Abstract
STUDY QUESTION
Is time to pregnancy (TTP) similar across successive pregnancy attempts among women experiencing pregnancy loss?
SUMMARY ANSWER
TTP after a loss may be longer compared with TTP before a loss.
WHAT IS KNOWN ALREADY
Two pregnancy cohort studies have reported that TTP is similar across pregnancy attempts in fertile women. However, this has not been investigated among women experiencing pregnancy losses.
STUDY DESIGN, SIZE, DURATION
Data for this analysis come from the Longitudinal Investigation of Fertility and the Environment Study, a population-based, preconception cohort of couples attempting pregnancy. During 2005–2009, recruitment was targeted to 16 counties in Michigan and Texas with reported exposures to persistent environmental chemicals. A total of 501 couples were recruited and followed for up to 12 months of pregnancy attempts allowing for continued participation of women with pregnancy losses until censoring.
PARTICIPANTS, SETTING, METHODS
We assessed TTP among 70 couples recruited upon discontinuing contraception for purposes of becoming pregnant and experiencing ≥1 prospectively observed pregnancy losses during 12 months of trying. There were 61 couples who contributed two pregnancy attempts and 9 who contributed three. Women were instructed in the use of urine-based home fertility monitors to time intercourse relative to ovulation and recorded their bleeding patterns in daily journals. TTP was defined as the number of menstrual cycles taken to achieve pregnancy. Women were also instructed in the use of home digital pregnancy tests and asked to begin pregnancy testing on the day of expected menses. Women recorded the results of their pregnancy tests in a daily journal with a single positive pregnancy test result indicating an hCG-confirmed pregnancy. Pregnancy losses were ascertained from a subsequent recorded negative pregnancy test or clinically confirmed loss. We estimated fecundability odds ratios (FORs) comparing subsequent to first TTP using discrete Cox models with robust standard errors, accounting for cycles off contraception before study entry and adjusting for maternal age, body mass index, reproductive history and time-varying cigarette, alcohol and caffeine usage while trying.
MAIN RESULTS AND THE ROLE OF CHANCE
The mean female age was 30.3 ± 4.3 years; 21% had a prior pregnancy loss before study entry. Of the second and third attempts, 59 and 43%, respectively, were longer compared with the first attempt. FORs <1 suggest reduced fecundability or a longer TTP when comparing the second with the first attempt (0.42, 95% confidence interval (CI): 0.28, 0.65), and similarly for the third relative to the first attempt (0.64, 95% CI: 0.18, 2.36). TTP in the second attempt was a median of 1 cycle longer (interquartile range: 0, 3 cycles) compared with TTP in the first attempt.
LIMITATIONS, REASONS FOR CAUTION
As this is the first study to investigate successive TTP exclusively among women experiencing pregnancy loss, our findings await corroboration since most losses occurred early in gestation. As such, the generalizability of our findings for all pregnancy losses awaits further research. We also had limited power to detect a reduction in fecundability for the third compared with first pregnancy attempt.
WIDER IMPLICATIONS OF THE FINDINGS
Unlike fertile women, TTP in women experiencing early pregnancy losses may trend towards longer subsequent attempts. If the findings are corroborated, women experiencing losses may benefit from counselling regarding trying times.
STUDY FUNDING/COMPETING INTERESTS
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-HD-3-3355, N01-HD-3-3356 and NOH-HD-3-3358). K.J.S. was supported by an Intramural Research Training Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research. The authors have no conflicts of interest to declare.
Keywords: time-to-pregnancy, spontaneous abortion, pregnancy loss
Introduction
Many adverse reproductive events are known to repeat within women, including pregnancy loss, gestational diabetes, pre-eclampsia and preterm birth (Heuser et al., 2010; Bramham et al., 2011; Melamed et al., 2012; Khambalia et al., 2013; Simonsen et al., 2013; Laughon et al., 2014). Time to pregnancy (TTP), or the number of menstrual cycles of unprotected sexual intercourse required to achieve pregnancy, is also reported to repeat within women (Basso et al., 1997; McLain et al., 2011). In the only two studies that have evaluated TTP across successive pregnancy attempts, analyses from the Collaborative Perinatal Project, a pregnancy cohort study conducted in the USA in the 1950s (McLain et al., 2011), and the European Studies of Infertility and Subfecundity, a population-based survey of reproductively aged women in five countries in Europe conducted in the 1990s (Basso et al., 1997), found that retrospectively reported TTP was similar across successive pregnancy attempts. The populations enrolled in these studies were primarily fertile, with most women reporting TTP for pregnancies ending in live births. Thus, the results from these studies may not hold for women experiencing fecundity impairments, such as pregnancy loss.
To our knowledge, no prior study has investigated TTP across pregnancy attempts exclusively among women with pregnancy loss despite the high incidence of loss. Between 20 and 50% of hCG-confirmed pregnancies end in loss (Miller et al., 1980; Edmonds et al., 1982; Whittaker et al., 1983; Sweeney et al., 1988; Wilcox et al., 1988b; Eskenazi et al., 1995; Hakim et al., 1995; Zinaman et al., 1996; Wang et al., 2003; Henriksen et al., 2004; Small et al., 2006; Buck Louis et al., 2009) and women experiencing losses may be particularly interested in their subsequent TTP as they have not yet achieved their goal of fertility. Therefore, to address this data gap and provide more information for women experiencing pregnancy loss and their clinicians, we used data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study to investigate prospectively observed TTP in successive pregnancy attempts among women with one or more pregnancy losses.
Materials and Methods
Study population and participants
The LIFE Study is a population-based prospective preconception cohort study comprising 501 couples in 16 counties in Michigan and Texas recruited upon discontinuation of contraception for purposes of becoming pregnant and followed for up to 1 year of trying, as fully described elsewhere (Buck Louis et al., 2011). The study sought to be inclusive of couples attempting pregnancy regardless of reproductive history. Only couples in which a partner had clinically diagnosed infertility were excluded from the study. The inclusion criteria for the study were (i) couples in a committed relationship, (ii) with an intention to begin attempting pregnancy or who had been off contraception for ≤2 months, (iii) where both partners could communicate in English or Spanish, (iv) where men were aged ≥18 years old and (v) where women were aged 18–40 years old, (vi) with reported menstrual cycle length between 21 and 42 days and (vii) no use of injectable contraceptives in the past year.
Importantly, couples were enrolled into the LIFE Study during the preconception period. At baseline, women had a urine pregnancy test to ensure they were not currently pregnant so that trying time could be observed. Couples were followed allowing for the prospective measurement of up to 12 months of trying across all attempts. Women not achieving pregnancy were censored. Women were given and instructed in the use of the ClearBlue Easy digital fertility monitor, a valid urine-based assessment of ovulation relative to the gold-standard, vaginal ultrasound (Behre et al., 2000). The monitor measures both estrone-3-glucuronide, a metabolite of estrogen, and luteinizing hormone to provide women with information on high and peak fertility days to facilitate intercourse timed to ovulation. Women in the LIFE Study were also given and instructed in the use of the ClearBlue Easy digital urine pregnancy test, which has a sensitivity of 25 IU/l of hCG and a digital readout of ‘pregnant’ or ‘not pregnant’ that reduces subjectivity in reading results. Women were instructed to test on the day of expected menses and were also provided with daily journals in which to record whether they had taken a pregnancy test, and if so, the test result. A single recorded positive pregnancy test was considered an hCG-confirmed pregnancy.
Couples experiencing a pregnancy loss during the study, which was defined as a subsequent negative urine pregnancy test or clinically confirmed pregnancy loss, were allowed to re-enter the study for a subsequent attempt as were couples experiencing a second loss. At the initial study enrolment, couples were informed that they could continue participating in the study if they experienced one or two losses. Couples with three losses met the clinical definition of recurrent pregnancy loss and were encouraged to seek medical care. Couples could decline the invitation to re-enter the study after a pregnancy loss. Therefore, this analysis of successive TTP includes only couples who experienced ≥1 pregnancy loss and chose to re-enter the study.
Descriptive analyses were undertaken to determine any differences by women's re-entry into the study or not, following a loss. Characteristics reported to affect TTP were assessed and included history of pregnancy loss prior to study entry (yes/no/never pregnant), maternal age (5-year intervals) maternal body mass index (BMI; categorical) at baseline and maternal preconception use of caffeine, cigarettes and alcohol (Wilcox et al., 1988a; Ford et al., 1994; Hakim et al., 1998; Jensen et al., 1998a,b; Hassan and Killick, 2005; Ramlau-Hansen et al., 2007; Buck Louis et al., 2009, 2012; Issa et al., 2010; Wise et al., 2012, 2013). Time-varying daily caffeine, cigarette and alcohol usage during the first pregnancy attempt was assessed using average cycle-level sums of consumption. We also compared gestational age based on the first day of the last menstrual period (LMP) for losses occurring to women who did and did not choose to re-enter. χ2 tests were used for categorical variables and non-parametric ANOVA tests were used for the time-varying covariates.
Prospectively observed TTP was counted from the first fully observed menstrual cycle in each attempt with Day 1 reflecting the first day of menses upon study entry or re-entry. For analysis, TTP includes both prospectively ‘observed’ cycles during the 12 months of longitudinal follow-up and ‘unobserved’ cycles, which denoted either the time couples were off contraception before the first pregnancy attempt, and also any elapsed time following a loss and resumption of trying in the second or third attempt. Couples who became pregnant after the baseline visit or pregnancy loss and before an observed menses were considered to have a TTP of zero. In these analyses, TTP denotes the number of cycles needed to achieve pregnancy or the last observed cycle for couples lost to follow-up or censored.
Statistical analysis
The Wilcoxon rank-sum test was used to determine the significance of the difference in TTP between the second and first attempts in the descriptive analysis. TTP for all pregnancy attempts includes both the observed and unobserved time as described above. For women who did not achieve a pregnancy in the second attempt, their second relative to first attempt was included in descriptive analysis if the TTP in the second attempt was the same or longer than the TTP in the first attempt. Women who were censored in the second attempt with a TTP that was shorter than their TTP in the first attempt were not included in descriptive analysis as it is unknown whether their TTP in the second relative to first attempt truly was shorter or if follow-up was simply not long enough to capture a longer TTP in the second attempt; however, these women were included in analyses that allow for right censoring. Survival probabilities for the first and second attempts were constructed using Lynden-Bell estimators (Keiding and Gill, 1990) that account for left-truncated (unobserved cycles prior to study entry or re-entry) and right-censored (unobserved cycles due to loss to follow-up or end of study period) data. Data from the third pregnancy attempt were not included in these descriptive analyses due to the small number of couples with a third attempt.
Cox proportional hazard models for discrete survival data (Cox, 1972) subjected to left truncation and right censoring were used to model TTP and estimate the fecundability odds ratio (FOR), which is the relative odds of achieving pregnancy in a cycle conditional on not becoming pregnant in the previous cycle. An FOR <1 is associated with a longer TTP, while an FOR >1 is associated with a shorter TTP. Robust standard errors were used in the construction of 95% confidence intervals (CIs) to account for repeated observations within women across attempts. Dummy variables were used to compare TTP for subsequent pregnancy attempts with TTP for the first pregnancy attempt. Multivariable models were adjusted for a priori confounders that are associated with TTP. Cigarette, alcohol and caffeine consumption were included as time-varying covariates using cycle-level sums for each. For women who had unobserved cycles during pregnancy attempts, the sums for the time-varying covariates in the first observed cycle of that attempt were used for the unobserved cycles in the same attempt. Maternal age and BMI at baseline (continuous variables) and history of pregnancy loss prior to study entry (yes/no/never pregnant) were included as fixed effects. Interactions between fixed effects and pregnancy attempt were assessed to determine whether the effects of maternal age, BMI or reproductive history on TTP differed by pregnancy attempt. All analyses were conducted in SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Ethical approval
Institutional Review Board approval was obtained from all participating institutions and all participants provided written informed consent prior to data collection.
Results
Of 501 couples enrolled in the LIFE Study, 347 (69%) achieved a pregnancy, of which 100 (29%) experienced a pregnancy loss. Of these, 70 eligible couples (70%) re-entered the study and 48 (69%) achieved a second pregnancy during the study period. Of these, 14 couples (29%) experienced a second loss and 9 (64%) re-entered the study for a third time. Of these, five couples (56%) achieved a third pregnancy and two (40%) couples experienced a third loss and were exited from the study. This analysis includes 70 couples, of whom 61 had two attempts and 9 had three attempts.
Among couples experiencing a first loss, there were no significant differences in reproductive history, maternal age or lifestyle between women who did or did not re-enter the study, with the exception of BMI being lower in women re-enrolling than in those who did not (Table I). Gestational age at the loss was also lower among women who re-enrolled than those who did not. Most women included in this analysis had no unobserved cycles at risk of pregnancy. In the first, second and third attempts, 70, 80 and 89% of women, respectively, had no unobserved cycles.
Table I.
Characteristics of women with pregnancy loss by re-entry status.
| Did not re-enter (n = 30) | Did re-enter (n = 70) | P-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Reproductive history at study entry | |||
| No prior loss in past pregnancies | 14 (47) | 24 (34) | 0.14 |
| History of prior loss in past pregnancies | 9 (30) | 15 (21) | |
| No prior pregnancies | 7 (23) | 31 (44) | |
| Maternal age (years) | |||
| 18–24 | 2 (7) | 3 (4) | 0.71 |
| 25–29 | 11 (37) | 34 (49) | |
| 30–34 | 11 (37) | 20 (29) | |
| 35–40 | 6 (20) | 13 (19) | |
| Body mass index | |||
| Underweight <18.5 kg/m2 | 1 (3) | 1 (1) | 0.02 |
| Normal 18.5–24.9 kg/m2 | 7 (23) | 35 (50) | |
| Overweight 25.0–29.9 kg/m2 | 6 (20) | 18 (26) | |
| Obese ≥30.0 kg/m2 | 16 (53) | 16 (23) | |
| Cigarette smoking preconception | |||
| None | 26 (87) | 55 (79) | 0.34 |
| ≥1 cigarette | 4 (13) | 15 (21) | |
| Mean (SD) | |||
| Average number of alcoholic drinks consumed in a cycle | 14.7 (18.4) | 15.6 (23.2) | 0.94 |
| Average number of caffeinated drinks consumed in a cycle | 45.3 (41.4) | 51.9 (39.8) | 0.35 |
| Median (IQR) | |||
| Gestational age at loss, days since LMP | 59 (45, 71) | 35 (30, 52) | <0.001 |
LMP, first day of last menstrual period.
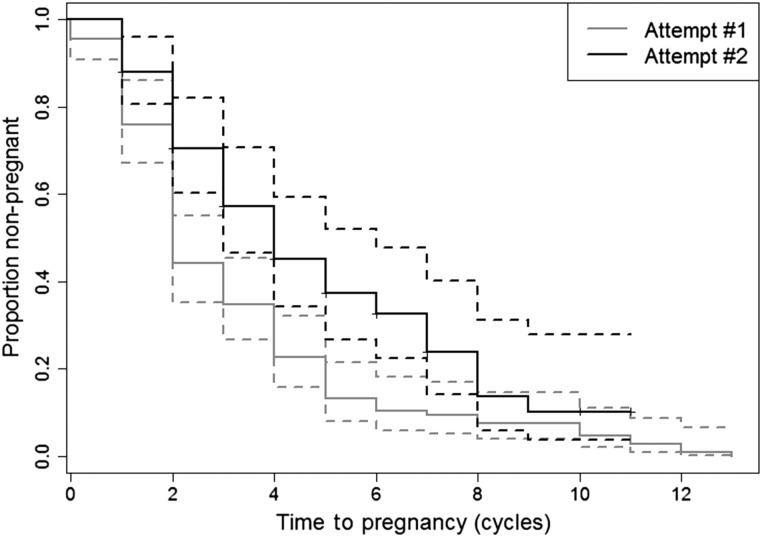
Figure 1 illustrates the longer TTP for the second attempt relative to the first. The proportion of women who were not pregnant in the second attempt was greater than those who were not pregnant in the first attempt across all cycles of trying with complete separation of the curves occurring during the third cycle of trying. The third attempt is not shown due to small numbers in the third attempt and to improve the clarity of the figure. In the first attempt, 69% of women had a TTP of 0–3 cycles, 25% had a TTP of 4–6 cycles and 6% had a TTP of ≥7 cycles. In the second attempt, 53% of women had a TTP of 0–3 cycles, 31% had TTP of 4–6 cycles and 16% had a TTP of ≥7 cycles.
Figure 1.
Survival probabilities for first and second pregnancy attempts including 95% CIs using Lynden-Bell estimators that account for left-truncated and right-censored data.
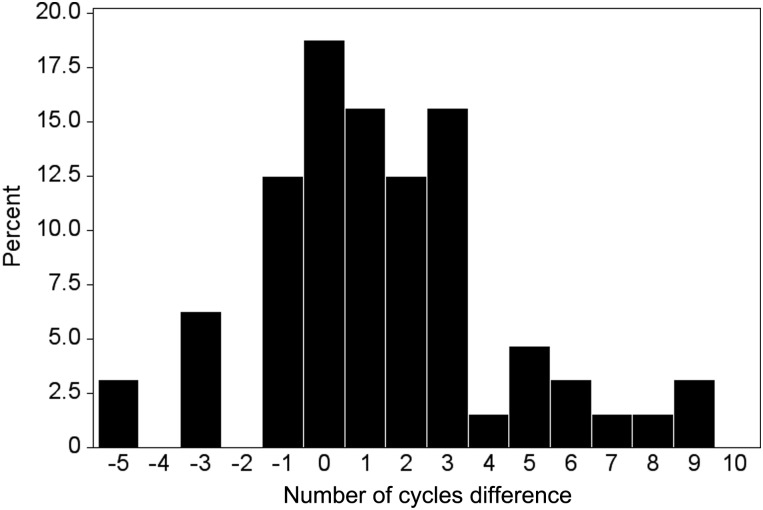
The differences in TTP between the second and first attempts are shown in Fig. 2. A positive integer represents a longer TTP in the second attempt relative to the first, while a negative integer reflects a shorter TTP in the second attempt. Six women who had not yet achieved a pregnancy at the time of censoring in the second attempt were not included in this descriptive analysis because the qualitative status of their TTP in the second attempt relative to the first attempt (longer, same, shorter) was unknown at the time of censoring. Women required a median of 1 additional cycle (interquartile range: 0, 3 cycles) to achieve a pregnancy in the second compared with first attempt (Wilcoxon rank-sum test P-value < 0.0001). Of the women, 59% had a longer TTP in the second attempt, 19% had the same TTP and 22% had a shorter TTP. Of the 38 women with a longer TTP in the second attempt, 26% took 1 cycle, 21% took 2 cycles, 26% took 3 cycles and 26% took ≥4 cycles longer. Among the 64 women for whom a qualitative status for the second relative to first attempt was known, the distribution of TTP for the second attempt among women with an initial TTP of 1 cycle was: 1 cycle (n = 1), 2 cycles (n = 3), 3–6 cycles (n = 9) and ≥7 cycles (n = 1). For women with an initial TTP of 2 cycles, the distribution among women was 1 cycle (n = 4), 2 cycles (n = 9), 3–6 cycles (n = 6) and ≥7 cycles (n = 6). Among women with an initial TTP of 3–6 cycles, 3, 4, 14 and 4 women had TTPs of 1, 2, 3–6 and ≥7 cycles, respectively, in the second attempt. For the six women for whom a qualitative status of TTP in the second relative to first attempt was not known, a long TTP in the first attempt was observed with four women taking ≥10 cycles to conceive. Of seven women with a known qualitative status of third relative to first attempt, three (43%) had a longer TTP, two (28%) had the same TTP and two (28%) had a shorter TTP in the third relative to first attempt.
Figure 2.
Difference in TTP between second and first attempts with positive integers indicating a longer second attempt. The histogram excludes six women who had not achieved a second pregnancy at the time of censoring and who had a shorter TTP in the second attempt.
TTP in the second attempt (FOR: 0.42 [95% CI: 0.29, 0.63]) and third attempt (FOR: 0.64 [95% CI: 0.18, 2.36]) were longer compared with the first attempt (Table II). After adjustment for maternal age, BMI, prior loss before study entry and time-varying cigarette, alcohol and caffeine consumption, the findings were essentially unchanged. The FORs for reproductive history at study entry changed from >1 to <1 after adjustment, although there was 95% CI overlap for both estimates and no result was statistically significant. We present FORs for the sums of time-varying exposures in a 28-day cycle for smoking a pack of cigarettes daily (560 cigarettes/cycle) and consuming one alcoholic drink (28 drinks/cycle) and two caffeinated beverages (56 drinks/cycle) daily to provide results for meaningful levels of consumption and context for the FORs for subsequent compared with first pregnancy attempts. Smoking and alcohol use resulted in reduced fecundability, but neither result was statistically significant. None of the interactions between fixed-effect covariates and pregnancy attempt were significant.
Table II.
Unadjusted and adjusted FORs.
| Unadjusted FOR [95% CI] | Adjusted FOR [95% CI]a | |
|---|---|---|
| Attempt | ||
| First | 1.00 | 1.00 |
| Second | 0.42 [0.29, 0.63] | 0.42 [0.28, 0.65] |
| Third | 0.64 [0.18, 2.36] | 0.56 [0.11, 2.79] |
| Reproductive history at study entry | ||
| Never pregnant | 1.00 | 1.00 |
| No prior loss | 1.07 [0.67, 1.70] | 0.92 [0.50, 1.69] |
| Prior loss | 1.13 [0.69, 1.85] | 0.97 [0.55, 1.72] |
| Maternal age (years) | 1.00 [0.96, 1.05] | 1.02 [0.96, 1.08] |
| Maternal BMI (unit) | 0.98 [0.95, 1.01] | 0.99 [0.95, 1.02] |
| Cigarettes smoked (560 cigarettes/cycle)b | 0.59 [0.30, 1.17] | 0.69 [0.26, 1.81] |
| Caffeine consumption (56 drinks/cycle)b | 0.95 [0.71, 1.28] | 1.02 [0.67, 1.56] |
| Alcohol consumption (28 drinks/cycle)b | 0.91 [0.74, 1.13] | 0.93 [0.71, 1.21] |
aAdjusted for all other covariates listed in the table.
bFor a 28-day cycle, the cycle-level sums for smoking a pack of cigarettes daily and consuming two caffeinated and one alcoholic drink daily.
Discussion
These data demonstrate that among women experiencing pregnancy loss and who took ≤6 cycles to conceive initially, TTP after compared with before an observed loss is significantly longer. The pregnancy loss itself, denoted by subsequent pregnancy attempts, is a stronger predictor of TTP than other traditional risk factors for delayed TTP, including smoking. This strong association is robust to procedures accounting for left truncation of unobserved cycles at risk of pregnancy and to adjustment for several well-measured confounders. It is important to note that the median post-LMP gestational age of losses was 35 days (5%: 26 days, 95%: 81 days) reflecting contemporary use of home pregnancy kits for the detection of pregnancy and ensuing losses. Of the couples who re-entered the study 69% conceived a second pregnancy within the observed follow-up period, of whom 63% experienced a live birth, while 29% experienced a second loss, and 8% were lost to follow-up.
The reason for the longer observed TTP in the subsequent pregnancy attempt is not readily apparent. We compared mean cycle-level sexual intercourse and contraceptive use frequency in the first pregnancy attempt to the first observed cycle immediately following the loss and found no differences in either of these behaviours as recorded in daily journals. Furthermore, we did not observe an increase in alcohol, cigarette or caffeine use after a loss. The longer observed TTP may be due to a biologic mechanism, such as delayed return of ovulation or changes in the endometrium following the loss. We examined the first cycle after a loss to determine whether the per cent of cycles that were detected as ovulatory by the fertility monitor was similar to the per cent of cycles prior to the loss that were detected as ovulatory. We found that a significantly lower percentage of first cycles following losses were ovulatory when compared with cycles preceding the losses. This finding agrees with prior studies that have found that ovulation returns at an average of 29–50 days following a loss (Ratten, 1972; Donnet et al., 1990) and results in pregnancy at an average of 9 weeks after a loss (Rud and Klunder, 1985), although ovulation has been reported to return as soon as 10–13 days following a loss (Ratten, 1972; Donnet et al., 1990) and result in pregnancy as soon as 2–4 weeks following a loss (Rud and Klunder, 1985; Wyss et al., 1994). Changes in the endometrium may also underlie the observed delay in TTP, though previous studies have found a reduction in endometrial thickness within 2 weeks of onset of a loss managed either expectantly or medically (Haines et al., 1994; Creinin et al., 2004) and return of secretory endometrium as soon as 2 weeks after a loss (Ratten, 1972; Haines et al., 1994). Unfortunately, we do not have sufficient data to evaluate this possible explanation, although we did consider whether TTP for the second attempt varied by whether or not a woman sought clinical care or underwent a dilation and curettage for the first loss. We did not observe any significant difference in TTP in the second attempt by either of these factors. The observed delay in TTP following these early losses is interesting, as women had been pregnant only for a short time prior to the loss. This finding suggests any biologic mechanism that may act to delay TTP following a loss is likely triggered early in gestation.
The strength of our findings was somewhat unexpected given the previously reported similarities in TTPs across pregnancy attempts among fertile women (Basso et al., 1997; McLain et al., 2011). However, our results corroborate the findings of another study (Hassan and Killick, 2005) that found a significantly longer TTP after a pregnancy loss compared with TTP after a live birth. An important difference between the previous study (Hassan and Killick, 2005) and ours is that the former compared TTP across women for a single pregnancy attempt, while we compared TTP within women for successive pregnancy attempts. Furthermore, we used prospectively observed TTP in a preconception cohort, while the previous study relied upon retrospectively reported TTP among pregnant women attending antenatal clinics (Hassan and Killick, 2005). Our findings also corroborate the results of a recent study that examined TTP following ≥2 unexplained recurrent miscarriages (Kaandorp et al., 2014). Among their sample of women with fecundity impairments, the cumulative incidence of conception was 56% after 6 months of trying and 74% after 12 months of trying. This is less than the cumulative incidence of pregnancy at 6 (76–92%) and 12 (90–95%) cycles of trying reported for women enrolled in preconception cohorts (Buck Louis, 2011), indicating a longer TTP for women with fecundity impairments relative to the general population of pregnancy planners.
Our findings are strengthened by many unique features of the LIFE Study. First, preconception recruitment of couples enabled us to prospectively measure TTP, which is the gold standard. Retrospectively reported TTP may be accurate for short (Zielhuis et al., 1992) but not long-term recall of retrospective TTP, given bidirectional reporting errors (Cooney et al., 2009). Furthermore, the use of highly accurate fertility monitors assisted couples in timing intercourse relative to ovulation, and the use of in-home, highly sensitive pregnancy tests allowed us to capture very early pregnancies and pregnancy losses. These technologies helped ascertain most pregnancies and losses, at least those detected by hCG. Secondly, this is a population-based sample of reproductive-aged women and their partners attempting pregnancy. Because our eligibility criteria only excluded couples with clinically diagnosed infertility, we believe that these results may be generalizable to a broad population of women experiencing pregnancy loss. Thirdly, our statistical models included potential confounders while accounting for left truncation and right censoring, giving us greater confidence in the findings. Accounting for cycles off contraception prior to study entry and any cycles off the study after a loss was critical to ensure that we accounted for all cycles at risk of pregnancy and that the longer TTP observed in subsequent attempts was not solely attributable to more complete observation of at-risk cycles. Finally, we were able to assess sexual intercourse and contraceptive use frequency in the cycles before and immediately following a loss to determine whether a behaviour change was the cause of the longer observed TTP.
Our findings should be interpreted within the limitations of the study and await corroboration. We had limited power to evaluate the third attempt as only nine women contributed a third attempt. Still, the association between the third compared with first attempt was in the same direction as the second attempt. Furthermore, as couples were only followed for 12 months of trying across all attempts, couples must have had a relatively short trying time (≤6 cycles) in the first attempt for their second attempt to be qualified as shorter, the same or longer at the time of censoring. Therefore, these results may not be generalizable to couples taking longer to conceive initially. Our findings are also limited to women who chose to re-enter the study. While these two groups did not differ on many of the characteristics we examined, women who chose to re-enter were less overweight and obese than women who chose not to re-enter. However, as women who are overweight and obese have longer TTP than women with normal weight (Ramlau-Hansen et al., 2007; Wise et al., 2012, 2013), our findings may underestimate the decrease in fecundability following a pregnancy loss. Women who chose to re-enter the study also experienced losses at earlier gestational ages than women who chose not to re-enter. As such, the extent to which our findings may be generalizable for losses across the spectrum of gestational age await further research. Still, we observed no differences in the TTP for the second attempt when comparing women whose initial loss was ≤6 or >6 weeks.
These results may suggest that at the population level, TTP in the pregnancy attempt following an early pregnancy loss is longer than TTP in the attempt preceding the loss for couples taking ≤6 cycles to conceive initially. The median difference in TTP between the second and first attempts was 1 cycle longer; however, more than 25% of women took ≥3 cycles longer in their second attempt. Such information may be relevant for clinicians in counselling women about successive pregnancy attempts following a pregnancy loss in light of the stress and sadness that may accompany a loss. Attempting pregnancy can be a stressful period for many couples, and pregnancy loss may exacerbate the anxiety surrounding conceiving and sustaining a successful pregnancy. Women who experience a loss, even very early in gestation, might best be counselled that the TTP in a subsequent attempt may be longer than the TTP in the initial attempt. Despite a longer TTP in the subsequent attempts, the good news is that most women in our study who had early pregnancy losses conceived again. Our data do not suggest that women and their partners should delay their attempts at conception following a pregnancy loss; rather, they provide couples with information, and hopefully some reassurance, that a longer TTP after a loss is not unexpected.
Authors' roles
K.J.S. assisted in conceptualizing the paper, performed statistical analyses and wrote the first draft. A.C.M. assisted in formation of the statistical analysis and provided substantive edits to the paper. J.M.M. was instrumental in implementing the statistical analysis and provided substantive edits to the paper. R.S. assisted in conceptualizing the paper, oversaw the statistical analysis and provided substantive edits to the paper. G.M.B.L. designed the study, assisted in conceptualizing the paper and provided substantive edits to the analytic plan and paper.
Funding
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-HD-3-3355, N01-HD-3-3356 and NOH-HD-3-3358). K.J.S. was supported by an Intramural Research Training Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Division of Intramural Population Health Research.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Basso O, Olsen J, Bisanti L, Bolumar F, Kuppers-Chinnow M. Repeating episodes of low fecundability. A multicentre European study. The European Study Group on Infertility and Subfecundity. Hum Reprod. 1997;12:1448–1453. doi: 10.1093/humrep/12.7.1448. [DOI] [PubMed] [Google Scholar]
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- Bramham K, Briley AL, Seed P, Poston L, Shennan AH, Chappell LC. Adverse maternal and perinatal outcomes in women with previous preeclampsia: a prospective study. Am J Obstet Gynecol. 2011;204:512. doi: 10.1016/j.ajog.2011.02.014. e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM. Fecundity and fertility. In: Buck Louis GM, Platt R, editors. Reproductive and Perinatal Epidemiology. New York: Oxford University Press; 2011. [Google Scholar]
- Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod. 2009;24:451–458. doi: 10.1093/humrep/den373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Chen Z, Kim S, Caldwell KL, Barr DB. Heavy metals and couple fecundity, the LIFE Study. Chemosphere. 2012;87:1201–1207. doi: 10.1016/j.chemosphere.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;20:187–220. [Google Scholar]
- Creinin MD, Harwood B, Guido RS, Fox MC, Zhang J. Endometrial thickness after misoprostol use for early pregnancy failure. Int J Gynaecol Obstet. 2004;86:22–26. doi: 10.1016/j.ijgo.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnet ML, Howie PW, Marnie M, Cooper W, Lewis M. Return of ovarian function following spontaneous abortion. Clin Endocrinol (Oxf) 1990;33:13–20. doi: 10.1111/j.1365-2265.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. Early embryonic mortality in women. Fertil Steril. 1982;38:447–453. [PubMed] [Google Scholar]
- Eskenazi B, Gold EB, Lasley BL, Samuels SJ, Hammond SK, Wight S, O’Neill Rasor M, Hines CJ, Schenker MB. Prospective monitoring of early fetal loss and clinical spontaneous abortion among female semiconductor workers. Am J Ind Med. 1995;28:833–846. doi: 10.1002/ajim.4700280615. [DOI] [PubMed] [Google Scholar]
- Ford JH, MacCormac L, Hiller J. PALS (pregnancy and lifestyle study): association between occupational and environmental exposure to chemicals and reproductive outcome. Mutat Res. 1994;313:153–164. doi: 10.1016/0165-1161(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Haines CJ, Chung T, Leung DY. Transvaginal sonography and the conservative management of spontaneous abortion. Gynecol Obstet Invest. 1994;37:14–17. doi: 10.1159/000292512. [DOI] [PubMed] [Google Scholar]
- Hakim RB, Gray RH, Zacur H. Infertility and early pregnancy loss. Am J Obstet Gynecol. 1995;172:1510–1517. doi: 10.1016/0002-9378(95)90489-1. [DOI] [PubMed] [Google Scholar]
- Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70:632–637. doi: 10.1016/s0015-0282(98)00257-x. [DOI] [PubMed] [Google Scholar]
- Hassan MA, Killick SR. Is previous aberrant reproductive outcome predictive of subsequently reduced fecundity? Hum Reprod. 2005;20:657–664. doi: 10.1093/humrep/deh670. [DOI] [PubMed] [Google Scholar]
- Henriksen TB, Hjollund NH, Jensen TK, Bonde JP, Andersson AM, Kolstad H, Ernst E, Giwercman A, Skakkebaek NE, Olsen J. Alcohol consumption at the time of conception and spontaneous abortion. Am J Epidemiol. 2004;160:661–667. doi: 10.1093/aje/kwh259. [DOI] [PubMed] [Google Scholar]
- Heuser C, Dalton J, Macpherson C, Branch DW, Porter TF, Silver RM. Idiopathic recurrent pregnancy loss recurs at similar gestational ages. Am J Obstet Gynecol. 2010;203:343. doi: 10.1016/j.ajog.2010.05.010. e341–343.e345. [DOI] [PubMed] [Google Scholar]
- Issa Y, Sallmen M, Nijem K, Bjertness E, Kristensen P. Fecundability among newly married couples in agricultural villages in Palestine: a prospective study. Hum Reprod. 2010;25:2132–2138. doi: 10.1093/humrep/deq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998a;12:289–295. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Hjollund NH, Henriksen TB, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ. 1998b;317:505–510. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaandorp SP, van Mens TE, Middeldorp S, Hutten BA, Hof MH, van der Post JA, van der Veen F, Goddijn M. Time to conception and time to live birth in women with unexplained recurrent miscarriage. Hum Reprod. 2014;29:1146–1152. doi: 10.1093/humrep/deu052. [DOI] [PubMed] [Google Scholar]
- Keiding N, Gill RD. Random truncation models and Markov processes. Ann Stat. 1990;18:582–602. [Google Scholar]
- Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabet Med. 2013;30:452–456. doi: 10.1111/dme.12124. [DOI] [PubMed] [Google Scholar]
- Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol. 2014;210:131. doi: 10.1016/j.ajog.2013.09.014. e131–1131.13e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLain AC, Sundaram R, Cooney MA, Gollenberg AL, Buck Louis GM. Clustering of fecundability within women. Paediatr Perinat Epidemiol. 2011;25:460–465. doi: 10.1111/j.1365-3016.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- Melamed N, Hadar E, Peled Y, Hod M, Wiznitzer A, Yogev Y. Risk for recurrence of preeclampsia and outcome of subsequent pregnancy in women with preeclampsia in their first pregnancy. J Matern Fetal Neonatal Med. 2012;25:2248–2251. doi: 10.3109/14767058.2012.684174. [DOI] [PubMed] [Google Scholar]
- Miller JF, Williamson E, Glue J, Gordon YB, Grudzinskas JG, Sykes A. Fetal loss after implantation. A prospective study. Lancet. 1980;2:554–556. doi: 10.1016/s0140-6736(80)91991-1. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Ratten GJ. Resumption of ovulation after incomplete abortion. Aust N Z J Obstet Gynaecol. 1972;12:217–219. doi: 10.1111/j.1479-828x.1972.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Rud B, Klunder K. The course of pregnancy following spontaneous abortion. Acta Obstet Gynecol Scand. 1985;64:277–278. doi: 10.3109/00016348509155129. [DOI] [PubMed] [Google Scholar]
- Simonsen SE, Lyon JL, Stanford JB, Porucznik CA, Esplin MS, Varner MW. Risk factors for recurrent preterm birth in multiparous Utah women: a historical cohort study. BJOG. 2013;120:863–872. doi: 10.1111/1471-0528.12182. [DOI] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, Marcus M. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17:52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- Sweeney AM, Meyer MR, Aarons JH, Mills JL, LaPorte RE. Evaluation of methods for the prospective identification of early fetal losses in environmental epidemiology studies. Am J Epidemiol. 1988;127:843–850. doi: 10.1093/oxfordjournals.aje.a114867. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–584. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- Whittaker PG, Taylor A, Lind T. Unsuspected pregnancy loss in healthy women. Lancet. 1983;1:1126–1127. doi: 10.1016/s0140-6736(83)92865-9. [DOI] [PubMed] [Google Scholar]
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988a;2:1453–1456. doi: 10.1016/s0140-6736(88)90933-6. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988b;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis AH, Hatch EE. A prospective cohort study of physical activity and time to pregnancy. Fertil Steril. 2012;97:1136–1142. doi: 10.1016/j.fertnstert.2012.02.025. e1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28:2856–2864. doi: 10.1093/humrep/det333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235–241. doi: 10.1515/jpme.1994.22.3.235. [DOI] [PubMed] [Google Scholar]
- Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol. 1992;21:1151–1156. doi: 10.1093/ije/21.6.1151. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]