Abstract
Because of increasing interest in at-risk enrollment of Medicare beneficiaries by health maintenance organizations, a number of modifications to the adjusted average per capita cost (AAPCC) formula employed by the Health Care Financing Administration have been proposed recently. Researchers have found that new models, which include measures of prior years' utilization and costs, predict Medicare payments significantly better than does the purely demographic formula currently used. In this article, we show that inclusion of instrumental activities of daily living (IADL), a measure of beneficiaries' functional health status, can further improve AAPCC models that already incorporate measures of previous-period utilization and costs. Various models for predicting Medicare payments were examined and compared using survey data and Medicare claims for a random sample of 1,934 beneficiaries. For these models, explained variation in subsequent Medicare payments (as indicated by R2 values) increased considerably when the IADL variable was included. Although actuarial concerns are associated with inclusion of the IADL score in the AAPCC, use of this measure is likely to offset other, possibly more serious, actuarial problems associated with including measures of previous utilization and costs.
Introduction
As Medicare at-risk contracts with health maintenance organizations (HMO's) become more prevalent, the validity of Medicare HMO capitation rates is acquiring increased importance. The Health Care Financing Administration (HCFA) is concerned that risk-sharing HMO's may be experiencing favorable selection in their Medicare enrollment (Eggers, 1980; Eggers and Prihoda, 1982) with consequent capitation overpayments. Juxtaposed against this concern are HMO fears of adverse selection and potential losses on Medicare contracts. At the heart of these concerns is the appropriateness of the adjusted average per capita cost (AAPCC), HCFA's formula for setting premiums for at-risk HMO's. The AAPCC is intended to represent an estimate of the costs that would have been incurred by a Medicare HMO enrollee had the beneficiary received care in the fee-for-service system. As such, it is an attempt by HCFA to calibrate HMO premiums to the expected cost of caring for a particular group of enrolled beneficiaries. Under the Tax Equity and Fiscal Responsibility Act of 1982, HMO's contracting on a risk basis with HCFA now receive capitation payments set at 95 percent of the AAPCC. This arrangement is designed to lower HCFA's expenditures for Medicare enrollees while providing HMO's incentives to deliver care more efficiently than the fee-for-service system does.
However, the actuarial factors currently used to calculate the AAPCC (age, sex, institutional status, and welfare status) have been shown to be poor predictors of costs for the Medicare population (Trieger, Galblum, and Riley, 1981). Alternative AAPCC models (that is, models that include other actuarial factors besides or in addition to the current ones) have been tested by researchers. In several studies, models that include measures of previous-period costs or utilization have been proven to provide significantly better financial predictions than does the purely demographic model now being used (e.g., Anderson, Resnick, and Gertman, 1982; Beebe, Lubitz, and Eggers, 1985; Eggers, 1981).
It has also been suggested that adding measures of beneficiary health status to the AAPCC would significantly improve its predictive capability (Luft et al., 1980; Trieger, Galblum, and Riley, 1981; Hornbrook, 1984; Anderson and Knickman, 1984). In an earlier article, we noted that the addition of health status measures might improve the AAPCC. We suggested direct measures of the beneficiary's physical health and functioning, such as measures of perceived health status and functional health status, as well as indirect measures, such as indicators of previous-period utilization and costs, which reflect beneficiaries' health-related behavior (Thomas et al., 1983).
In a recent study (Thomas et al., 1985), we tested various health status measures as predictors of future Medicare costs. Of the direct health status measures considered, the best single predictor was the instrumental activities of daily living (IADL) score, based on the Rosow-Breslau Functional Health Status Test (Rosow and Breslau, 1966). This measure explained 3.2 percent of the variance in Medicare payments when used alone (compared with 0.3 percent for the current AAPCC) and 3.9 percent when used with the demographic factors currently comprising the AAPCC formula.
The purpose of this article is to show that inclusion of the IADL score can further improve AAPCC models that already incorporate measures of previous-period utilization and costs. We use data from a randomly selected sample of Medicare beneficiaries in Michigan to examine several models based on utilization and costs that have been proposed by other researchers. Beneficiary IADL score is then added as another independent variable in each model to determine the degree to which predictions of Medicare payments are improved by including this health status measure. Based on these results, we suggest new models for more accurately predicting Medicare capitation rates.
Methods
Data
A random sample of 3,000 individuals was selected from the approximately 1,050,000 Medicare beneficiaries in Michigan. Data on demographic characteristics and self-rated health status of beneficiaries were obtained through a mail survey. Measures of medical services utilization and costs were developed by analyzing Medicare claims data, and data on beneficiary welfare status were provided by the Michigan Department of Social Services.
At the end of calendar year 1982, a survey instrument containing questions on health perceptions, functional ability, chronic illnesses, and living situation was mailed to the beneficiaries in the sample. Standard mail survey procedures, including several followup mailings to nonrespondents, were employed. Beneficiaries who had died or moved out of Michigan prior to the survey were dropped, as were those who could not respond because of mental disability. The survey yielded 2,123 responses for an adjusted response rate of 81.2 percent.
Medicare claims data for sample beneficiaries were provided by Blue Cross/Blue Shield of Michigan, the Medicare intermediary and carrier for the State. The year prior to the survey (January 1, 1982, through December 31, 1982) and the 6-month period following the survey (January 1, 1983, through June 30, 1983) were covered. Receipt of claims data for the January-June 1983 period was postponed until December 1983 to accommodate routine delays in claims filing and processing. The choice of 6 months as the length of the postsurvey data collection period was influenced by these delays and represented a compromise between the quantity of claims data obtainable and the need for timely analytical results.
Because available claims data covered only services provided in Michigan, respondents were resurveyed in late 1983 to determine whether medical services had been received in other States during the study period. Not surprisingly, utilization outside Michigan was found to be concentrated among residents of border counties, i.e., those adjacent to neighboring States. To eliminate bias from underreporting of utilization, beneficiaries having any hospitalizations or more than three physician visits outside Michigan were dropped from the study, leaving data on 1,934 persons for the analyses.
The AAPCC currently includes welfare status (which is actually operationalized as Medicaid eligibility) as an actuarial factor. HCFA determines Medicaid eligibility by identifying beneficiaries for whom the Medicare supplementary medical insurance (Part B) premium is paid by Medicaid. For this study, data on Medicaid eligibility were obtained directly from the Michigan Department of Social Services, the Medicaid agency.
Variables
In the analyses presented in this article, the dependent variable, total Medicare payments made on behalf of the beneficiary during January-June 1983, is regressed on beneficiary IADL score, demographic characteristics, and prior-year utilization and costs.
Dependent variable
Claims data used in this study cover periods before implementation of the prospective payment system. Medicare payments, which then were based on retrospective cost reimbursement, could have varied for equivalent services from hospital to hospital. To eliminate the effect of interinstitutional rate and cost differences, each discharge was assigned to a diagnosis-related group (DRG) using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), Medicare Provider Analysis and Review (MEDPAR) Grouper algorithm (Public Health Service and Health Care Financing Administration, 1980). Based on actual Medicare payments for these discharges, an average payment was calculated for each DRG represented in the data set. Standardized inpatient service payments then were tabulated for each beneficiary using these DRG averages.
No method comparable to DRG's exists for standardizing other categories of Medicare payments. Thus, the dependent variable for the study, January-June 1983 standardized total Medicare payments, was created as the sum of standardized hospital insurance (Part A) inpatient service payments (representing 69.5 percent of total payments) plus actual payments for supplementary medical insurance (Part B) services, Part A outpatient services, Part A home health services, and Part A skilled nursing facility services. For the 1,934 beneficiaries in the final sample, the minimum value of standardized total Medicare payments for January-June 1983 was $0, the maximum was $26,416, and the mean standardized payment for the 6-month period was $932.
Demographic characteristics
Independent variables used in the analyses are described in Table 1. Examination of current AAPCC factors shows that the mean age of sample beneficiaries was 76 years, 61 percent were women, only 2.1 percent resided in institutions, and 5.6 percent were covered under Medicaid.
Table 1. Minimum, maximum, and mean of independent variables for regressions on January-June 1983 standardized payments.
| Variable | Minimum | Maximum | Mean |
|---|---|---|---|
| Age1 | 68 | 100 | 76.18 |
| Sex (1 = female) | 0 | 1 | .61 |
| Institutional status (1 = yes) | 0 | 1 | .02 |
| Medicaid (1 = yes) | 0 | 1 | .06 |
| IADL2 score | 0 | 5 | 3.04 |
| Number of Part B claims, 1982 | 0 | 450 | 21.47 |
| Number of emergency room visits, 1982 | 0 | 8 | .17 |
| Number of inpatient admissions, 1982 | 0 | 7 | .34 |
| Number of inpatient days, 1982 | 0 | 196 | 3.52 |
| 1982 standardized payments (in dollars) | 0 | 46,818 | 1,518.80 |
| Hospitalization in 1982 (1 = yes) | 0 | 1 | .22 |
| 1 hospitalization, 1982 (1 = yes) | 0 | 1 | .15 |
| More than 1 hospitalization, 1982 (1 = yes) | 0 | 1 | .07 |
| Hospitalization in 1982: | |||
| Non-high-cost diagnosis only (1 = yes) | 0 | 1 | .07 |
| Secondary diagnosis (1 = yes) | 0 | 1 | .06 |
| High-cost diagnosis, 20 days or less (1 = yes) | 0 | 1 | .06 |
| High-cost diagnosis, more than 20 days (1 = yes) | 0 | 1 | .04 |
| Reimbursement but no hospitalization (1 = yes) | 0 | 1 | .54 |
| Part B deductible met (1 = yes) | 0 | 1 | .74 |
As of June 1983.
Instrumental activities of daily living.
NOTE: Information shown is based on answers of 1,934 survey respondents.
IADL score
The instrumental activities of daily living score is based on responses to the questions listed in Table 2. As may be observed from the right-hand column of the table, these responses form a Guttman scale with a coefficient of reproducibility equal to 0.9301. Scale values range from 0 for respondents who reported a health problem and could perform none of the functions without assistance to 5 for those who could perform all listed activities and reported no bothersome physical or health problem.
Table 2. Questions and responses relating to instrumental activities of daily living.
| Question | Yes | No |
|---|---|---|
|
| ||
| Percent | ||
| Can you walk up and down stairs to the second floor without help? | 86.6 | 13.4 |
| Can you go out to a movie, a meeting, to church or synagogue, or to visit friends without help? | 81.5 | 18.5 |
| Can you walk half a mile without help? | 67.4 | 32.6 |
| Can you do heavy work around the house, like shoveling snow or washing walls, without help? | 37.6 | 62.4 |
| Is there any physical condition, illness, or health problem that bothers you now?1 | 68.9 | 31.1 |
For scaling purposes, this item is reverse scored.
NOTE: Information shown is based on answers of 1,934 survey respondents.
Prior-period utilization and costs
Standardized total Medicare payments for calendar year 1982, the year preceding the mail survey, were computed for each beneficiary using the DRG procedure described earlier. Total numbers of inpatient admissions, acute inpatient days, hospital emergency room visits, and Part B claims submitted during the period were also tabulated for each beneficiary.
In addition to these counts of 1982 utilization, several binary (0/1) measures were constructed to allow testing of models previously proposed by Beebe, Lubitz, and Eggers (1985) and Anderson, Resnick, and Gertman (1982). In one of the models examined by Beebe, Lubitz, and Eggers (1985), a binary measure indicating whether a beneficiary's Part B deductibles had been met during either of the 2 previous years was included as an independent variable. Our data base contained utilization data for only 1 prior year, so we were unable to replicate this variable exactly. Instead, the variable in Table 1 relating to the Part B deductible was constructed by assigning a value of 1 if any Part B payments were made on behalf of a beneficiary in 1982 and a value of 0 otherwise. For 74 percent of the sample beneficiaries, the Part B deductible was satisfied in 1982.
In another model tested by Beebe, Lubitz, and Eggers (1985), a binary variable was included to indicate beneficiaries who had been hospitalized during the preceding year. As shown in Table 1, this variable is assigned a value of 1 for beneficiaries hospitalized for any reason during 1982 and a value of 0 for those not hospitalized. Of the sample beneficiaries 22 percent were admitted to a hospital during 1982.
Among the independent variables tested by Anderson, Resnick, and Gertman (1982) was a measure that was assigned a value of 1 if Medicare payments were made on the beneficiary's behalf during the prior year but the beneficiary was not hospitalized during that year. In the same model, a second binary variable was used to identify beneficiaries who had been hospitalized once during the previous year and a third variable to indicate those who had been admitted two or more times to a hospital. Of the 1,934 beneficiaries in the Michigan sample, 15.2 percent were hospitalized one time, 7.1 percent were hospitalized two or more times, and 53.9 percent were not hospitalized but received services for which Medicare payments were made.
For another model, Anderson, Resnick, and Gertman (1982) developed a list of hospital discharge diagnoses that were found to be associated with high costs in the subsequent year. Most cancers and heart conditions and many other chronic diseases were included in the list of high-cost diagnoses. Using this list, Medicare hospital claims were analyzed and beneficiaries who had been hospitalized during the previous year were assigned to one of the following categories:
One or more hospitalizations in prior year but none involving a diagnosis on the high-cost list or a secondary diagnosis.
One or more hospitalizations in prior year, at least one involving a secondary diagnosis, but none involving a diagnosis on the high-cost list.
One or more hospitalizations in prior year, with at least one diagnosis on the high-cost list, but with total hospital days not exceeding 20.
One or more hospitalizations in prior year, with at least one diagnosis on the high-cost list and with more than 20 total hospital days.
In their analyses, Anderson, Resnick, and Gertman (1982) employed 1974-75 Medicare data in which diagnoses were identified using the coding system of the Eighth Revision International Classification of Diseases, Adapted for Use in the United States (ICDA-8) (National Center for Health Statistics, 1967). For use with 1982-83 Michigan data, the three-digit ICDA-8 codes defining the list of high-cost diagnoses were translated into three-digit ICD-9-CM codes. Discharge diagnoses and lengths of stay for 1982 hospitalizations then were used to classify sample beneficiaries into the categories defined above. As shown in Table 1, 7 percent of sample beneficiaries were hospitalized in 1982 without a high-cost diagnosis and with no secondary diagnosis, 6 percent had a secondary diagnosis and fell into the second group, and 6 percent and 4 percent were classified into the third and fourth groups, respectively. Correlations among the independent variables are shown in Table 3.
Table 7. Performance (R2) of selected models using untransformed and log transformed dependent variable1.
| Model and model variables | R2 untransformed dependent variable | R2 log transformation of dependent variable |
|---|---|---|
| Model 42 | ||
| Age; sex; institutional status; Medicaid; reimbursement but no hospitalization; 1 hospitalization; more than 1 hospitalization | .071 | .173 |
| Model 4a2 | ||
| Model 4 plus IADL3 score | .087 | .189 |
| Model 52 | ||
| Age; sex; institutional status; Medicaid; reimbursement but no hospitalization; hospitalization with non-high-cost diagnosis; hospitalization with secondary diagnosis; hospitalization with high-cost diagnosis, 20 days or less; hospitalization with high-cost diagnosis, more than 20 days | .063 | .169 |
| Model 5a2 | ||
| Model 5 plus IADL3 score | .080 | .185 |
| Model 64 | ||
| Age; sex; 1982 standardized payments5 | .058 | .217 |
| Model 6a4 | ||
| Model 6 plus IADL3 score | .075 | .230 |
| Model 7 | ||
| Age; sex; 1 hospitalization; more than 1 hospitalization; number of Part B claims; number of emergency room visits; reimbursement but no hospitalization; IADL3 score | .096 | .236 |
| Model 8 | ||
| Age; sex; 1 hospitalization; more than 1 hospitalization, number of Part B claims; IADL3 score | .092 | .198 |
January-June 1983 standardized payments.
Instrumental activities of daily living.
(Eggers, 1981).
For regression on log transformation of dependent variable, 1982 standardized payments variable is also transformed.
NOTE: Information shown is based on answers of 1,934 survey respondents.
Table 3. Correlations (Pearson's r) among potential independent variables.
| Variable | Age | Sex | Institutional status | Medicaid | IADL1 | Number of— | 1982 standardized payments | Hospitalization | Hospitalization diagnosis | Reimbursement no hospitalization | Part B deductiblemet | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||||||
| Part B claims | Emergency room visits | Inpatient admissions | Inpatient days | Non-high-cost only | Secondary | High-cost | |||||||||||||
|
|
|
||||||||||||||||||
| Any | 1 | More than 1 | 20 Days or less | More than 20 days | |||||||||||||||
| Age | 1.00 | ||||||||||||||||||
| Sex | .08 | 1.00 | |||||||||||||||||
| Institutional status | .12 | .02 | 1.00 | ||||||||||||||||
| Medicaid | .06 | .08 | .36 | 1.00 | |||||||||||||||
| IADL1 score | −.22 | −.20 | −.24 | −.24 | 1.00 | ||||||||||||||
| Number of Part B claims, 1982 | .02 | .03 | .13 | .09 | −.25 | 1.00 | |||||||||||||
| Number of emergency room visits, 1982 | .03 | .00 | .06 | .10 | −.11 | .16 | 1.00 | ||||||||||||
| Number of inpatient admissions, 1982 | .06 | −.03 | .12 | .12 | −.27 | .44 | .25 | 1.00 | |||||||||||
| Number of inpatient days, 1982 | .04 | −.02 | .18 | .14 | −.26 | .45 | .26 | .84 | 1.00 | ||||||||||
| 1982 standardized payments | .04 | −.03 | .15 | .13 | −.29 | .55 | .30 | .90 | .90 | 1.00 | |||||||||
| Hospitalization in 1982 | .08 | −.05 | .09 | .06 | −.26 | .38 | .15 | .81 | .61 | .70 | 1.00 | ||||||||
| 1 hospitalization, 1982 | .07 | −.05 | .04 | .00 | −.16 | .19 | .04 | .36 | .24 | .28 | .79 | 1.00 | |||||||
| More than 1 hospitalization, 1982 | .03 | −.01 | .10 | .10 | −.20 | .35 | .20 | .81 | .65 | .74 | .52 | −.12 | 1.00 | ||||||
| Non-high-cost diagnosis only | .03 | −.06 | .00 | .02 | −.07 | .10 | .03 | .29 | .15 | .19 | .51 | .53 | .08 | 1.00 | |||||
| Secondary diagnosis | .06 | −.01 | .07 | .01 | −.14 | .14 | .03 | .32 | .21 | .24 | .46 | .41 | .17 | −.07 | 1.00 | ||||
| High-cost diagnosis, 20 days or less | .03 | −.01 | .01 | .00 | −.13 | .18 | .07 | .31 | .17 | .27 | .48 | .43 | .17 | −.07 | −.06 | 1.00 | |||
| High-cost diagnosis, more than 20 days | .03 | −.01 | .10 | .10 | −.17 | .31 | .17 | .62 | .67 | .65 | .36 | −.01 | .61 | −.05 | −.05 | −.05 | 1.00 | ||
| Reimbursement but no hospitalization | .05 | .−07 | −.02 | −.01 | .07 | −.01 | .00 | −.47 | −.35 | −.37 | −.58 | −.46 | −.30 | −.29 | −.27 | −.28 | −.21 | 1.00 | |
| Part B deductible met | .02 | .01 | .06 | .02 | −.18 | −.38 | .13 | .25 | .19 | .26 | .31 | .24 | .16 | .16 | .13 | .15 | .11 | .55 | 1.00 |
Instrumental activities of daily living.
NOTES: For consistency, the Pearson's r statistic is reported for all variable combinations in the table. Although not reported, Kendall's tau was checked for nominal/ordinal scaled variables. Information shown is based on answers of 1,934 respondents.
Results
Table 4 shows results from analyses of Michigan data using models proposed by Beebe, Lubitz, and Eggers (1985), Anderson, Resnick, and Gertman (1982), and Eggers (1981). Although other models have also been suggested in the literature, we believe those identified in Table 4 to be representative of current AAPCC-related research. In selecting models, we of course limited our consideration to those based on variables that were available or could be constructed from our data base.
Table 4. Regressions on January-June 1983 standarized payments using variables from models proposed by other researchers.
| Variable | Model 11 | Model 21 | Model 32 | Model 42 | Model 52 | Model 63 |
|---|---|---|---|---|---|---|
| R2 | .046 | .050 | .039 | .071 | .063 | .058 |
| Coefficient | ||||||
| Age | −.026 | −.015 | −.015 | −.023 | −.025 | −.018 |
| Sex | −.023 | −.030 | −.028 | −.029 | −.028 | −.024 |
| Institutional status | −.001 | −.017 | −.014 | −.009 | −.011 | — |
| Medicaid | .031 | .023 | .022 | .021 | .024 | — |
| Hospitalization in 1982 | ***.209 | — | — | — | — | — |
| 1 hospitalization, 1982 | — | — | — | ***.163 | — | — |
| More than 1 hospitalization, 1982 | — | — | — | ***.275 | — | — |
| Hospitalization in 1982: | ||||||
| Non-high-cost diagnosis | — | — | — | — | ***.128 | — |
| Secondary diagnosis | — | — | — | — | ***.151 | — |
| High-cost diagnosis, 20 days or less | — | — | — | — | ***.127 | — |
| High-cost diagnosis, more than 20 days | — | — | — | — | ***.210 | — |
| Number of inpatient days, 1982 | — | ***.174 | ***.193 | — | — | — |
| 1982 standardized payments | — | — | — | — | — | ***.240 |
| Reimbursement but no hospitalization | — | — | — | ***.105 | ***.105 | — |
| Part B deductible met | — | ***.104 | — | — | — | — |
Significant at the 0.01 level.
(Eggers, 1981).
NOTE: Information shown is based on answers of 1,934 survey respondents.
Model 1 includes current AAPCC factors plus a binary variable that indicates whether the beneficiary was admitted to a hospital during the previous year. Using a sample of approximately 21,000 records from the Medicare Health Insurance Master Accretions file, Beebe, Lubitz, and Eggers (1985) found this model (excluding institutional status, on which they had no information) to explain 2.2 percent of the variance in 1976 Medicare payments per enrollee. With 1982-83 Michigan data, this model explains 4.6 percent of dependent variable variance (Table 4). Although Beebe, Lubitz, and Eggers (1985) found the regression coefficients for age, sex, and Medicaid buy-in to be significant at the 0.05 level with Model 1, coefficients for these variables in our analysis were not significant at even the 0.1 level. This may be attributable to our smaller sample size.
Using a formulation similar to Model 2, Beebe, Lubitz, and Eggers (1985) measured prior utilization with two variables, one indicating the number of hospital days used during the previous 2 years and the other indicating whether the Part B deductible was met during this period. With their sample, Beebe, Lubitz, and Eggers (1985) found this model to yield an R2 value of 0.043. As noted earlier, the variables in our study representing number of hospital days and Part B deductible relate to the 12 months of calendar year 1982 instead of the 24-month period used by Beebe, Lubitz, and Eggers (1985). Nevertheless, this model explains 5.0 percent of the variance in January-June 1983 standardized payments, a value relatively close to that found by Beebe, Lubitz, and Eggers (1985) with their larger data set.
Models 3, 4, and 5 were proposed and tested by Anderson, Resnick, and Gertman (1982) using a sample of approximately 13,500 cases from Los Angeles County and data from the Medicare history file for 1974-75. Model 3 is similar to Model 2 except that it does not include a variable indicating whether the Part B deductible was met. With Los Angeles data, Anderson, Resnick, and Gertman (1982) found that this model yielded an R2 value of 0.055, with age, sex, welfare status, and number of hospital days all having significant coefficients at the 0.05 level or better. With our sample, the model explains 3.9 percent of the variance in January-June 1983 standardized payments, and none of the demographic variables has significant coefficients (Table 4).
Model 4 includes several more detailed measures of utilization than those employed in Model 3. With the data used by Anderson, Resnick, and Gertman (1982), Model 4 explained 6.2 percent of the variance in subsequent-year payments, a figure close to the 7.1 percent shown in Table 4.
Model 5 was proposed by Anderson, Resnick, and Gertman (1982) and includes information on discharge diagnoses for hospitalizations occurring during the previous year. With Michigan data, all utilization and cost variables in this model are significant at the 0.01 level or better, but none of the demographic variables is significant. The model explains 6.3 percent of the variance in subsequent-year payments, a figure similar to the 6.6 percent found by Anderson et al.
Model 6 was suggested by Eggers (1981) and involves only age, sex, and prior-year payments as predictors of subsequent-year payments. Using this model to analyze data from the Medicare history file for a random sample of approximately 13,600 beneficiaries for 1978-79, Eggers found the model to explain 4.9 percent of variance in subsequent-year payments for all beneficiaries in the sample and 4.2-7.3 percent for selected subarea samples. These results are comparable with the 5.8-percent figure shown in Table 4.
Models supplemented by IADL score
In Table 5, results are shown for a set of regression analyses using the models from Table 4 supplemented by the IADL measure of beneficiary health status. Several interesting observations can be made from these results. First, the R2 for every model is increased by adding the IADL measure. The smallest increase is for Model 4, in which R2 rises from 0.071 to 0.087; the largest is for Model 3, with an increase in the R2 value from 0.039 to 0.062. Coefficients for the utilization and cost variables remain significant, although the magnitude of these coefficients decreases with the introduction of IADL into the model. Lastly, although coefficients for the age and sex variables were not significant in any of the models in Table 4, age and/or sex are significant at the 0.05 level or better in every one of the models in Table 5. Apparently, controlling for functional health status reveals relationships between Medicare payments and sex (and, to a lesser degree, age) that are not otherwise apparent.
Table 5. Regressions on January-June 1983 standarized payments using variables from models proposed by other researchers supplemented by IADL1 score.
| Variable | Model 1a2 | Model 2a2 | Model 3a3 | Model 4a3 | Model 5a3 | Model 6a4 |
|---|---|---|---|---|---|---|
| R2 | .066 | .069 | .062 | .087 | .080 | .075 |
| Coefficient | ||||||
| Age | **−.052 | *− .042 | *− .043 | **−.046 | **−.048 | **.044 |
| Sex | **− .052 | **− .057 | **−.058 | **−.053 | **−.054 | −.051 |
| Institutional status | −.024 | −.037 | −.036 | −.029 | −.031 | — |
| Medicaid | .007 | .001 | −.002 | −.000 | .003 | — |
| Hospitalization in 1982 | ***.171 | — | — | — | — | — |
| 1 hospitalization, 1982 | — | — | — | ***.130 | — | — |
| More than 1 hospitalization, 1982 | — | — | — | ***.242 | — | — |
| Hospitalization in 1982: | ||||||
| Non-high-cost diagnosis only | — | — | — | — | ***.109 | — |
| Secondary diagnosis | — | — | — | — | ***.126 | — |
| High-cost diagnosis, 20 days or less | — | — | — | — | ***.100 | — |
| High-cost diagnosis, more than 20 days | — | — | — | — | ***.183 | — |
| Number of inpatient days, 1982 | — | ***.145 | ***.158 | — | — | — |
| 1982 standardized payments | — | — | — | — | — | ***.200 |
| Reimbursement but no hospitalization | — | — | — | ***.090 | ***.090 | — |
| Part B deductible met | — | ***.085 | — | — | — | — |
| IADL1 score | ***−.160 | ***−.157 | ***−.169 | ***−.144 | ***.146 | ***−.142 |
Significant at the 0.10 level.
Significant at the 0.05 level.
Significant at the 0.01 level.
Instrumental activities of daily living.
(Eggers, 1981).
NOTE: Information shown is based on answers of 1,934 survey respondents.
We can conclude from Tables 4 and 5 that even models that already include indirect measures of health status in the form of variables representing prior utilization and/or reimbursement can provide more accurate predictions of future Medicare payment levels if supplemented by a direct health status measure.
Other models
For this sample of Michigan beneficiaries, payments for acute inpatient services represented 62 percent of total Medicare costs in 1982 and 70 percent in the first 6 months of 1983. The emphasis on hospitalization-related utilization variables in the models shown in Tables 4 and 5 thus appears appropriate. However, Part B and Part A outpatient payments also accounted for significant fractions of total Medicare costs, 36.5 percent in 1982 and 28.6 percent in January-June 1983. Therefore, the predictive validity of the models might be improved by including variables more specifically related to outpatient activities.
In Table 6, ambulatory utilization is represented by three variables: number of Part B claims filed during 1982, number of emergency room visits in 1982, and the binary variable indicating whether Medicare payments were made during 1982 for an individual who had no hospitalizations. Inpatient utilization is indicated by two binary variables from Model 4: one is assigned a value of 1 if the beneficiary had exactly one hospitalization in the previous year; the other is assigned a value of 1 if two or more hospitalizations occurred. Age and sex variables are included in these models because they were observed to be significant for models which also incorporate IADL.
Table 6. Regressions on January-June 1983 standardized payments using age, sex, IADL1 score, and measures of utilization and cost.
| Variable | Model 7 | Model 8 |
|---|---|---|
| R2 | .096 | .092 |
| Coefficient | ||
| Age | **−.045 | **−.045 |
| Sex | **−.053 | **−.053 |
| 1 hospitalization, 1982 | ***.089 | ***.060 |
| More than 1 hospitalization, 1982 | ***.184 | ***.167 |
| Number of Part B claims, 1982 | ***.096 | ***.113 |
| Number of emergency room visits, 1982 | **.050 | — |
| Reimbursement but no hospitalization | *.054 | — |
| IADL1 score | ***−.122 | ***−.128 |
Significant at the 0.10 level.
Significant at the 0.05 level.
Significant at the 0.01 level.
Instrumental activities of daily living.
NOTE: Information shown is based on answers of 1,934 survey respondents.
Variables used in Model 7 for prior utilization and costs were selected based on their contributions to explained variance and their lack of correlation with other independent variables. On the basis of these considerations, Model 7 represents the best set of independent variables identified thus far in that it produces the highest R2, 0.096.
Model 8 is more parsimonious than Model 7 in that two of the utilization-cost variables are dropped. The reduction in R2 because of eliminating the number of emergency room visits and the binary variable for Medicare reimbursement with no hospitalizations is only 0.004. The resulting model predicts Medicare payments based only on age and sex, number of Part B claims, IADL, and two variables for inpatient hospitalizations. Except for Model 7, Model 8 explains more variance in future payments than any of the models previously considered.
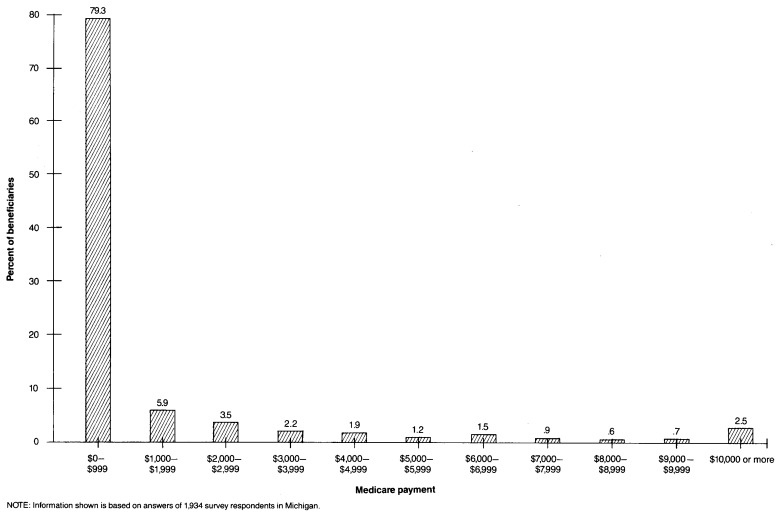
Although Model 7 has a higher R2 value than the other models for predicting Medicare payment levels, it still accounts for less than 10 percent of variance in payments. The primary reason for the relatively small R2 values achieved in this and other AAPCC-related studies is the skewness of the distribution of Medicare payments. As shown in Figure 1, a few beneficiaries incur high costs that are reimbursed by Medicare, and many other beneficiaries incur no reimbursable expenses. The extreme skewness of the Medicare payments distribution violates one of the assumptions of ordinary least squares regression, that of a normally distributed dependent variable. As a result, regression models tend to underestimate payment levels for those at the high end of the distribution. Because R2 reflects squared deviations between actual and predicted values, the effects of these underestimates may be quite large. Thus R2 values with the untransformed dependent variable are lower than those generally encountered in other research using appropriately specified models.
Figure 1. Percent distribution of Medicare beneficiaries, by January-June 1983 standardized payments.
Perhaps a better sense of the accuracy of various models for predicting subsequent-year reimbursement is gained when the distribution of the dependent variable is normalized by using the logarithm of January-June 1983 standardized payments as the dependent variable instead of the actual value.
Table 7 lists R2 values achieved for Models 4-6 (Table 4), Models 4a-6a (Table 5), and Models 7 and 8 (Table 6) when the natural log of January-June 1983 standardized payments is used as the dependent variable. For every model, the percentage of explained variation in standardized payments is much greater when the log transform is used. In terms of R2 value, Model 7 is better than the other models using either the untransformed dependent variable or the log transform dependent variable. With the exception of Models 6 and 6a, all of the models listed in Table 7 are based on measures of prior utilization, and the relative rankings of these models in terms of R2 values are not changed by use of the transformed dependent variable.
The predictive power of Models 6 and 6a, which include measures of prior payments instead of prior utilization, is greatly enhanced through use of the log transformation of the payment variables. With the untransformed variables, Model 6 yields an R2 of 0.058, the lowest of all of the models listed in Table 7, and R2 for Model 6a ranks near the middle. However, when the independent and dependent payment variables are transformed, Models 6 and 6a are among the best, with Model 6a producing an R2 value of 0.230, only slightly less than the R2 achieved in Model 7. A likely explanation for this improvement in the predictive power of models based on prior-year payments is that these models predict reasonably well for all cases except those involving very high payment levels. With the log transforms, the tail of the payment variable distribution is “shortened,” and differences between actual and predicted values are relatively smaller. This suggests that these models, particularly 6a, are likely to be better predictors of future Medicare payments than is indicated by their R2 values in Tables 4 and 5.
Discussion
Depending on the characteristics of enrolled beneficiaries, a risk-sharing HMO can experience large and systematic profits or losses if capitation levels are based on an inadequately specified AAPCC formula. From HCFA's perspective, neither of these outcomes is desirable. If they are to be avoided and if calculated capitation payments are to relate more closely to HMO's costs of providing services to Medicare enrollees, a more accurate and precise AAPCC formula must be devised.
In presenting the regression results, we have examined and compared the ability of alternative AAPCC models to predict future Medicare payments, predictive ability being measured by model R2. In terms of this criterion, the results indicate that models incorporating measures of prior-period utilization and costs are superior to the purely demographic model currently used by HCFA. The results also show that predictions of future payments are even further improved by adding beneficiary IADL score to AAPCC models that incorporate measures of utilization and/or costs.
Although ability to predict future costs is a principal criterion for selecting new factors for the AAPCC, other actuarial characteristics of these factors must be considered as well. Primary among these characteristics is the degree to which a factor is potentially subject to direct or indirect manipulation by HMO's or other providers. Any payment formula involves implicit financial incentives. In revising the AAPCC, HCFA must consider the ways in which a potential actuarial factor may influence these incentives and how providers and patients may behave in response. The potential benefits and problems of incorporating a new factor must be weighed against the benefits and problems that are likely to occur if it is not included.
Information on beneficiary IADL scores is not currently collected for the Medicare population. If IADL were to be included in the AAPCC, HCFA would have to develop a method for acquiring the necessary data. Data might be obtained by having the HMO's survey their enrollees directly. However, HMO providers might have both the motivation and the ability to influence enrollees' responses to IADL questions, thereby increasing the HMO's AAPCC payments. Therefore, procedures for detecting such abuses would be necessary. Other means of collecting this information, such as through third parties or through direct mailings by HCFA or its intermediaries, may be more costly to implement but would be less subject to manipulation.
Although collection of IADL data for all Medicare HMO enrollees may present administrative difficulties, HCFA must also consider the problems of not including IADL in the AAPCC formula. Because IADL has been shown to be a good predictor of future health services use and costs, HMO's might employ this indicator of functional health to their own advantage when marketing to Medicare beneficiaries. For example, an HMO might advertise that it is currently enrolling Medicare beneficiaries at its office on the second floor of its downtown building. Beneficiaries who could not walk up and down stairs without help or who could not go out without help (two IADL functions) would be less likely to respond to this solicitation, leaving the HMO with a biased selection of enrollees. Yet the current AAPCC formula would not take account of this bias, and the HMO would be paid as if it enrolled a cross-section of the elderly community. Incorporating IADL into the AAPCC formula would eliminate the advantage to HMO's of implicitly or explicitly using this type of selective marketing strategy because the HMO would be paid lower premiums for healthier enrollees.
Similar issues must be considered if measures of previous use and costs are to be included in the AAPCC. First, HCFA would have to engage in special data collection activities to determine the prior use and cost experience of beneficiaries when they become eligible for the program for the first time at age 65. Although these data may be readily available through HMO or insurance company records for some new enrollees, they would be difficult to collect for others.
Second, variables for prior use and cost could also be used by HMO's to obtain favorable selection. A higher AAPCC rate associated with hospitalization in the previous year could encourage HMO's to identify and enroll Medicare patients who had been admitted to a hospital for elective procedures or other nonrecurrent problems. Based on prior hospitalization, the HMO would then receive the higher capitation rate for patients who are no more likely to be hospitalized than other beneficiaries are. Depending on the size of the incremental payment, a capitation increment associated with prior hospitalization might also reduce the HMO's incentive to keep patients out of the hospital because a portion of hospitalization costs would be recouped through subsequent increases in capitation. Defining hospitalization factors on the basis of high-cost diagnoses, as suggested by Anderson, Resnick, and Gertman (1982) and shown in Models 5 and 5a, reduces the incentives for, and likelihood of, this type of manipulation.
In general, including any prior-utilization factor in the AAPCC could promote unnecessary use if the associated capitation increment exceeds the HMO's marginal cost of providing the service. If the number of hospital days in the previous year were included as a factor, for example, the AAPCC increment associated with an additional day (or block of days) should not exceed the marginal cost to the HMO of extending the patient's stay. This is particularly relevant for ambulatory use measures, such as reimbursement, Part B deductible met, or number of Part B claims, for which associated marginal costs are likely to be low.
The incremental change in the AAPCC that is appropriate for a unit change in utilization is indicated in the tables by the coefficient for the particular utilization measure selected. Coefficients of the utilization variables are smaller in magnitude for models that include IADL along with one or more measures of utilization (Table 5) than for models that do not include IADL (Table 4). For example, the coefficient for hospitalization with non-high-cost diagnosis only in Model 5 is 0.128 (Table 4), but the coefficient for the same variable in Model 5a, which includes IADL, is 0.109. The coefficient for 1982 standardized payments is 0.240 in Model 6 but only 0.200 in Model 6a. Thus, if measures of prior utilization or costs are included in the AAPCC, adding the IADL score is likely to reduce the utilization increment, thereby reducing the financial incentive for HMO's to “game” the system.
In summary, incorporating the IADL score as an AAPCC adjusting factor may improve predictions of beneficiary costs and reduce the likelihood of HMO's selectively marketing to promote favorable selection. If used in conjunction with measures of prior utilization or costs, it may reduce incentives for HMO's to manipulate the capitation rate through promoting unnecessary use of services. HCFA must weigh these benefits against the likely costs and potential difficulties involved in obtaining IADL data from beneficiaries.
Acknowledgments
This report was funded by the Health Care Financing Administration (Grant No. 18-P-98179/5-01).
Footnotes
Reprint requests: J. William Thomas, Department of Medical Care Organization, School of Public Health, University of Michigan, Ann Arbor, Michigan 48109.
References
- Anderson G, Knickman J. Implications for adverse selection under a voucher system: The grouping of Medicare recipients by level of expenditure. Inquiry. 1984;21(2):135–143. [PubMed] [Google Scholar]
- Anderson JJ, Resnick AL, Gertman PM. Prediction of Subsequent Year Reimbursement Using the Medicare History File. Boston University Medical Center, University Health Policy Consortium; Boston: 1982. Unpublished document. [Google Scholar]
- Beebe J, Lubitz J, Eggers P. Health Care Financing Review. No. 3. Vol. 6. Washington: U.S. Government Printing Office; Spring. 1985. Using prior utilization information to determine payments for Medicare enrollees in health maintenance organizations. HCFA Pub. No. 03198. Office of Research and Demonstrations, Health Care Financing Administration. [PMC free article] [PubMed] [Google Scholar]
- Eggers P. Health Care Financing Review. No. 3. Vol. 1. Washington: U.S. Government Printing Office; Winter. 1980. Risk differential between Medicare beneficiaries enrolled and not enrolled in an HMO. HCFA Pub. No. 03027. Office of Research, Demonstrations, and Statistics, Health Care Financing Administration. [PMC free article] [PubMed] [Google Scholar]
- Eggers P. Analysis of the Relationship Between Reimbursements in One Year and Reimbursements in a Subsequent Year. Office of Research, Demonstrations, and Statistics, Health Care Financing Administration; Baltimore, Md.: 1981. Working Paper OR-33. [Google Scholar]
- Eggers PW, Prihoda R. Health Care Financing Review. No. 1. Vol. 4. Washington: U.S. Government Printing Office; Sept. 1982. Pre-enrollment reimbursement patterns of Medicare beneficiaries enrolled in “at-risk” HMO's. HCFA Pub. No. 03146. Office of Research, Demonstrations, and Statistics, Health Care Financing Administration. [PMC free article] [PubMed] [Google Scholar]
- Hornbrook MC. Examination of the AAPCC methodology in an HMO prospective payment demonstration project. The Group Health Journal. 1984;5(1):13–21. [PubMed] [Google Scholar]
- Luft HS, Feder J, Holahan J, Lennox KD. Health maintenance organizations. In: Feder J, Holahan J, Marmor T, editors. National Health Insurance: Conflicting Goals and Policy Choices. Washington, D.C.: The Urban Institute; 1980. [Google Scholar]
- National Center for Health Statistics. Eighth Revision International Classification of Diseases, Adapted for Use in the United States. Washington: U.S. Government Printing Office; 1967. DHEW Pub. No. (PHS) 1693. Public Health Service. [Google Scholar]
- Public Health Service and Health Care Financing Administration. International Classification of Diseases, 9th Revision, Clinical Modification. Washington: U.S. Government Printing Office; Sept. 1980. DHHS Pub. No. (PHS) 80-1260. Public Health Service. [Google Scholar]
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Lichtenstein R, Wyszewianski L, Berki SE. Increasing Medicare enrollment in HMO's: The need for capitation rates adjusted for health status. Inquiry. 1983;20(3):227–239. [PubMed] [Google Scholar]
- Thomas JW, Lichtenstein R, Wyszewianski L, Berki SE. Final Report: A Health Status Measure for Adjusting the HMO Capitation Rates of Medicare Beneficiaries. Ann Arbor, Mich.: Department of Medical Care Organization, School of Public Health, The University of Michigan; 1985. Prepared for Health Care Financing Administration. Grant No. 18-P-98179/5-01. [Google Scholar]
- Trieger S, Galblum TW, Riley G. Health Care Financing Issues. Washington: U.S. Government Printing Office; Apr. 1981. HMO's: Issues and alternatives for Medicare and Medicaid. HCFA Pub. No. 03107. Office of Research, Demonstrations, and Statistics, Health Care Financing Administration. [Google Scholar]