Abstract
Context:
Telomerase promoter mutations (TERT) were recently described in follicular cell-derived thyroid carcinomas (FCDTC) and seem to be more prevalent in aggressive cancers.
Objectives:
We aimed to evaluate the frequency of TERT promoter mutations in thyroid lesions and to investigate the prognostic significance of such mutations in a large cohort of patients with differentiated thyroid carcinomas (DTCs).
Design:
This was a retrospective observational study.
Setting and Patients:
We studied 647 tumors and tumor-like lesions. A total of 469 patients with FCDTC treated and followed in five university hospitals were included. Mean follow-up (±SD) was 7.8 ± 5.8 years.
Main Outcome Measures:
Predictive value of TERT promoter mutations for distant metastasization, disease persistence at the end of follow-up, and disease-specific mortality.
Results:
TERT promoter mutations were found in 7.5% of papillary carcinomas (PTCs), 17.1% of follicular carcinomas, 29.0% of poorly differentiated carcinomas, and 33.3% of anaplastic thyroid carcinomas. Patients with TERT-mutated tumors were older (P < .001) and had larger tumors (P = .002). In DTCs, TERT promoter mutations were significantly associated with distant metastases (P < .001) and higher stage (P < .001). Patients with DTC harboring TERT promoter mutations were submitted to more radioiodine treatments (P = .009) with higher cumulative dose (P = .004) and to more treatment modalities (P = .001). At the end of follow-up, patients with TERT-mutated DTCs were more prone to have persistent disease (P = .001). TERT promoter mutations were significantly associated with disease-specific mortality [in the whole FCDTC (P < .001)] in DTCs (P < .001), PTCs (P = .001), and follicular carcinomas (P < .001). After adjusting for age at diagnosis and gender, the hazard ratio was 10.35 (95% confidence interval 2.01–53.24; P = .005) in DTC and 23.81 (95% confidence interval 1.36–415.76; P = .03) in PTCs.
Conclusions:
TERT promoter mutations are an indicator of clinically aggressive tumors, being correlated with worse outcome and disease-specific mortality in DTC. TERT promoter mutations have an independent prognostic value in DTC and, notably, in PTC.
Telomerase activation is known to be a hallmark of cancer (1), being detected in up to 80% of malignant tumors (2, 3). Some tumors may maintain their telomeres by an alternative mechanism, which is telomerase independent, designated as alternative lengthening of telomeres, which appears to maintain telomeres through recombination-based interchromosomal exchange of sequence information (4, 5). The maintenance of telomere length in cancer cells is thought to result more frequently from telomerase reexpression than from alternative lengthening of telomeres, although the mechanisms underlying such process remain largely unknown.
Thyroid tissue is a conditionally renewing tissue, which proliferates rarely in adult life (6). We have proposed that the embryonic remnants of the ultimobranchial body, the so-called solid cell nests of the thyroid, may represent the pool of thyroid stem cells because they express several stem cell markers, including telomerase (7). At variance with this, normal thyroid tissue is thought to be telomerase negative, thus raising the possibility that the reactivation of telomerase may be a useful marker of tumor development (8).
Several studies have examined telomerase activity in thyroid lesions and surrounding normal tissues using a PCR-based telomeric repeat amplification protocol assay for detection of telomerase activity and RT-PCR or quantitative real-time RT-PCR for the detection of TERT mRNA (for a review see reference 9). Thyroid carcinomas apparently display less frequent telomerase activation than other human carcinomas. Approximately 66% of all the thyroid carcinomas analyzed to date display telomerase activation that is more frequent in undifferentiated thyroid carcinomas than in differentiated carcinomas (9). Putting together the results obtained in the evaluation of telomerase activity by several authors, telomerase activity/expression is reported in 48% of papillary thyroid carcinomas (PTCs) and 71% of follicular thyroid carcinomas (FTCs). TERT copy number gain was described in familial PTCs (10). Capezzone et al (11) observed telomerase activity in most sporadic and familial malignant thyroid tumors as well as in some adenomas. No telomerase activity was observed in hyperplastic nodules or in normal thyroid tissue of patients with sporadic PTCs (11). Altogether, the aforementioned findings suggest that telomerase activity may contribute to a more aggressive behavior of thyroid tumors (12–14).
Recently highly frequent mutations in the promoter region of TERT were reported in melanomas, gliomas, and bladder and thyroid cancers (15–18). These mutations occur in two hot spot positions, located −124 and −146 bp upstream from the ATG start site (−124G>A and −146G>A, C>T on the opposite strand) and confer enhanced TERT promoter activity (1, 2) putatively by generating a consensus binding site (GGAA) for ETS transcription factors within the TERT promoter region. In thyroid tumors, these mutations were shown to be associated with aggressive features and with the presence of BRAF or BRAF/RAS mutations (15, 19, 20). The role played by TERT promoter mutations in the clinical course of thyroid tumors, response to the therapy and survival of cancer patients, remains to be addressed.
In the present study, we searched for the presence of telomerase promoter mutations in a large series of thyroid tumors and investigated the putative clinical significance of such somatic alterations.
Materials and Methods
All the procedures described in this study were in accordance with national and institutional ethical standards. Patients signed an informed consent form approved by the internal reviewing board.
Patient tissue samples
Six hundred forty-seven formalin-fixed, paraffin-embedded tissue samples from tumors and tumor-like lesions of the thyroid and from normal thyroid parenchyma localized at distance from the respective tumors were collected from the files of the Institute of Molecular Pathology and Immunology of the University of Porto (Porto, Portugal), corresponding to patients followed up in five university hospitals in Portugal and Spain. The histology of all tumor samples was revised by three pathologists (C.E., J.M.C.-T., M.S.-S.) according to the World Health Organization criteria (21). Data on the histological characteristics of the 647 samples and the frequency of TERT promoter mutations in each group are summarized in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Twelve follicular adenomas and 27 PTCs from the Chernobyl-irradiated setting that we had previously studied (22) were also searched for the presence of TERT promoter mutations. Due to their etiopathogenic specificity (irradiation induced tumors) and lack of complete clinical information, they were not included in the subsequent clinicopathological analysis.
DNA extraction
DNA from formalin-fixed, paraffin-embedded tissues was retrieved from 10-μm sections after careful microdissection. DNA extraction was performed using the Ultraprep tissue DNA kit (AHN Biotechnologie) following the manufacturer's instructions.
PCR and Sanger sequencing
The genetic characterization of part of the tumors series regarding BRAF, NRAS, RET/PTC, and PAX8/PPARG had been previously reported; mutations were screened as previously described (23–25). To screen for TERT promoter mutations, we analyzed the hot spots previously identified by PCR followed by Sanger sequencing. TERT promoter mutation analysis was performed with the pair of primers forward TERT, CAGCGCTGCCTGAAACTC; and reverse TERT, GTCCTGCCCCTTCACCTT. Amplification of genomic DNA (25–100 ng) was performed by PCR using the QIAGEN multiplex PCR kit following the manufacturer's instructions (further details on the amplification will be given upon request). Sequencing reaction was performed with the ABI Prism BigDye terminator kit (PerkinElmer), and the fragments were run in an ABI prism 3100 genetic analyzer (PerkinElmer). The sequencing reaction was performed in a forward direction, and all the detected mutations were further validated by a new independent analysis in both strands (Supplemental Figure 1).
Patients' follow-up
Patients were treated and followed up in accordance with international protocols available at the time. Data regarding the number of radioiodine treatments and cumulative activity were retrieved from hospital records, together with other therapeutic procedures. For statistical analysis, we defined the category, additional treatments, in which we included other treatment modalities in addition to radioiodine, including surgery, external beam irradiation, and treatment with tyrosine kinase inhibitors (Supplemental Table 2). Patients were defined as being disease free at the end of follow-up if they had undetectable stimulated thyroglobulin (in the absence of thyroglobulin antibodies) and no imagiological evidence of disease. In the survival analysis, we have considered only deaths attributable to the thyroid carcinoma (disease-specific mortality).
Statistical analysis
Statistical analysis was conducted with SPSS version 20.0 (SPSS Inc). The results are expressed as a percentage or mean ± SD. Statistical analysis was performed both on the whole series of follicular cell-derived thyroid carcinomas (FCDTCs) and considering the different groups of tumors. A Fisher's exact test, t test (unpaired, two tailed), and ANOVA were used when appropriate. The predictive value of TERT promoter mutations and other factors [age, gender, histologic category, extrathyroidal extension, vascular invasion, lymph node metastases, staging I–IV (Union for International Cancer Control/American Joint Committee on Cancer [AJCC]), BRAF mutations, RAS mutations] for distant metastases and disease-free status at the end of follow-up were assessed using univariate and multivariate logistic regression models. Survival curves were plotted by the Kaplan-Meier method with the log-rank statistics. Multivariate survival analysis was performed using Cox regression. In the regression models, all the variables significantly associated with the specified outcome in the univariate model were included in the multivariate analysis. Mortality was expressed as a percentage and rate per person-year, the latter obtained dividing the number of thyroid cancer-specific deaths by the total follow-up time. Results were considered statistically significant at P < .05.
Results
TERT promoter mutations were not detected in normal parenchyma (n = 30) or in benign lesions (n = 81), such as a nodular goiter (hyperplastic lesions), lymphocytic thyroiditis, or follicular adenomas. TERT promoter mutations were also not detected in medullary thyroid carcinomas (n = 28) or in any of the 39 tumors from the Chernobyl series (Supplemental Table 1).
TERT promoter mutations were detected in 58 FCDTCs. The mutations were detected in 25 PTCs (7.5%), 12 FTCs (17.1%), nine poorly differentiated thyroid carcinomas (PDTCs; 29.0%), and 12 anaplastic thyroid carcinomas (ATCs; 33.3%) (Table 1). In differentiated thyroid carcinomas (DTCs), the overall prevalence of TERT promoter mutations was 9.2%. TERT promoter mutations were slightly more frequent in conventional PTCs (8.3%) than in cases of follicular variant of PTCs (6.8%). All PTCs and FTCs with oncocytic features as well as tall-cell PTCs and Warthin-like PTCs did not harbor TERT promoter mutations (Supplemental Table 1). Most of the mutated cases (48 of 58) presented the −124G>A mutation and the remaining 10 cases presented the −146 G>A.
Table 1.
Epidemiological, Histological, and Clinical Data of Patients With FCDTCs Included in the Study
| Total | PTC | FTC | PDTC | ATC | |
|---|---|---|---|---|---|
| Total number | 469 | 332 | 70 | 31 | 36 |
| Age at diagnosis, y, n | 469 | 332 | 70 | 31 | 36 |
| Mean, y | 48.2 ± 16.9 | 44.8 ± 15.6 | 51.8 ± 16.4 | 53.2 ± 18.4 | 68.7 ± 10.4 |
| <45 | 211 (45.0) | 178 (53.6) | 23 (32.9) | 8 (25.8) | 2 (5.6) |
| ≥45 | 258 (55.0) | 154 (46.4) | 47 (67.1) | 23 (74.2) | 34 (94.4) |
| Gender, n | 459 | 327 | 68 | 29 | 35 |
| Female | 342 (74.5) | 254 (77.7) | 50 (73.5) | 16 (55.2) | 22 (62.9) |
| Male | 117 (25.5) | 73 (22.3) | 18 (26.5) | 13 (44.8) | 13 (37.1) |
| Tumor size, cm, n | 418 | 315 | 61 | 26 | 16 |
| <2a | 151 (36.1) | 142 (45.0) | 7 (11.5) | 2 (7.7) | 0 |
| 2–4 | 161 (38.5) | 129 (41.0) | 23 (37.7) | 7 (26.9) | 2 (12.5) |
| >4 | 106 (25.4) | 44 (14.0) | 31 (50.8) | 17 (65.4) | 14 (87.5) |
| Extrathyroidal extension, n | 373 | 268 | 49 | 20 | 36 |
| Present | 231 (61.9) | 176 (65.7) | 8 (16.3) | 12 (60.0) | 35 (97.2) |
| Vascular invasion, n | 325 | 257 | 53 | 8 | 7 |
| Present | 157 (48.3) | 103 (40.1) | 39 (73.6) | 8 (100.0) | 7 (100.0) |
| Lymph node metastasis, n | 391 | 298 | 47 | 24 | 22 |
| Present | 234 (59.8) | 202 (67.8) | 8 (17.0) | 14 (58.3) | 10 (45.5) |
| Distant metastasis, n | 337 | 263 | 31 | 20 | 23 |
| Present | 76 (22.6) | 36 (13.7) | 8 (25.8) | 14 (70.0) | 18 (78.3) |
| Stage (sixth UICC/AJCC), n | 310 | 225 | 29 | 20 | 36 |
| I | 134 (43.2) | 124 (55.1) | 8 (27.6) | 2 (10.0) | 0 |
| II | 36 (11.6) | 25 (11.1) | 8 (27.6) | 3 (15.0) | 0 |
| III | 45 (14.5) | 36 (16.0) | 5 (17.2) | 4 (20.0) | 0 |
| IV | 95 (30.7) | 40 (17.8) | 8 (27.6) | 11 (55.0) | 36 (100.0) |
| TERT promoter, n | 469 | 332 | 70 | 31 | 36 |
| Wild-type | 411 (87.6) | 307 (92.5) | 58 (82.9) | 22 (71.0) | 24 (66.7) |
| Mutation | 58 (12.4) | 25 (7.5) | 12 (17.1) | 9 (29.0) | 12 (33.3) |
Abbreviations: n, number of patients with available data for each feature; UICC, Union for International Cancer Control. Numbers in parentheses represent percentages within each category.
Number of microcarcinomas (≤1.0 cm): total, 47 (11.2%); PTC, 43 (13.7%); FTC, 4 (6.6%); PDTC, 0, ATC, 0.
Because TERT promoter mutations were only detected in FCDTCs, the subsequent clinicopathological analysis was restricted to these tumors (Table 1).
Relationship between TERT mutation and clinicopathological features
In the group of patients with DTCs, the presence of TERT promoter mutations was significantly associated with older age (P < .001) and larger tumor size (P = .002) (Table 2). Patients with tumors harboring TERT mutations had more distant metastases (P < .001) and higher stage (P < .001). No association was found with the presence of vascular invasion, extrathyroidal extension, or lymph node metastases.
Table 2.
Summary of Clinicopathological and Molecular Associations With TERT Promoter Mutations in Thyroid Cancers
| Tumor Type | All Patients | TERT Wild Type | TERT Mutated | P Value |
|---|---|---|---|---|
| DTCs (n = 402) | ||||
| Clinicopathological | ||||
| Age at diagnosis, y (n = 402) | 46.0 ± 16.0 | 44.5 ± 15.6 | 60.2 ± 12.7 | <.001 |
| Gender (n = 395) | ||||
| Female | 304 (77.0) | 278 (77.7) | 26 (70.3) | NS (.31) |
| Male | 91 (23.0) | 80 (22.3) | 11 (29.7) | |
| Mean tumor size, cm (n = 376) | 2.7 ± 1.9 | 2.6 ± 1.8 | 3.6 ± 2.3 | .002 |
| Extrathyroidal extension (n = 317)a | ||||
| Present | 184 (58.0) | 167 (57.4) | 17 (65.4) | NS (.43) |
| Vascular invasion (n = 310)b | ||||
| Present | 142 (45.8) | 129 (45.4) | 13 (50.0) | NS (.65) |
| LN metastases (n = 345) | ||||
| Present | 210 (60.9) | 189 (61.0) | 21 (60.0) | NS (.91) |
| Distant metastases (n = 294) | ||||
| Present | 44 (15.0) | 34 (12.5) | 10 (43.5) | <.001 |
| Stage (n = 254) | ||||
| I | 132 (52.0) | 129 (55.1) | 3 (15.0) | <.001 |
| II | 33 (13.0) | 31 (13.3) | 2 (10.0) | |
| III | 41 (16.1) | 37 (15.8) | 4 (20.0) | |
| IV | 48 (18.9) | 37 (15.8) | 11 (55.0) | |
| Other genetic alterations | ||||
| BRAF mutation (n = 357) | 148 (41.5) | 130 (40.2) | 18 (52.9) | NS (.15) |
| NRAS mutation (n = 365) | 29 (7.9) | 24 (7.3) | 5 (14.3) | NS (.15) |
| PTCs (n = 332) | ||||
| Clinicopathological | ||||
| Age at diagnosis, y (n = 332) | 44.8 ± 15.6 | 43.6 ± 15.3 | 58.4 ± 13.2 | <.001 |
| Gender (n = 327) | ||||
| Female | 254 (77.7) | 237 (78.5) | 17 (68.0) | NS (.23) |
| Male | 73 (22.3) | 65 (21.5) | 8 (32.0) | |
| Mean tumor size, cm (n = 315) | 2.4 ± 1.5 | 2.3 ± 1.4 | 3.2 ± 2.2 | .005 |
| Extrathyroidal extension (n = 268)a | ||||
| Present | 176 (65.7) | 162 (64.8) | 14 (77.8) | NS (.26) |
| Vascular invasion (n = 257)b | ||||
| Present | 103 (40.1) | 98 (40.7) | 5 (31.3) | NS (.46) |
| LN metastases (n = 298) | ||||
| Present | 202 (67.8) | 184 (66.9) | 18 (78.3) | NS (.26) |
| Distant metastases (n = 263) | ||||
| Present | 36 (13.7) | 31 (14.7) | 5 (27.8) | NS (.07) |
| Stage (n = 225) | ||||
| I | 124 (55.1) | 121 (57.3) | 3 (21.4) | .02 |
| II | 25 (11.1) | 24 (11.4) | 1 (7.1) | |
| III | 36 (16.0) | 32 (15.2) | 4 (28.6) | |
| IV | 40 (17.8) | 34 (16.1) | 6 (42.9) | |
| Other genetic alterations | ||||
| BRAF mutation (n = 301) | 148 (49.2) | 130 (46.9) | 18 (75.0) | .008 |
| NRAS mutation (n = 301) | 15 (5.0) | 14 (5.1) | 1 (4.2) | NS (.85) |
| FTCs (n = 70) | ||||
| Clinicopathological | ||||
| Age at diagnosis, y (n = 70) | 51.8 ± 16.4 | 49.3 ± 16.3 | 63.8 ± 11.0 | .004 |
| Gender (n = 68) | ||||
| Female | 50 (73.5) | 41 (73.2) | 9 (75.0) | NS (.90) |
| Male | 18 (26.5) | 15 (26.8) | 3 (25.0) | |
| Mean tumor size, cm (n = 61) | 4.4 ± 2.6 | 4.4 ± 2.6 | 4.4 ± 2.5 | NS (.96) |
| Extrathyroidal extension (n = 49)a | ||||
| Present | 8 (16.3) | 5 (12.2) | 3 (37.5) | NS (.08) |
| Vascular invasion (n = 53)b | ||||
| Present | 39 (73.6) | 31 (72.1) | 8 (80.0) | NS (.61) |
| LN metastases (n = 47) | ||||
| Present | 8 (17.0) | 5 (14.3) | 3 (25.0) | NS (.39) |
| Distant metastases (n = 31) | ||||
| Present | 8 (25.8) | 3 (11.5) | 5 (100.0) | <.001 |
| Stage (n = 29) | ||||
| I | 8 (27.6) | 8 (34.8) | 0 | .007 |
| II | 8 (27.6) | 7 (30.4) | 1 (16.7) | |
| III | 5 (17.2) | 5 (21.7) | 0 | |
| IV | 8 (27.6) | 3 (13.1) | 5 (83.3) | |
| Other genetic alterations | ||||
| BRAF mutation (n = 56) | 0 | 0 | 0 | c |
| NRAS mutation (n = 64) | 14 (21.9) | 10 (18.9) | 4 (36.4) | NS (0.20) |
| Undifferentiated thyroid carcinomas (poorly differentiated + anaplastic) (n = 67) | ||||
| Clinicopathological | ||||
| Age at diagnosis, y (n = 67) | 61.5 ± 16.2 | 58.3 ± 17.6 | 68.7 ± 9.6 | .003 |
| Gender (n = 64) | ||||
| Female | 38 (59.4) | 22 (50.0) | 16 (80.0) | .02 |
| Male | 26 (40.6) | 22 (50.0) | 4 (20.0) | |
| Mean tumor size, cm (n = 42) | 6.5 ± 3.2 | 6.3 ± 3.4 | 6.8 ± 2.9 | NS (.62) |
| Extrathyroidal extension (n = 56)a | ||||
| Present | 47 (83.9) | 32 (82.1) | 15 (88.2) | NS (.56) |
| Vascular invasion (n = 15)b | ||||
| Present | 15 (100.0) | 11 (100.0) | 4 (100.0) | c |
| LN metastases (n = 46) | ||||
| Present | 24 (52.2) | 16 (57.1) | 8 (44.4) | NS (.40) |
| Distant metastases (n = 43) | ||||
| Present | 32 (74.4) | 19 (70.4) | 13 (81.3) | NS (.43) |
| Stage (n = 56) | ||||
| I | 2 (3.6) | 2 (5.3) | 0 | NS (.39) |
| II | 3 (5.4) | 3 (7.9) | 0 | |
| III | 4 (7.1) | 2 (5.3) | 2 (11.1) | |
| IV | 47 (83.9) | 31 (81.5) | 16 (88.9) | |
| Other genetic alterations | ||||
| BRAF mutation (n = 65) | 10 (15.4) | 6 (13.6) | 4 (19.0) | NS (0.57) |
| NRAS mutation (n = 61) | 17 (27.9) | 9 (22.5) | 8 (38.1) | NS (0.20) |
Abbreviations: LN, lymph node; n, number of patients with available data for each feature; NS, not significant. Numbers in parentheses represent percentages within each category. Bold values indicate the result was statistically significant.
Extrathyroidal extension was defined as having disease extending beyond the thyroid capsule, including either microscopic or macroscopic invasion.
Vascular invasion was defined as the presence of intravascular tumor cells either covered by endothelium or associated with thrombus, affecting lymphatic or blood vessels within or beyond the tumor capsule.
No statistics were computed due to constant numbers of one feature.
A regression model was performed for factors associated with distant metastases in DTCs (Table 3). A total of 44 patients (15.0%) with DTCs had distant metastases detected during follow-up; the metastases were located in the lung (n = 28), bone (n = 8), lung and bone (n = 6), brain (n = 1), and lung and kidney (n = 1). TERT promoter mutations [odds ratio (OR) 5.36; P < .001], gender (OR 2.31; P = .02), tumor size (OR 1.28; P = .009), and vascular invasion (OR 3.94; P < .001) were associated with distant metastases. TERT promoter mutations were found to be a predictor of distant metastases irrespectively of gender and vascular invasion. When all the features associated with distant metastases in the univariate model were introduced in the multivariate regression, vascular invasion became the only independent predictive factor of distant metastases.
Table 3.
Predictive Factors for Distant Metastases and Disease Persistence at the End of Follow-Up in Differentiated Thyroid Carcinomas
| Distant Metastases (n = 294) |
Persistent Disease (n = 211) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presence (%) | Univariate Analysis |
Multivariate Analysis |
Presence (%) | Univariate Analysis |
Multivariate Analysis |
|||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Total | 44 (15.0) | 58 (27.5) | ||||||||
| Age, y | ||||||||||
| <45 | 17 (11.5) | 1 (Referent) | NS | 25 (22.9) | 1 (Referent) | NS | ||||
| ≥45 | 27 (18.5) | 1.75 (0.91–3.37) | (.09) | 33 (32.4) | 1.61 (0.87–2.96) | (.13) | ||||
| Gender | ||||||||||
| Female | 28 (12.4) | 1 | .02 | 1 | .03 | 42 (24.9) | 1 | .04 | 1 | .03 |
| Male | 16 (24.6) | 2.31 (1.16–4.60) | 2.32 (1.10–4.90) | 16 (41.0) | 2.10 (1.02–4.35) | 2.46 (1.11–5.47) | ||||
| Tumor sizea | 1.28 (1.07–1.54) | .009 | 1.18 (0.97–1.44) | .10 | 1.18 (0.97–1.43) | NS (.10) | ||||
| Extrathyroidal extension | ||||||||||
| No | 14 (15.1) | 1 | NS | 22 (24.4) | 1 | NS | ||||
| Yes | 22 (14.3) | 0.94 (0.46–1.94) | (.87) | 28 (35.9) | 1.73 (0.89–3.27) | (.11) | ||||
| Vascular invasion | ||||||||||
| No | 10 (7.2) | 1 | <.001 | b | 16 (20.0) | 1 | NS | |||
| Yes | 22 (23.4) | 3.94 (1.77–8.78) | 22 (32.8) | 1.96 (0.93–4.13) | (.08) | |||||
| Lymph node metastases | ||||||||||
| N0/Nx | 17 (17.9) | 1 | NS | 21 (23.3) | 1 | .04 | 1 | .03 | ||
| N1 | 27 (15.7) | 0.85 (0.44–1.66) | (.64) | 35 (37.2) | 1.95 (1.03–3.71) | 2.16 (1.10–4.25) | ||||
| BRAFc | ||||||||||
| wt | 21 (17.2) | 1 | NS | 20 (22.7) | 1 | NS | ||||
| V600E | 14 (12.1) | 0.66 (0.32–1.37) | (0.26) | 26 (37.1) | 2.00 (1.00–4.03) | (.05) | ||||
| TERT | ||||||||||
| wt | 34 (12.5) | 1 | <.001 | 1 | .002 | 48 (24.6) | 1 | .001 | 1 | .007 |
| mut | 10 (43.5) | 5.36 (2.18–13.18) | 4.60 (1.73–12.21) | 10 (62.5) | 5.10 (1.76–14.78) | 4.68 (1.54–14.27) | ||||
Abbreviations: mut, mutated; n, number of patients with available data for each feature; NS, not significant; wt, wild type. Bold values indicate the result was statistically significant.
Tumor size was included in the model as a continuous variable.
Vascular invasion was not considered in the multivariate model displayed in the table. The OR of TERT promoter mutations for distant metastases adjusted for gender was 5.61 (2.24–16.06) (P = .001); the OR of TERT promoter mutations adjusted for tumor size was 4.30 (1.66–11.13) (P = .003); the OR of TERT promoter mutations adjusted for vascular invasion was 3.54 (1.06–11.82) (P = .04); the OR of TERT promoter mutations adjusted for vascular invasion and gender was 3.51 (1.05–11.72) (P = .04). When all the features associated with distant metastases in the univariate model (gender, tumor size, TERT mutational status, and vascular invasion) were included in the multivariate model, vascular invasion was the only independent predictor of distant metastases, with an OR adjusted for age, tumor size, gender, and TERT of 3.38 (1.42–8.04).
BRAF analysis was performed only in the group of PTC.
In patients with PTCs, the presence of TERT promoter mutations was associated with older age (P < .001), larger tumor size (P = .005), and higher stage (P = .02) (Table 2). Although patients with TERT mutation-positive PTCs had distant metastases more frequently than patients harboring TERT mutation-negative PTCs (27.8% vs 14.7%, respectively), the difference was not statistically significant (P = .07).
In patients with FTCs, the presence of TERT promoter mutations was associated with older age (P = .004), higher stage (P = .007), and distant metastases (P < .001).
In patients with PDTCs and ATCs, the presence of TERT promoter mutations was associated with older age (P = .003) and female gender (P = .02).
Taken BRAF and RAS together, 60.3% of TERT-mutated tumors were also mutated for BRAF or RAS. In PTCs, there was a significant association between the presence of TERT promoter mutations and the presence of BRAF mutation (P = .008) (Table 2). The BRAF mutation was not associated with increased lymph node or distant metastases or with patients' outcome (survival) (see Figure 2H). In patients with TERT-mutated tumors, the coexistence of BRAF mutations was not associated with more aggressive clinicopathological features or worse outcome (Supplemental Table 3 and Supplemental Figure 2). No associations were found concerning RAS mutated tumors.
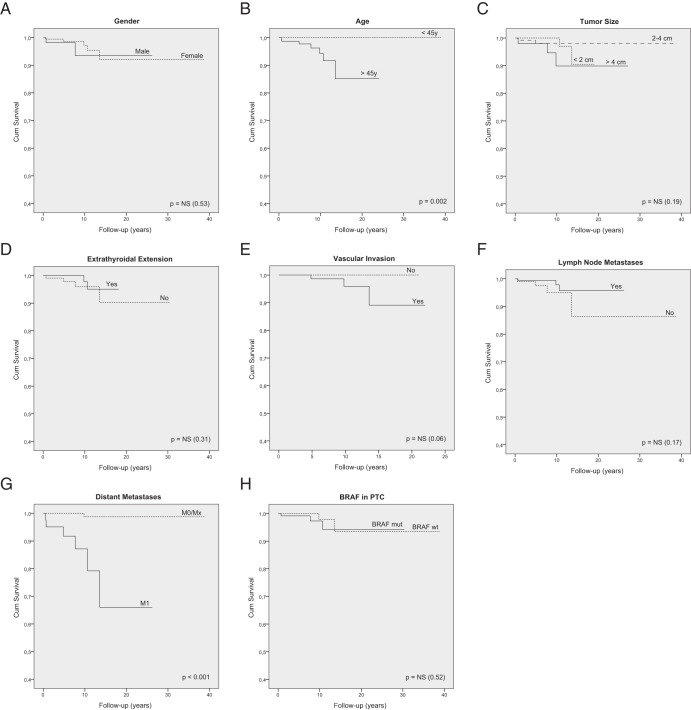
Figure 2.
Kaplan-Meier survival curves of DTC-specific survival according to the different clinicopathological and molecular features: gender (A), age (B), tumor size (C), extrathyroidal extension (D), vascular invasion (E), lymph node metastases (F), distant metastases (G), and BRAF status in PTCs (H).
None of the cases presenting RET/PTC (12 of 75) or PAX8/PPARG rearrangement (5 of 62) had TERT promoter mutations.
Relationship between TERT promoter mutations, clinicopathological features, and outcome
Mean follow-up time (±SD) of the patients was 7.8 ± 5.8 years (range 0.1–38.9 y). The total follow-up for the whole of FCDTC patients was 2967.5 person-years, 2760.8 person-years for DTC patients, 2342.9 person-years for PTC patients, and 417.9 person-years for FTC patients.
Patients with TERT promoter-mutated DTCs were submitted to more radioiodine treatments (2.7 ± 1.8 vs 1.8 ± 1.2; P = .009) with higher cumulative doses (15.8 ± 12.4 vs 9.0 ± 8.1 GBq; P = .004) as well as to more treatment modalities of other types (2.6 ± 2.5 vs 1.1 ± 1.5; P = .001) (Supplemental Table 2). A similar analysis was not performed for PDTCs and ATCs due to the heterogeneity of the therapeutic management of these two groups of patients.
At the end of follow-up, patients with DTCs harboring TERT-mutated tumors were more prone to have persistent disease (OR 5.10; P = .001) (Table 3). Male gender (OR 2.10; P = .04) and lymph node metastases (OR 1.95; P = .04) were other factors associated with disease persistence. In the multivariate model, TERT promoter mutations were independent predictors of persistent disease at the end of follow-up.
During follow-up, the overall disease-specific mortality was 11.7% in FCDTCs, 2.2% in DTCs, and 1.8% in the group of patients with PTCs (Table 4). The disease-specific mortality rate was 14.83 per 1000 person-years in FCDTCs, 2.53 per 1000 person-years in DTCs, and 2.13 per 1000 person-years in PTCs.
Table 4.
Thyroid Cancer-Specific Mortality and Hazard Ratios for Patients With TERT wt vs TERT Mutated Tumors
| Type of Cancer | Mortality, n, % |
Mortality Rate (Deaths per 1000 Person-Years) |
Hazard Ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | TERT wt | TERT Mutated | P Valuea | TERT wt | TERT Mutated | Unadjusted | P Value | Adjustedb | P Value | |
| DTC | 7/323 (2.2) | 3/298 (1.0) | 4/25 (16.0) | <.001 | 1.17 | 21.17 | 19.42 (4.30–87.64) | <.001 | 10.35 (2.01–53.24) | .005 |
| PTC | 5/284 (1.8) | 3/265 (1.1) | 2/19 (10.5) | .001 | 1.36 | 13.64 | 11.63 (1.93–70.15) | .007 | 23.81 (1.36–415.76) | .03 |
| FTC | 2/70 (2.8) | 0/58 | 2/12 (16.7) | <.001 | 0 | 47.23 | c | c | c | c |
Abbreviations: mut, mutated; wt, wild type.
Log-rank P values.
Adjusted for age and gender (Cox regression).
Due to the low numbers, a regression analysis was not performed for FTC patients.
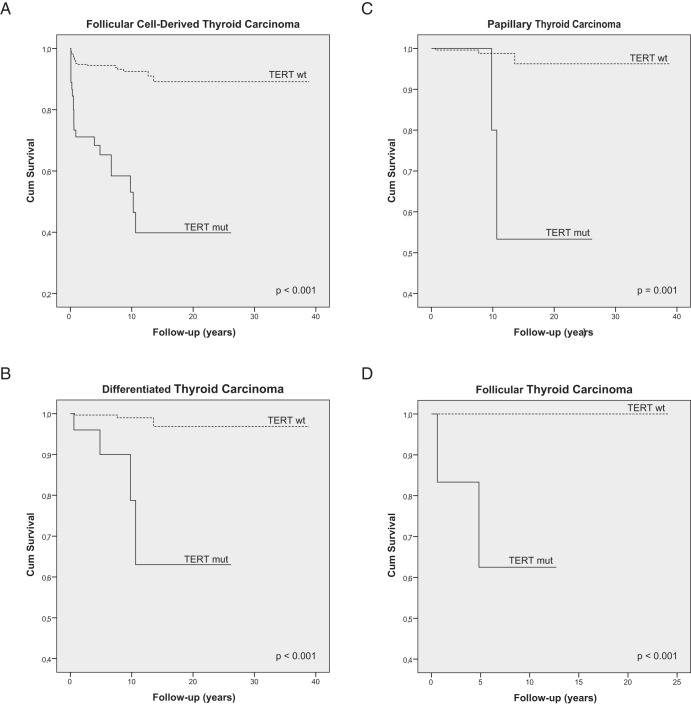
The Kaplan-Meier survival analysis showed that the presence of TERT promoter mutations was associated with increased disease-specific mortality in DTCs (log rank P < .001), PTCs (log rank P = .001), and FTCs (log rank P < .001) (Figure 1 and Table 4). Age at diagnosis and the presence of distant metastases were the other clinicopathological features significantly associated with patients' survival (Figure 2).
Figure 1.
Kaplan-Meier survival curves of thyroid carcinoma-specific survival by TERT mutation status representing the whole follicular cell-derived thyroid carcinoma series (A), the group of patients with differentiated thyroid carcinoma (B), the subgroups of patients with PTCs (C), and the subgroup of patients with FTCs (D). TERT mut, TERT promoter mutation; TERT wt, TERT promoter wild type.
In the Cox regression analysis, the hazard ratio (HR) for mortality from DTC in patients harboring tumors with TERT promoter mutation was 19.42 [95% confidence interval (CI) 4.30–87.64]; after adjustment for age at diagnosis and gender, the HR was 10.35 (95% CI 2.01–53.24). In the subgroup of patients with PTCs with TERT promoter mutations, the HR for disease-specific mortality was 11.63 (95% CI 1.93–70.15); after adjustment for age at diagnosis and gender, the HR was 23.81 (95% CI 1.36–415.76). The low number of deaths in the group of patients with FTCs precluded a similar regression analysis.
Discussion
In the present study, we show that TERT promoter mutations are an indicator of tumor aggressiveness in DTCs, being associated with distant metastases, worse response to treatment, and poor outcome.
TERT promoter mutations were present in 12.4% of the FCDTC tumors, being detected in PTCs and FTCs (7.5% and 17.1%, respectively), PDTCs and ATCs (29.0% and 33.3%, respectively). The frequencies of TERT promoter mutations in DTCs are similar to those recently reported by Liu et al (19) and lower than those reported by Landa et al (20) and Liu et al (26). TERT promoter mutations were not detected in normal thyroid or in benign lesions, such as nodular goiter, adenoma, or thyroiditis, or in medullary thyroid carcinomas. These negative findings fit with previous reports (15, 19, 20). No TERT promoter mutations were detected in benign or malignant tumors from Chernobyl-irradiated patients, suggesting that irradiation is not a strong etiological factor for the occurrence of such mutations.
We did not detect TERT promoter mutations in any of the carcinomas displaying oncocytic features (Supplemental Table 1). The absence of mutations may reflect the small size of the series regarding oncocytic variant of PTCs, tall-cell PTCs, and Warthin-like PTCs. This does not hold true, however, regarding FTCs in which TERT promoter mutations were not detected in any of the 27 oncocytic cases (0%), in contrast to their presence in 12 of the 43 conventional FTCs (27.9%). We do not know how to explain this finding, which has to be confirmed in larger series, namely of oncocytic PTCs, but we believe it contributes to reinforce the assumption that oncocytic tumors of the thyroid differ from their nononcocytic counterparts with regard to several genetic and metabolic pathways (25, 27).
The relatively low number of microcarcinomas (11.2%; Table 1), all negative for TERT promoter mutations, and the higher percentage of cases with distant metastases (15% of DTCs) in comparison with data reported in some recent series (28, 29) support the assumption that one may be dealing in the present study with higher-stage cases referred to academic centers. Further studies including lower-risk patients are necessary to establish a more accurate evaluation of the frequency of TERT promoter mutations in the different histotypes of FCDTCs.
In DTCs, the presence of TERT promoter mutations was associated with older age at diagnosis, larger tumors, higher frequency of distant metastases, and higher tumor stage. These results are in line with previous reports indicating that TERT promoter mutations were more prevalent in histological categories of thyroid carcinomas classically associated with more aggressive clinical behavior (15, 19, 20). The most important added value of the present study is that we found evidence showing that patients with TERT mutated tumors had decreased survival when compared with patients with tumors harboring wild-type TERT and, moreover, that this holds true for the whole DTC series as well as for PTCs and FTCs independently. The usefulness of TERT mutations as a prognostic marker is particularly relevant in DTCs due to the combination of two main reasons: only a small percentage of such carcinomas behave aggressively and may turn ultimately lethal, and there is a lack of good prognostic indicators in this setting.
TERT promoter mutations were more prevalent in PDTCs (29.0%) and ATCs (33.3%) than in DTCs. Although we did not find an association between TERT promoter mutations and mortality in this subset of less differentiated tumors (data not shown), the dismal outcome of most patients with poorly differentiated/undifferentiated carcinomas and their aggressive clinicopathological characteristics raises doubts about the prognostic utility of molecular markers, such as TERT promoter mutations, from a clinical standpoint. Furthermore, the small number of patients in each of the two categories does not allow a definitive conclusion on this issue. We also believe that the predictive value of TERT promoter mutations for disease-specific mortality in patients with FTCs should be clarified in larger series because we acknowledge the small number of events that occurred in our series due to the relatively low number of FTCs patients with appropriate follow-up.
Although the overall prognosis of patients with DTCs is usually good, with a 10-year survival rate higher than 85% in most series (30), the outcome of older patients with distant metastases is guarded, with a 5-year survival of approximately 30%–40% (31). Due to the clinical relevance of distant metastases, we performed a multivariate logistic regression evaluating the clinicopathological and molecular factors associated with distant metastasization. The presence of TERT promoter mutations was a significant predictor of distant metastases after adjusting for gender, tumor size, or vascular invasion, independently. When all the features associated with distant metastases in the univariated model (gender, tumor size, vascular invasion, and TERT mutational status) were included in the regression, vascular invasion became the only independent predictor. From the clinical standpoint, vascular invasion is information available only after surgery and all the others [age, gender, tumor size (estimated by ultrasound), and TERT mutational status (if feasible in fine needle aspiration cytology)] may in the near future be available for physicians before surgery, allowing a better risk stratification before the first treatment approach. For this reason, we decided to present the results of the multivariate model with and without the input of vascular invasion (Table 3).
The association of TERT promoter mutations with decreased survival in DTCs was kept irrespectively of age at diagnosis and gender. In addition to TERT promoter mutations and distant metastases, age at diagnosis was the only factor significantly associated with decreased survival in the present study. Age at diagnosis is a well-established predictor of mortality in DTCs, and this has been recognized by the Union for International Cancer Control/AJCC, setting age at diagnosis as a major determinant in the staging system (32). Even though male gender was not associated with decreased survival in our study, a large population-based cancer registry (Surveillance, Epidemiology and End-Results) showed that male gender may be associated with increased mortality (33). This finding fits with our results showing that male gender was significantly associated with distant metastases and disease persistence at the end of follow-up. These were the reasons that we decided to adjust the HR for both age at diagnosis and gender. The independent association of TERT promoter mutations with decreased survival holds true if we adjust the HR either for age at diagnosis (data not shown) or for both age at diagnosis and gender (Table 4).
Liu et al (26) recently published a report studying the frequency of TERT promoter mutations in a series of 107 FCDTCs (51 PTCs, 36 FTCs, and 20 ATCs) and its relationship with older age and shorter telomeres. The authors also found an association between TERT promoter mutations and distant metastases as well as shorter disease-specific survival in the whole FCDTC series and in PTCs. The results of this study fit in general with our findings. However, it should be emphasized that the report of Liu et al (26) was conducted in a small group of patients with extremely aggressive DTCs (51 PTCs and 36 FTCs, with a disease-specific mortality of 24.1%). At variance with the study by Liu et al, the disease-specific mortality in the present study is similar to those reported in most of the large recent series (34, 35). Furthermore, our study allowed a regression analysis in which the effect of TERT mutational status on survival could be determined and adjusted for age at diagnosis and gender. Taken together, we may say that, although performed in different populations, both studies indicate that TERT promoter mutations may serve as a marker of aggressive disease and poor outcome in DTCs.
Our data show that DTC patients with TERT promoter-mutated tumors were less prone to be free of disease at the end of follow-up and were submitted to more radioiodine treatments with higher cumulative activities as well as to a greater number of other treatment modalities. Combining this information with the worse outcome of the patients, it is likely that, in the future, TERT promoter mutational status may be used to individualize treatment decisions, namely the type of surgery (if the molecular study proves to be feasible in fine needle aspiration cytology), the decision to perform radioiodine ablation, and the amount of 131I to administer to patients. The latter issue is particularly relevant due to the current trend to reduce the number of patients submitted to radioiodine ablation and to use lower doses (36–38). International consensus and guidelines may also take into account TERT promoter mutational status to establish the best follow-up strategy for the individual patient.
Considering that DNA or RNA of TERT has been used as vaccines to induce T-helper cells and specific cytotoxic T lymphocyte responses against TERT-positive tumors (39, 40), it seems possible that, in the future, TERT-based immunotherapies may be tailored for patients with TERT-mutated carcinomas because these tumors carry a poor outcome, possibly because of a worse response to current therapies. Further studies are necessary to address this issue.
All the institutions involved in the present study followed international guidelines, but there were discrepancies regarding prophylactic central lymph node dissection. Taking into consideration that the role of such dissection is currently under debate and the existing differences from institution to institution of the present study, we did not investigate the implications of prophylactic lymph node dissection on the outcome.
TERT mutations were associated with BRAF mutations, highlighting the coexistence of activation of BRAF and of TERT genes, previously reported in melanoma (16) and thyroid (15). Horn et al (16) advanced that the mutation creates newly consensus binding sites for TCF subfamily transcription factors (Elk1 and Elk4) that can be activated by BRAF. Both in thyroid carcinoma and in melanoma, it seems that a background status of activated BRAF enhances the effects of TERT promoter mutation. Our previous results in TERT mRNA expression corroborated this assumption showing an increased TERT expression in tumors harboring BRAF and TERT mutation (15). However, the concurrence or coexistence in the current study of TERT and BRAF mutations was not associated with increased aggressiveness and worse outcome in comparison with the presence of TERT mutations alone (Supplemental Table 3 and Supplemental Figure 2). Nonetheless, these results should be viewed with caution due to the low number of patients in each of the two groups.
It remains to be fully clarified the biological meaning of the association between TERT promoter mutations and the metastatic nature of thyroid tumors. Being one of the hallmarks of cancer and enabling replicative immortality of cancer cells, TERT activation appears to be independent of the tumor microenvironment because there is no substantive evidence for stromal contributions to telomere stabilization in cancer cells (41). Our findings suggest that a link between telomerase activity and metastatic capacity may exist. We can speculate that this link may be inherent to the extended survival of TERT-mutated cells during the circulation phase and/or the homing in distant places, rather than to the increased invasiveness of such cells, but the discussion of this issue is beyond the scope of the present study.
We conclude that TERT promoter mutations are an indicator of clinical aggressiveness of follicular cell-derived thyroid carcinomas, being associated with distant metastases, worse response to treatment, and poor outcome. The detection of such mutations appears to be, per se, a promising prognostic indicator in DTCs and PTCs.
Acknowledgments
We thank Margarida Marques (Department of Technology and Information of the University Hospital of Coimbra) for the valuable review of the statistical analysis.
We acknowledge GENZYME for funding our work through a research project.
This study was supported by the Portuguese Foundation for Science and Technology through PhD Grant SFRH/BD/81940/2011 (to J.V.); PhD Grant SFRH/BD/87887/2012 (to C.T.); PhD Grant SFRH/BD/79135/2011 (to A.A.); and the Scientific Investigation Project PIC/IC/83037/2007. Further funding was obtained from the project “Microenvironment, Metabolism and Cancer,” partially supported by Programa Operacional Regional do Norte (ON.2-O Novo Norte), under the Quadro de Referência Estratégico Nacional, and through the European Regional Development Fund. The work of J.M.C.-T. was supported by Grant PI12/00749-FEDER from the Instituto de Salud Carlos III, the Ministry of Economy and Competitiveness (Madrid, Spain).
The Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP) is an associate laboratory of the Portuguese Ministry of Science, Technology, and Higher Education, which is partially supported by the Foundation for Science and Technology.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AJCC
- American Joint Committee on Cancer
- ATC
- anaplastic thyroid carcinoma
- CI
- confidence interval
- DTC
- differentiated thyroid carcinoma
- FCDTC
- follicular cell-derived thyroid carcinoma
- FTC
- follicular thyroid carcinoma
- HR
- hazard ratio
- OR
- odds ratio
- PDTC
- poorly differentiated thyroid carcinoma
- PTC
- papillary thyroid carcinoma.
References
- 1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70 [DOI] [PubMed] [Google Scholar]
- 2. Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015 [DOI] [PubMed] [Google Scholar]
- 3. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791 [DOI] [PubMed] [Google Scholar]
- 4. Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274 [DOI] [PubMed] [Google Scholar]
- 5. Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dumont JE, Maenhaut C, Pirson I, Baptist M, Roger PP. Growth factors controlling the thyroid gland. Baillieres Clin Endocrinol Metab. 1991;5:727–754 [DOI] [PubMed] [Google Scholar]
- 7. Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, et al. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol. 2004;17:819–826 [DOI] [PubMed] [Google Scholar]
- 8. Matthews P, Jones CJ, Skinner J, Haughton M, de Micco C, Wynford-Thomas D. Telomerase activity and telomere length in thyroid neoplasia: biological and clinical implications. J Pathol. 2001;194:183–193 [DOI] [PubMed] [Google Scholar]
- 9. Soares P, Lima J, Preto A, et al. Genetic alterations in poorly differentiated and undifferentiated thyroid carcinomas. Curr Genomics. 2011;12:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cantara S, Pisu M, Frau DV, et al. Telomere abnormalities and chromosome fragility in patients affected by familial papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E1327–E1331 [DOI] [PubMed] [Google Scholar]
- 11. Capezzone M, Cantara S, Marchisotta S, et al. Telomere length in neoplastic and nonneoplastic tissues of patients with familial and sporadic papillary thyroid cancer. J Clin Endocrinol Metab. 2011;96:E1852–E1856 [DOI] [PubMed] [Google Scholar]
- 12. Hoang-Vu C, Boltze C, Gimm O, et al. Expression of telomerase genes in thyroid carcinoma. Int J Oncol. 2002;21:265–272 [PubMed] [Google Scholar]
- 13. Takano T, Ito Y, Matsuzuka F, et al. Quantitative measurement of telomerase reverse transcriptase, thyroglobulin and thyroid transcription factor 1 mRNAs in anaplastic thyroid carcinoma tissues and cell lines. Oncol Rep. 2007;18:715–720 [PubMed] [Google Scholar]
- 14. Brousset P, Chaouche N, Leprat F, et al. Telomerase activity in human thyroid carcinomas originating from the follicular cells. J Clin Endocrinol Metab. 1997;82:4214–4216 [DOI] [PubMed] [Google Scholar]
- 15. Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 16. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961 [DOI] [PubMed] [Google Scholar]
- 17. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Related Cancer. 2013;20:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeLellis RA, Lloyd RV, Heitz PU, Eng C. WHO Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004 [Google Scholar]
- 22. Lima J, Trovisco V, Soares P, et al. BRAF mutations are not a major event in post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4267–4271 [DOI] [PubMed] [Google Scholar]
- 23. Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580 [DOI] [PubMed] [Google Scholar]
- 24. Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595 [DOI] [PubMed] [Google Scholar]
- 25. de Vries MM, Celestino R, Castro P, et al. RET/PTC rearrangement is prevalent in follicular Hurthle cell carcinomas. Histopathology. 2012;61:833–843 [DOI] [PubMed] [Google Scholar]
- 26. Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas [published online October 21, 2013]. Oncogene. doi:10.1038/onc.2013.446 [DOI] [PubMed] [Google Scholar]
- 27. Jacques C, Guillotin D, Fontaine JF, et al. DNA microarray and miRNA analyses reinforce the classification of follicular thyroid tumors. J Clin Endocrinol Metab. 2013;98:E981–E989 [DOI] [PubMed] [Google Scholar]
- 28. Elisei R, Molinaro E, Agate L, et al. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab. 2010;95:1516–1527 [DOI] [PubMed] [Google Scholar]
- 29. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276–E285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53 856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 31. Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–1456 [DOI] [PubMed] [Google Scholar]
- 32. Sobin LH, Wittekind Ch. UICC: TNM Classification of Malignant Tumors. 6th ed New York: Wiley-Liss; 2002 [Google Scholar]
- 33. Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid. 2013;23:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37 [DOI] [PubMed] [Google Scholar]
- 36. Mallick U, Harmer C, Yap B, et al. Ablation with low-dose radioiodine and thyrotropin α in thyroid cancer. N Engl J Med. 2012;366:1674–1685 [DOI] [PubMed] [Google Scholar]
- 37. Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673 [DOI] [PubMed] [Google Scholar]
- 38. Mallick U, Harmer C, Hackshaw A, Moss L, Io NTMG. Iodine or Not (IoN) for low-risk differentiated thyroid cancer: the next UK National Cancer Research Network randomised trial following HiLo. Clin Oncol. 2012;24:159–161 [DOI] [PubMed] [Google Scholar]
- 39. Liao ZL, Tang XD, Lu MH, et al. Antitumor effect of new multiple antigen peptide based on HLA-A0201-restricted CTL epitopes of human telomerase reverse transcriptase (hTERT). Cancer Sci. 2012;103:1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dosset M, Vauchy C, Beziaud L, Adotevi O, Godet Y. Universal tumor-reactive helper peptides from telomerase as new tools for anticancer vaccination. Oncoimmunology. 2013;2:e23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322 [DOI] [PubMed] [Google Scholar]