within the United States to the influence of profits on medical decisions (Relman, 1980; Relman and Rennie, 1980). Starkly put, the logic of the proposition is that where nephrologists have a pecuniary interest in placing patients on dialysis, they are more inclined to do so. Buttressing this position is the observation that proprietary dialysis services in the United States have, until recently at least (Tinsley, 1981), been very profitable ventures and that, where they exist, some local nephrologists have a financial interest in them.
Statistical support for this proposition is weak. Only when States with very small populations were excluded, and the prevalence of proprietary dialysis facilities dichotomized, was a statistically significant difference (p < 0.05) of 40 patients per million found. This relatively weak finding forced the conclusion that “social, cultural and economic factors rather than purely medical or epidemiologic consideration” (Relman and Rennie, 1980) must explain the observed interstate variations.
Even this overstates the existing empirical support for the profit motivated medical practice argument. Analyzing interstate data in another way, it can be shown that the percentage of a State's population that is black explains 49 percent of the variance in dialysis use (p < .001, r = .698, r2 = .49).2 Additionally, the logic of the argument is weak. The marginal patients, the ones least likely to benefit from dialysis (the aged, those with severely complicating conditions) are also the least likely to be placed on dialysis at profit-making facilities because they are the most costly patients to treat. As dialysis is by all accounts an exceedingly unpleasant experience, it is difficult to imagine that patients who are not clinically in need of this form of treatment could be started or kept on dialysis.
One might add that non-profit medical care may also be said to earn a profit. This “profit” can be in the form of revenue, net of expenses, or an increased cost base upon which to claim overheads. Much has been written about cross-subsidization among hospital departments and of the effects of cost-based reimbursements on hospital expenditures.3 Moreover, physician fees, narrowly defined in this instance as the money government pays attending physicians for the care of ESRD patients, are no lower in a nonprofit dialysis unit than in a profit-making unit. Therefore, biases claimed can only be a matter of degree.
The hypothesis that U.S. dialysis rates are driven by proprietary interests is unpersuasive empirically and theoretically. It also is untestable in a comparative context because of the unique structure of the U.S. health care system. Only the United States permits major participation in dialysis services by profit-making organizations. Thus, one must examine demographic and policy effects to explain variations in dialysis rates. (Of course, national policies can be expected to influence the behavior of medical practitioners. When dialysis resources are limited, doctors can be expected to attempt to treat renal failure in other ways, through dietary regimens, for example. Such efforts may postpone the need for dialysis but, aside from transplantation, there is no other treatment for permanent kidney failure.) First, we briefly outline several alternative hypotheses, describing how they might be tested. Succeeding sections will explore and attempt to test these hypotheses.
Hypotheses
Demographic Explanations
Before attempting to explain the prevalence of a treatment modality, it is obviously necessary to consider the prevalence of the illnesses for which the treatment may be appropriate. The direct measurement of illness rates is impractical in this case, partly because kidney failure may result from a number of prior circumstances and partly because good international data are unavailable. However, a number of surrogates can effectively be used. Statistics on race provide one such source of data.
Clinical and demographic evidence indicates that rates of kidney failure vary strikingly between whites and blacks. This must affect dialysis rates. In like manner, age data help explain differences in dialysis rates, since age is positively correlated with kidney failure. Although, the prevalence of some illnesses is correlated with demographic factors, national lifestyles may account, in known or unknown ways, for part of the variation. Given the purpose of this paper, the sources of any found variations do not concern us, but their existence as an explanation for variations in dialysis rates does.
Racial Composition of the Population
The Western European nations for which we present data are relatively homogeneous racially and ethnically as compared with the United States. There are a number of suggestions in the literature that U.S. blacks have much higher rates of ESRD and some of its precursor diseases, hypertension for example.4 There seems to be no evidence that other large American ethnic or racial groups have a comparably high incidence and prevalence of ESRD or its precursors.5 Although certain European nations, particularly the United Kingdom and France, have significant (within the European context) non-white populations, the size of these populations is small in comparison to that in the United States. Precise data do not appear to be available on the racial compositions of European populations, nor the racial composition of their dialysis rolls. These data are available for the U.S., however. It appears to be a valid assumption that adjustment of the U.S. prevalence rate for the influence of the black population is warranted, and that this “race adjusted” prevalence estimate is the more appropriate one for comparison with Europe. The population not affected by this adjustment is still quite heterogeneous, and should certainly be considered to overstate, at the very least, any existing European ethnic and racial heterogeneity and its possible effects on prevalence.
A test of this hypothesis will involve a process of adjustment similar to the so-called “direct” method of age adjustment used by epidemiologists (Friedman, 1980). If the adjusted U.S. rate, based on census rather than sample data, is substantially lower than the overall prevalence rate, this will constitute strong support for the hypothesis that U.S. racial composition contributes to the United States' high renal dialysis prevalence.
Age
End-stage renal disease is not evenly distributed across population cohorts (Brunner et al., 1979) so it is appropriate to adjust dialysis prevalence data for age. Such adjustments, of course, might leave the U.S. rate higher than before, but they would still allow a more reasonable cross-national comparison of prevalence rates. Unfortunately, the data necessary for such adjustments are not available.
An alternative, albeit less satisfactory exercise, is to compare the relative sizes of the population cohorts that contain most dialysis patients, and determine if the U.S. differs significantly from the other nations under study. A higher than normal proportion of the population in these “at risk” cohorts would suggest that the U.S. population structure contributes to its high prevalence rate.
Epidemiological
Apart from, although obviously related to, race and age differences in nations, cross-national differences in the extent of renal and renal-related diseases might account for some of the variation in dialysis prevalence. Using morbidity data for renal disease, hypertension, and diabetes mellitus (the illnesses most associated with kidney failure), the possible relationships between dialysis prevalence and disease variations can be explored. Because the mortality and morbidity data for these conditions are not likely to be very accurate or complete, positive findings will be interesting, although only suggestive (Friedman, 1980). Thus, as we do not know how accurately these mortality data reflect the true extent of disease, negative findings will not be sufficient cause to reject the hypothesis that cross-national differences in renal or related diseases account for the observed variation in dialysis prevalence.
Public Policy Explanations
The second category of explanations has to do with policy differences among nations. Once the variations in the incidence of renal disease are accounted for, the variations that exist in rates of dialysis treatment could be explained by official policies that restrict (or extend) access to treatment.
Selection Criteria
If nations differ in their patient selection criteria for dialysis treatment, one would certainly expect these differences to affect overall prevalence rates for dialysis. Selection criteria are particularly relevant with respect to patient age and possible complicating conditions, such as diabetes mellitus (Moorhead, 1975). Selection could also be based on judgments regarding a given individual's prospects for withstanding and benefiting from dialysis treatment (Moorhead, 1975; Swazey, 1974), as it is a form of treatment that can be quite debilitating, both physically and mentally (Gutman et al., 1981). There are also associated diet regimens which demand a high degree of personal discipline (Gardner, 1981).
Suggestions have been made that certain nations, Great Britain in particular, have made conscious decisions to restrict dialysis to those most likely to benefit from it (British Medical Journal, 1978; Golding and Tosey, 1980). Selection criteria seem less a product of direct governmental fiat than the result of resource constraints which make selection necessary. Of course, it must be kept in mind that patient selection is ultimately a medical decision. Even in the absence of severe resource constraints, there are and have been accepted medical reasons for selecting patients according to age or complicating condition (Moorhead, 1975). Yet, it appears impossible to separate the medical decision from the resource constraint issue. The latter concern is ultimately one of public policy. The consequences of resource constraints on the availability and use of renal dialysis services are not difficult to anticipate.
There are some cross-national data available on center-specific selection policies.6 Even without information on the specific links between national health policy and center selection criteria, the actual selection criteria are the most useful proximate variables to relate to dialysis prevalence. Stated governmental policies are only meaningful to the extent to which they have an effect. Thus, actual center policies are the most valid indicators of the consequences of governmental policy. It is, after all, the selection policies of the centers, a combination of governmental resource allocation and physician decisions, that will actually affect prevalence of treatment.
The policy hypothesis will be tested using the selection criteria information listed in Table 4. We will first relate selection criteria to their proximate outcomes (that is, age selection criteria to mean age of the dialysis population). We will then relate the selection criteria to the variation in prevalence for the European nations and ultimately for the United States as well.
Table 4. Center Specific Selection Criteria and Patient Mean Age Data.
| Nation (Dialysis Prevalence per Million) | Percent1 Centers Excluding 55 + | Percent1 Centers Excluding 65 + | Percent1 Centers Excluding All Diabetics | Percent1 Centers Allowing All Diabetics | Mean Age2 Patients on Hospital Hemodialysis | Mean Age2 New Patients on Hospital Hemodialysis |
|---|---|---|---|---|---|---|
| Austria (69) | 0 | 41 | 18 | 29 | 44.9 | 44.9 |
| Belgium (123) | 0. | 32 | 7 | 33 | 49.9 | 49.1 |
| Denmark (86) | 11 | 56 | 11 | 22 | 46.3 | 42.7 |
| Finland (36) | 66 | 89 | 11 | 16 | 41.0 | 40.0 |
| France (133) | 0 | 6 | 3 | 66 | 48.4 | 47.9 |
| West Germany (117) | 0 | 2 | 1 | 75 | 49.4 | 48.9 |
| Greece (61) | 4 | 26 | 4 | 48 | 47.3 | 47.0 |
| Ireland (47) | 33 | 67 | 0 | 0 | 41.5 | 39.3 |
| Israel (144) | 0 | 10 | 5 | 30 | 45.8 | 46.9 |
| Italy (120) | 1 | 12 | 1 | 75 | 48.2 | 48.2 |
| Luxembourg (80) | 0 | 0 | 0 | 100 | 50.5 | 51.4 |
| Netherlands (92) | 0 | 40 | 8 | 28 | 48.2 | 46.3 |
| Norway (31) | 0 | 56 | 0 | 25 | 51.3 | 48.5 |
| Spain (78) | 15 | 54 | 21 | 22 | 41.1 | 41.4 |
| Sweden (65) | 0 | 42 | 18 | 14 | 49.3 | 47.4 |
| Switzerland (127) | 0 | 30 | 0 | 56 | 51.3 | 49.3 |
| United Kingdom (53) | 35 | 80 | 33 | 10 | 40.6 | 39.8 |
| United States (209) | 03 | 03 | 03 | 100 | 52.0 | —4 |
A. J. Wing et al., Combined Report on Regular Dialysis Transplantation in Europe, VIII, 1977, Tables VI and VIII, in B. H. B. Robinson and J. B. Hawkins, eds., Dialysis Transplantation Nephrology (Turnbridge Wells: Pittman Medical, 1978).
F. P. Brunner et al., Combined Report on Regular Dialysis and Transplantation in Europe, IX, 1978, Table VIII.
There are approximately 15 to 20 pediatric dialysis centers in the United States (source: Pediatric Renal Unit, The Children's Hospital, Boston; telephone conversation).
This information was not available from HCFA.
Economics
All things being equal, a wealthier nation is likely to have more resources to support very expensive ESRD treatments. Presumably, economic factors will influence, although not wholly determine, the resources which a government and a society will commit to renal dialysis. As discussed with regard to the policy hypothesis, these resource allocation decisions will have a significant effect on the ultimate selection policies of centers (and the referral policies of physicians). Gross Domestic Product per capita will be related to dialysis prevalence to test this hypothesis.
Tests of the Hypotheses
Demographic
Racial
As we indicated previously, race appears to be a potential factor in explaining variations in dialysis rates among populations. Blacks, in particular, suffer more from the illnesses leading to renal failure. For example, blacks have a higher rate of hypertension than do whites (McDonough et al., 1967; Boyle et al., 1967), and hypertension appears to contribute significantly to the risk of renal failure. (Moorhead, 1975). Given that blacks constitute nearly 12 percent of the U.S. population, though only a negligible percent of European populations, there is a strong supposition that the U.S. is likely to have a higher prevalence of dialysis cases because of the composition of its population.
Two epidemiological studies, using patients receiving treatments for ESRD (dialysis and transplant) as the basis for morbidity statistics, found the incidence and prevalence of dialysis utilization to be substantially higher for blacks than for whites (Mausner et al., 1978; Easterling, 1977). Age adjusted prevalence was approximately three times greater for blacks than for whites. This was true for both sexes. Easterling found that the incidences for both glomerulonephritis, a major cause of ESRD, and for diabetic nephropathy, were approximately three times greater for blacks than for whites. The black incidence for renal disease due to hypertension was 17 times that for whites.
Statistical evidence exists documenting the relationship between size of the black population and size of the dialysis population. Our calculations, using data from the Health Care Financing Administration (HCFA), reveal that 53 percent of the variance in dialysis prevalence among the 50 States and the District of Columbia can be explained by racial differences in the population.7 This would be expected given the existence of black/white ESRD prevalence and incidence differences of the scale reported.
Y = Dialysis prevalence per million persons (1979)
X = Percentage of the population of the State which is black (1979)
bo = 114.8 b = 7.6
It is interesting to note that if the percentage of the United States population which is black (11.7 percent) is inserted into the equation, the predicted dialysis prevalence is 204, which is only 5 per million less than the actual U.S. number, 209 per million.
Surveys of the dialysis population have found substantially higher percentages of black patients in renal treatment units (Evans et al., 1981). One of these studies, using a sample of 18 dialysis providers and 2,481 patients, found that 42 percent of dialysis patients sampled were black (Gutman et al., 1981). The authors caution that the geographic distribution of participating dialysis units may have biased their results with respect to race. A second study, using a more complex sampling technique, found that blacks constituted 34.9 percent of the dialysis population (Evans et al., 1981). However, the nature of their sampling methodology may “… slightly overrepresent the proportion of blacks in the dialysis population.” Neither of these studies calculated prevalence figures. The nature of these studies does not allow the calculation of accurate national prevalence figures; however, they strongly suggest that black dialysis prevalence is higher than white dialysis prevalence.
The most definitive available evidence is the census of the 1978 ESRD Medicare dialysis population, which found that blacks constituted 27.1 percent of those being treated. Because the total U.S. dialysis population is greater than the Medicare dialysis population, it would be useful to have racial censuses for the non-Medicare populations, such as those whose care is financed by Medicaid, the Veterans Administration, and the Department of Defense, but such censuses are unavailable from these agencies. Using the Medicare census black/white ratio, we used a procedure similar to the “direct” method of age adjustment to adjust the U.S. dialysis prevalence for the high rate of renal disease among blacks. (Friedman, 1980). The prevalence estimate for blacks was 482/million, and for whites and others, 172/million. This ratio, 172/million, holds race constant and thus is the more appropriate prevalence estimate to use in comparison with European nations.
Thus there is apparent strong support for the hypothesis that race accounts for much of the high prevalence of dialysis in the United States. The percent of blacks in the U.S. population accounts for 37 patients per million in the U.S. dialysis rate, or 49 percent of the difference between the U.S. dialysis rate and the highest rate in Europe, that of France.
We must point out that this finding is best understood as a powerful indicator of the direction and order of magnitude of the relationship. Race as a variable in the United States correlates with far too many other social and economic characteristics to permit a precise estimation of its impact on disease prevalence. Indeed the strong statistical connection between race and renal failure raises almost as many questions as it answers. The medical literature suggests three possible explanations for this relationship: 1) population genetics hypothesis, 2) diet hypothesis, and 3) central nervous system activity hypothesis. The first suggests that the higher U.S. dialysis rate is in fact partially explained simply by the presence of a particularly susceptible population. The second is a lifestyle explanation. Here the interesting issue is whether the lifestyle in question is ethnically or socioeconomically defined. The evidence is inconclusive, since white rates of hypertension are themselves somewhat related to socioeconomic levels, although it appears that the higher black prevalence remains when one compares black and white socioeconomic cohorts.
The third hypothesis suggests that stresses attendant with life as a black in American society lead to the heightened activity of the central nervous system which is associated with increased risk of hypertension, a prime precursor of ESRD. To these hypotheses we would add a fourth: limited access to preventive medical care. It may be that the U.S. medical system puts less emphasis or provides fewer opportunities for the poor to obtain preventive medical care. This might lead to a later complex relationship between class, race, and end-stage renal disease that record-keeping picks up as a race/disease relationship.
These explanations remain largely in the realm of speculation. In regard to an analysis of dialysis rates, however, all are equally external to the ESRD program itself. Whatever the underlying causality of the relationship, it is clear that a large part of the variation between American and European dialysis rates has to do with variations in the demand for the treatment among the respective populations. The ESRD program obviously cannot be held accountable for, nor can it alter, those variations.
Age
Age is positively related to the need for renal dialysis (Brunner et al., 1979). A nation with an older population would therefore be expected to have a higher at-risk population and so a potentially higher dialysis incidence rate. The age composition of the U.S. population might explain part of its high dialysis rate. An examination of the age cohort columns in Table 2, however, does not support this hypothesis. On the contrary, they indicate that the U.S. population is slightly younger than is the European. (The data represent the percentage of the total population that is within the designated ages and male. Males are more likely to be on dialysis than are females, so this is a better test of the at-risk population than percent of total population, Brunner et al., 1979). An examination of the data shows that the U.S. at-risk population is smaller than average and smaller than or equal to any of the nations closest to it in prevalence, except Israel. Therefore, it does not appear warranted to suppose that the United States' population cohort structure contributes to its high dialysis prevalence.
Table 2. Selected Male Population Cohorts.
| Nation (Dialysis Prevalence per Million) |
Age Cohort1 Males 35-54 (% of total population) |
Age Cohort1 Males 55-64 (% of total population) |
Age Cohort1 Males 65 + (% of total population) |
|---|---|---|---|
| Austria (69) | 12 | 4 | 6 |
| Belgium (123) | 12 | 5 | 6 |
| Denmark (96) | 11 | 5 | 6 |
| Finland (36) | 12 | 4 | 4 |
| France (133) | 12 | 4 | 5 |
| West Germany (117) | 14 | 4 | 6 |
| Greece (61) | 13 | 4 | 6 |
| Ireland (47) | 10 | 5 | 5 |
| Israel (144) | 9 | 4 | 4 |
| Italy (120) | 13 | 4 | 5 |
| Luxembourg (80) | 14 | 5 | 5 |
| Netherlands (92) | 11 | 4 | 5 |
| Norway (31) | 11 | 6 | 6 |
| Spain (78) | 12 | 4 | 4 |
| Sweden (65) | 12 | 6 | 7 |
| Switzerland (127) | 12 | 5 | 5 |
| United Kingdom (53) | 12 | 5 | 6 |
| United States (209) | 10 | 4 | 4 |
World Health Statistics Annual, 1979 (Geneva: World Health Organization, 1979), Table 2.
Epidemiological
No statistically significant relationships are found between dialysis prevalence and mortality rates for hypertension, diabetes mellitus, or nephropathies for Europe (Table 3). This lack of relationship held when the U.S. was included in the calculations. These findings do not support the hypothesis that cross-national variations in diseases associated with renal failure explain variance in dialysis prevalence. On the other hand, given the problems with the use of mortality (and morbidity) data to accurately reflect actual disease prevalence and incidence (Friedman, 1980), these findings are not a sufficient basis to reject this hypothesis. Data inadequacies preclude its definitive test.
Table 3. Mortality Rates for Diseases Associated with Renal Failure (per 100,000, per year).
| Nation (Dialysis Prevalence per Million) | Mortality1 Diabetes | Mortality1 Hypertensive Disease | Mortality1 Diseases of the Genito-Urinary System | Mortality1 Other Nephritis and Nephrosis |
|---|---|---|---|---|
| Austria (69) | 17.8 | 24.2 | 21.3 | 3.5 |
| Belgium (123) | 34.2 | 12.1 | 19.2 | 2.0 |
| Denmark (86) | 11.0 | 7.0 | 17.1 | 1.4 |
| Finland (36) | 16.4 | 15.2 | 15.3 | 4.2 |
| France (133) | 15.5 | 13.3 | 16.0 | 2.5 |
| West Germany (117) | 27.5 | 21.5 | 18.9 | 2.7 |
| Greece (61) | 31.6 | 13.8 | 21.1 | 9.7 |
| Ireland (47) | 10.8 | 15.0 | 16.7 | 6.5 |
| Israel (144) | 10.0 | 5.0 | 16.5 | 6.6 |
| Italy (120) | 22.1 | 27.6 | 14.6 | 3.8 |
| Luxembourg (80) | 37.8 | 35.6 | 12.1 | 0.8 |
| Netherlands (92) | 11.1 | 6.5 | 15.0 | 1.9 |
| Norway (31) | 8.9 | 14.5 | 13.7 | 2.5 |
| Spain (78) | 19.0 | 5.9 | 16.4 | 7.3 |
| Sweden (65) | 15.1 | 4.0 | 16.2 | 3.0 |
| Switzerland (127) | 19.2 | 24.0 | 12.5 | 3.6 |
| United Kingdom (53) | 10.4 | 15.2 | 16.4 | 5.5 |
| United States (209) | 19.1 | 7.5 | 12.1 | 3.2 |
World Health Statistics Annual, 1980 (Geneva: World Health Organization, 1980), Table 7.
Public Policy Explanations
Corrections for demographic differences have reduced the variation between the U.S. and European dialysis rates. We now need to consider the degree to which the remaining differences can be accounted for by public policies. The ideal approach to this issue would be first to determine the official government policies affecting access to renal dialysis in each nation and second, to determine how those policies are implemented in practice. Precise information on these factors can only be obtained through field research. Surrogate data, however, are available from published sources.
The major medical contraindications to renal dialysis have been age and the presence of complicating diseases, particularly diabetes mellitus (Moorhead, 1975; Chester et al., 1979; Rathaus and Bernheim, 1978; Kjellstrand, 1978; Massry et al., 1979; Hosten et al., 1981). For this reason, we have used the percentage of dialysis centers in a nation that exclude patients over a certain age, and the percentage that include all diabetics, as tests of the degree of publicly imposed restrictions on access to treatment. These data are available for all European nations. Their exact equivalents are not available in the United States, but here we do have direct information about policy. Because P.L. 92-603 assured all Americans covered by social security access to dialysis regardless of age, medical condition, or ability to pay, the exclusion rate in the United States is effectively zero. (Actually, there are 15 to 20 pediatric dialysis centers in the U.S. that exclude patients based on age as an official policy, but because of their small number we have not considered their impact in our analysis.)
One very large advantage of using these data is that it allows us to see if our approach can account for differences within Europe as well as the differences between the U.S. and Europe. If the model can be so broadly applied, confidence in its validity is increased. There is, fortunately, substantial variation in renal treatment rates among the member nations of the European Dialysis and Transplant Association.
The United Kingdom, which has one of the lowest dialysis prevalence rates (53/million—Table 1), has been rationing treatment for renal failure (De Wardener, 1977; British Medical Journal, 1978; Golding and Tosey, 1980). It appears that this rationing is induced by general constraints on the resources allocated to dialysis, and is not, in its specifics, a mandated government policy (Golding and Tosey, 1980). Resource constraints lead to constraints on the number of dialysis service units, and these in turn lead to a dependence on medically grounded selection criteria as a rationing tool (DeWardener, 1977; Golding and Tosey, 1980; British Medical Journal, 1978). As the British Medical Journal put it, “Pressure on limited facilities for new patients must result in more stringent selection.” It is evident that age is a prime selection criterion, one which is defended in a recent British text on dialysis (Moorhead, 1975). In 1977, 35 percent of British dialysis centers had a policy to exclude patients who were over 55, and 80 percent excluded patients over 65 (Table 4; Wing et al., 1978). Selection also appears to operate against diabetics, or patients with other multisystem diseases (Moorhead, 1975; Robinson and Hawkins, 1978). The fact that the British rely quite heavily on home dialysis is also said to result in more restrictive selection policies, as requirements for admission to home dialysis are evidently more stringent than those for hospital dialysis (British Medical Journal, 1978). Examination of Table 4 indicates that only 10 percent of dialysis centers in the United Kingdom will treat all diabetics, the second lowest percentage for the countries studied, and 33 percent exclude ail diabetics, the highest percentage for the countries in our sample.
Israel, which ranks second to the United States in dialysis prevalence, has a policy quite opposite that of the United Kingdom (Boner and Elster, 1977; Eliahou and laina, 1979). Its program philosophy is reported to be to “… prevent death at any cost” (Eliahou and laina, 1979). Older patients apparently make up a significant portion of Israel's dialysis population (Boner and Elster, 1977). Examination of Table 4 indicates that no centers exclude patients over 55, and only 10 percent exclude patients over 65. Only 5 percent of centers exclude all diabetic patients, while 30 percent allow all diabetics.
Age-specific criteria operate in 30 percent of European dialysis centers; this means that these centers have a general rule to reject patients who are older than 65. No such formal criteria appear to operate in the United States, where Medicare funding of ESRD treatment guarantees treatment to nearly all who will medically benefit from dialysis. Sixty percent of the 1980 U.S. ESRD program facility dialysis population was 51 or older; approximately 35 percent of the European hospital dialysis population was 55 or older (HCFA raw data, 1981). The mean European ages for hospital hemodialysis and home hemodialysis were 45.3 and 41.1, respectively (Brunner et al., 1979). The U.S. mean age for all dialysis patients was 52 (HCFA raw data, 1981). Comparing individual European mean patient ages for hospital dialysis (Table IV), which tend to be higher than those for home dialysis, we see that no country is as high as the United States, and only two are over 50 (Luxembourg and Switzerland). Thus, not only do a significant number of European dialysis centers discriminate against older patients, but actual mean patient ages are higher in the U.S. than in Europe. Additionally, a much larger proportion of the U.S. dialysis population is over 51-55, indicating that selection policies are effective in excluding older patients from treatment abroad. This, of course, further strengthens the validity of using center data as a surrogate of public policy.
European dialysis centers also discriminate against patients with diabetes mellitus (Table 4). Fifty percent of dialysis centers discriminate to some degree against diabetics, and 10 percent take no diabetics at all. Diabetes as such is not a cause for exclusion from renal dialysis in the United States.
Having established that selection policies and ages of dialysis populations vary, and having asserted that age-specific selection policies should in fact lower prevalence and incidence in older age cohorts and exert a dampening effect on total prevalence, it will be useful to examine directly the relationship between selection policies and average ages of total and new dialysis populations. As expected, these relationships are present, moderately strong, and statistically significant. Table 5 presents these correlation statistics for Europe alone, and for all 18 countries as well. The highest correlations are for the selection variables with the mean age of the total dialysis population. It is interesting to note that correlations are stronger for total dialysis population age than for new population age. This suggests that the reported age-specific selection policies, which were for the previous year, may in fact capture past selection policies (which have contributed to total mean age) better than the selection policy for 1978. It is highly speculative, but this may also mean that exclusionary policies against older patients are diminishing in Europe. This is consistent with the trend in medical opinion which suggests that older patients tolerate dialysis better than anticipated (Chester et al., 1979; Rathaus and Bernheim, 1978).
Table 5. Correlations: Selection Policies and Population (Dialysis) Age1.
| Exclude Patients 55+ | Exclude Patients 65+ | ||
|---|---|---|---|
|
|
|
||
| Mean Age of Patients on Hospital Hemodialysis | −0.782 | −0.672 | Europe (N = 17) |
| −0.772 | −0.712 | Europe and United States (N = 18) | |
| Mean Age of New Patients on Hospital Hemodialysis | −0.603 | −0.643 | Europe (N = 17) |
1977 and 1978 EDTA Combined Reports, Tables VI, VII, and VIII.
p < 0.01
p < 0.05
Pearson Product Moment Correlation Coefficient (r)
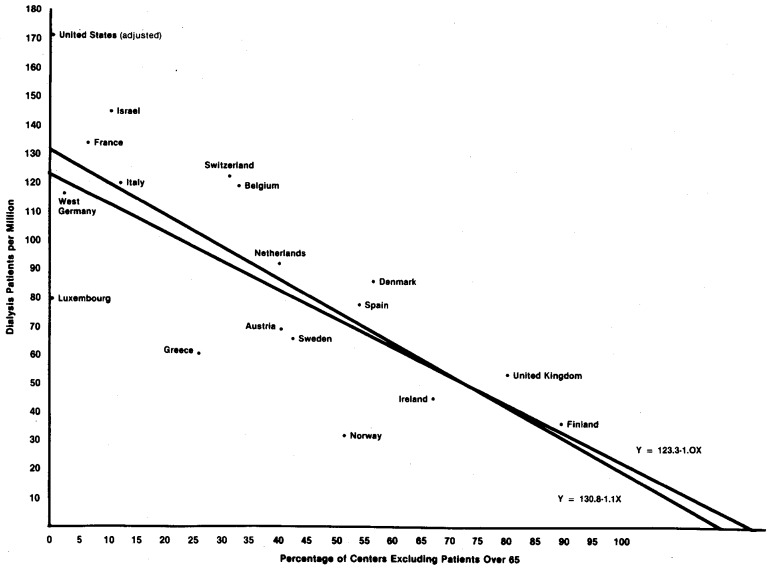
Because the United States is an outlier for selection policies and dialysis prevalence, the effect of these policies on dialysis treatment rates will first be explained for Europe alone. The correlation coefficients relating dialysis patients per million with percentage of units excluding patients older than 55 years and older than 65 years are r = − .56 (p < .05) and r = − .75 (p < .01), respectively (N = 17). We estimated the regression equations using SAS's PROC GLM (SAS Users Guide, 1979).
Y = Dialysis patients per million population
X = Percentage of centers excluding patients over 65
bo = 123.3 b = − 1
95% confidence intervals: 101.1 <bo< 145.4, −.5< b< −1.5
Having established a strong relationship, with the 65 or over exclusion variable explaining 56 percent of the variance in dialysis prevalence, we estimated the model for all 18 countries listed in Table 1 (which includes the U.S.):
bo = 130.8 b = −1.1
95% confidence intervals: 109.1 < bo < 152.5, −.64< b< −1.62
Again the model performed well. Both bo and b, slope and intercept, are within the confidence intervals of the first model. The model does not appear inordinately sensitive to the addition of the United States, with bo's, b's, and R2's remaining relatively stable. Table 6 lists the observed and predicted values for this model, and Figure 1 presents their scatter plot. It should be remembered that the U.S. dialysis prevalence is adjusted for race.
Table 6. Observed, Predicted, and Residual Dialysis Prevalence.
| Nation | Observed Value | Predicted Value | Residual |
|---|---|---|---|
| Austria | 69.0 | 84.8 | −15.8 |
| Belgium | 123.0 | 95.2 | 27.8 |
| Denmark | 80.0 | 67.5 | 12.5 |
| Finland | 36.0 | 29.5 | 6.5 |
| France | 133.0 | 125.2 | 7.8 |
| West Germany | 117.0 | 129.8 | −12.9 |
| Greece | 61.0 | 102.1 | −41.2 |
| Ireland | 47.0 | 54.8 | −7.8 |
| Israel | 144.0 | 120.5 | 23.4 |
| Italy | 120.0 | 118.2 | 1.8 |
| Luxembourg | 80.0 | 132.1 | −52.1 |
| Netherlands | 92.0 | 85.9 | 6.0 |
| Norway | 31.0 | 67.5 | −36.5 |
| Spain | 78.0 | 69.8 | 8.2 |
| Sweden | 65.0 | 83.6 | −18.6 |
| Switzerland | 127.0 | 97.5 | 29.5 |
| United Kingdom | 53.0 | 39.8 | 13.1 |
| United States | 172.0 | 130.8 | 41.1 |
Figure 1. Dialysis Patients per Million (1978) by Percentage of Dialysis Centers Excluding All Patients Older than 65 (1977).
We must conclude that, for the European countries examined, age selection policies are highly related to dialysis prevalence. The U.S. prevalence, despite being a good deal higher than its predicted value, does not weaken this model. Thus, the U.S. case fits generally in a model that relates age exclusion (or its absence) to dialysis prevalence. Although it is impossible to account precisely for the prevalence of an individual case, the hypothesis that the U.S. high incidence is highly related to its lack of exclusion policies seems strongly supported.11
One caveat is in order. The particular measure used to capture exclusion policies is a crude one. It does not take into account the size of the particular centers with a given policy and hence the number of particular patients or potential patients affected by these policies. Nor does it measure the actual policies precisely. Nevertheless, this measure does offer a general measure of a country's exclusion policies. The percentage of centers excluding patients over 65 is highly correlated with, and inversely related to, the percentage of centers dialyzing all diabetics (r = − .84, r2 = .71, p < .0001). Thus the age exclusion variable captures more general exclusionary inclinations quite nicely. Countries that exclude older patients are also more likely to exclude diabetics. This is congruent with our assumptions that exclusionary policies stem from the same general sources. Finally, given the crude nature of the age selection variable, the fact that it still explains 56 to 60 percent of the variance suggests that the relationship between prevalence and some “true” measure of exclusionary policy is a strong one.12
Economics
One would suppose that wealthier nations have more resources to commit to the treatment of chronic renal failure, all other things being equal. These resource commitments are presumed to have a direct effect on actual dialysis prevalence, with selection practices acting as a mediating variable. We examined the relationship between Gross Domestic Product (GDP) per capita and dialysis prevalence. This relationship was not statistically significant however (r = .18, p > .05). Added to the univariate model previously tested, the addition of GDP per capita did not increase its predictive ability. Nor was GDP per capita related to dialysis incidence. Given this analysis, there is no evidence to support the hypothesis that a nation's wealth is positively related to its dialysis prevalence within the range of wealth existing in the North Atlantic community.
Conclusions
Much criticism of the ESRD program implies that the United States dialyzes too many of its citizens. The United States, it is noted, has a much higher rate of dialysis treatments than have nations of comparable wealth and medical sophistication. Some have suggested that this extra care may be unnecessary and that it may exist because the United States permits a market in ESRD services. The thrust of their analysis of the dialysis rate is that the United States is now a victim of a “medical industrial complex” created in dialysis and other health care services.
This is a powerful call for reform perhaps, but one not supported by the evidence presented. It cannot be demonstrated that private involvement in dialysis services substantially alters dialysis treatment rates among American States. Only by omitting consideration of racial composition of the U.S. dialysis population can even a weak correlation between profit-making and dialysis be found.
But when one considers demographic and policy differences between the United States and European nations, the sources of much of the variation become readily apparent. Racial differences alone reduce the comparable U.S. rate by 37 per million (or 18 percent). Adding this to the U.S. prevalence predicted by our selection policy model, we have an expected prevalence for the U.S. of 168 per million. If the selection policies and their precise effects were better known, it is likely that the observed U.S. prevalence would be still closer to the expected. And equally important, if the epidemiological consequences of the racial mixture of the U.S. population were better understood with regard to renal disease, it is likely that the underlying prevalence of renal disease, and hence the need for dialysis, would be somewhat higher in the United States than in Europe.
One could argue that it is compassion, not profit-making, that is the cause of the extra care provided in the United States. The ESRD program was established largely because we were unwilling to tolerate the tragic choices required to distribute scarce dialysis resources. The program provides government financed care to all who might possibly benefit from it. As expensive as this care might be, it is not likely to be made scarce again.
Footnotes
| a) | Medicare population in the week ending December 16, 1978. Reported in End-Stage Renal Disease Program, Annual Report to Congress, 1979 (Washington, D.C.: DHEW, 1979). | 36,463 |
| b) | Medicaid, Federal employee insurance, private insurance, etc., for 1979 discounted by the 1978-79 growth in the Medicare population. (This seemed the best estimation of the 1978 figure.) Provided by the ESRD program data division. | 3,359 |
| c) | Population pending Medicare eligibility (on dialysis) for 1979. Discounted as in b), and provided by the same source. | 3,662 |
| d) | VA dialysis population, December 1978 (excluding contract/fee patients presumably picked up by HCFA 1979 census of dialysis facilities). | 3,084 |
|
|
||
| e) | Total estimated dialysis patients, end of year, 1978. | 46,568 |
| The relevant U.S. population was estimated as follows: | ||
| a) | Population of all residents of the U.S. (50 States and D.C.) on December 1, 1978. Source: U.S. Bureau of the Census, Current Population Reports, Population Estimates and Projections, p. 25, No. 874, January 1980 (Washington, D.C: Department of Commerce, 1980). |
219,554,000 |
| b) | Population of Puerto Rico, July 1976. Source: U.S. Bureau of the Census, Current Population Reports, Federal-State Cooperative Program for Population Estimates, p. 26, No. 76-51, January 1979 (Washington, D.C: Department of Commerce, 1979). |
3,334,000 |
| c) | Population of the Virgin Islands, 1977. Source: U.S. Bureau of the Census, Statistical Abstract of the United States (Washington, D.C: Department of Commerce, 1979). |
93,000 |
|
|
||
| d) | Total 1978 approximate population relevant to jurisdiction of ESRD program. | 222,981,000 |
Letters to the Editor on “Treatment of End-Stage Renal Disease,” The New England Journal of Medicine 304 (1981):355-357. See in particular the letter from Edmund G. Lowrie, M.D.
See for example Fuchs, Victor R., Who Shall Live? (New York: Basic Books, 1974), Chapter 4; Dowling, William L., “Prospective Reimbursement of Hospitals,” Inquiry 11 (1974):163-180; Harris, Jeffrey E., “The Internal Organization of Hospitals: Some Economic Implications,” The Bell Journal of Economics 8 (1977):467-482.
See for example, Mausner, Judith S., M.D. et al., “An Area-wide Survey of Treated End-Stage Renal Disease,” American Journal of Public Health 68-2:166-169 and Easterling, R.E., “Racial Factors in the Incidence and Causation of End-Stage Renal Disease (ESRD),” Transactions of the American Society of Artificial Internal Organs 33 (1977):28-32. Also, with regard to hypertension, see Stamler, Jeremiah, M.D. et al., The Epidemiology of Hypertension (New York: Grune and Stratton, 1967), containing McDonough, John R. et al., “Blood Pressure and Hypertensive Disease Among Negroes and Whites in Evans County, Georgia,” pp. 167-187 and Boyle, Edwin W., Jr., et al., “An Epidemiologic Study of Hypertension Among Racial Groups of Charleston County, South Carolina, The Charleston Heart Study, Phase II,” pp. 193-203.
There is, however, some speculation that Japanese Americans experience a relatively high rate of kidney failure or at least dialysis care. Federal data do not reveal whether this is the case. In any event, the size of the Japanese American population is not large enough to significantly affect the overall U.S. rate.
Wing, A. J. et al., Combined Report on Regular Dialysis and Transplantation in Europe, VIII, 1977, in Robinson B.H.B. and J. B. Hawkins, eds. Dialysis Transplantation Nephrology, Turnbridge Wells: Pittman Medical, 1978, pp. 13-16. The Europeans use the term “center” to describe the providing services and we retain the term when referring to the European or comparative situation.
From data supplied by the ESRD program for ESRD population by State, 1979. Population data from U.S. Bureau of the Census, Current Population Reports, p. 23, No. 334 (Washington, D.C.: Department of Commerce, 1979). Data processed using SAS's PROC GLM.
† statistic
p < ( )
† statistic
p < ( )
† statistics
p < ( )
The choice between transplant and dialysis, while to a large extent fixed by the availability of live donor and cadaveric kidneys, and by general patient condition, may nevertheless have enough inherent discretion so that systematic cross-national variation might exist and be systematically related to dialysis prevalence. Given some “true” prevalence of ESRD patients, treatment must lie in one mode or the other. There is indeed a great deal of variance in transplant prevalence, but this prevalence is not statistically significant in its relationship with dialysis prevalence (r = − .30, p > .05, U.S. excluded), nor does the transplant prevalence add to the univariate model in a statistically significant manner. Thus, although some countries with low dialysis prevalence have relatively high transplant prevalence (Denmark, Finland, Norway, Sweden), the overall pattern is not systematic in this direction. It may be the case, however, that these four countries, all Nordic, do in fact have medical practice styles which tend to favor transplant over dialysis. The precise nature of this relationship remains to be explored. While good data on transplant prevalence were not readily available for the United States, there are indications that U.S. annual transplant rates are not particularly low. (See ESRD Annual Report, 1979, p. 9.)
lt will also be useful to examine the relationship between the age exclusion variable and the incidence of dialysis in 1978. In fact, there is no statistically significant relationship between the two variables. This would be explained if the relationship between dialysis incidence and prevalence was not very strong, and this is the case, (r = .54, p < .05). In fact, some of the countries with the lowest prevalence figures have reasonably high incidence figures (Finland, Sweden, Spain). These data, as do the data on mean age for incidence and prevalence, point to a relative relaxation of selection criteria, and a possible convergence of prevalence at some point in the future. This convergence could of course reverse itself if prevalence begins to strain resources. However, decreasing medical bases for selection might make this reversal quite difficult.
References
- Boner G, MD, Elster Z. Renal Transplantation and Dialysis in Israel, 1974-75: Report of the Israel Transplantation and Dialysis Association. Israel Journal of Medical Science. 1977;13:65–75. [PubMed] [Google Scholar]
- Selection of Patients on Dialysis and Transplantation (editorial) British Medical Journal. 1978;2-6150:1449–1450. doi: 10.1136/bmj.2.6150.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Medical Journal. 1978;2-6154 (editorial):1785–1786. doi: 10.1136/bmj.2.6154.1785-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner FP, Brynger H, Chantler C, Donckerwolcke RA, Hathway RA, Jacobs C, Selwood NH, Wing AJ. Combined Report on Regular Dialysis and Transplantation in Europe, IX, 1978. In: Robinson BHB, Hawkins JB, Naik RB, editors. Proceedings of the European Dialysis and Transplant Association. Turnbridge Wells: Pittman Medical; 1979. [PubMed] [Google Scholar]
- Chester Alexander C, Rakowski Thomas A, MD, Argy William P, Jr, MD, Giacalone Anna, MD, Schreiner George E., MD Hemodialysis in the Eighth and Ninth Decades of Life. Archives of Internal Medicine. 1979;139:1001–1005. [PubMed] [Google Scholar]
- De Wardener Hugh. Shortage of Dialysis Facilities. British Medical Journal. 1977;1-6064:835. doi: 10.1136/bmj.1.6064.835. Letter in the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliahou HE, laina A. Dialysis Requirements in Israel. Israel Journal of Medical Science. 1979;15:1011–1013. [PubMed] [Google Scholar]
- Evans Roger W, Blagg Christopher R, MD, Bryan Fred R. Implications for Health Care Policy: A Social and Demographic Profile of Hemodialysis Patients in the United States. Journal of the American Medical Association. 1981;245:487–491. doi: 10.1001/jama.245.5.487. [DOI] [PubMed] [Google Scholar]
- Fox Renee C, Swazey Judith P. The Courage to Fail. Chicago: The University of Chicago Press; 1974. [Google Scholar]
- Friedman Gary D., MD, SM, FACP . Primer of Epidemiology. 2nd. New York: McGraw-Hill; 1980. [Google Scholar]
- Gardner Jean L., RD Hyperdietism—Its Prevention, Control, and Relation to Compliance in Dialysis and Transplant Patients. Dialysis and Transplantation. 1981;10:57–68. [Google Scholar]
- Golding AMB, Tosey D. The Cost of High-Technology Medicine. The Lancet. 1980 Jul 26;:195–197. doi: 10.1016/s0140-6736(80)90073-2. [DOI] [PubMed] [Google Scholar]
- Gutman Robert A, MD, Stead William W, MD, Robinson Roscoe R., MD Physical Activity and Employment Status of Patients on Maintenance Dialysis. The New England Journal of Medicine. 1981;304:309–313. doi: 10.1056/NEJM198102053040601. [DOI] [PubMed] [Google Scholar]
- Hosten Adrian O, MD, Mamidanna S Rao. Dialysis and Transplantation. 1981;10:206–209. [Google Scholar]
- Kjellstrand Carl M., MD, FACP Treatment Strategies of Diabetic Patients with End-Stage Renal Failure (editorial) Mayo Clinic Proceedings. 1978;53:819–820. [PubMed] [Google Scholar]
- Massry Shaul G, Feinstein Eben I, Goldstein David A. Early Dialysis in Diabetic Patients With Chronic Renal Failure (editorial) Nephron. 1979;23:2–5. doi: 10.1159/000181597. [DOI] [PubMed] [Google Scholar]
- Moorhead JF. Chronic Renal Failure: A Continuing Medical, Social and Economic Problem. In: Jones NF, editor. Recent Advances in Renal Disease. New York: Churchill Livingstone; 1975. [Google Scholar]
- Rathaus Mauro, MD, Bernheim Jacques L., MD Are Your Elderly Patients Good Candidates for Dialysis? Geriatrics. 1978;33:56–59. 63–66. [PubMed] [Google Scholar]
- Relman Arnold S., MD The New Medical Industrial Complex. The New England Journal of Medicine. 1980;303:963–970. doi: 10.1056/NEJM198010233031703. [DOI] [PubMed] [Google Scholar]
- Relman Arnold S, MD, Rennie Drummond., MD Treatment of End-Stage Renal Disease: Free But Not Equal (editorial) The New England Journal of Medicine. 1980;303:996–998. doi: 10.1056/NEJM198010233031708. [DOI] [PubMed] [Google Scholar]
- Rettig Richard A. The Politics of Health Cost Containment: End-Stage Renal Disease. Bulletin of the New York Academy of Medicine. 1980 Jan-Feb;56(No. 1):115–138. [PMC free article] [PubMed] [Google Scholar]
- SAS User's Guide. 1979. Raleigh: SAS Institute, Inc.; 1979. [Google Scholar]
- Tinsley Elisa. Pitfalls in Public Funding. New York Times. 1981 Oct 25;:8–9F. [Google Scholar]