Abstract
Background
We aimed to examine the correlation among nighttime blood pressure, heart rate variability, and left atrium peak systolic global longitudinal strain among patients with subjective tinnitus.
Material/Methods
Eighty patients with tinnitus were assigned to Group 1 and 80 healthy individuals were assigned to Group 2. Clinical blood pressure measurements, ambulatory blood pressure monitoring, and Holter electrocardiography monitoring were performed. All of the cases included in the study were examined with conventional echocardiography and 2-dimensional speckle tracking echocardiography.
Results
Mean nighttime systolic blood pressure (130.3±5.4) and mean nighttime diastolic blood pressure (82.8±3.9) in Group 1 were higher than in Group 2 (125.1±5.4 and 80.7±4.7, respectively) (p<0.05). Mean heart rate in Group 1 was significantly lower than in Group 2 but there was no statistically significant difference between the groups in terms of heart rate variability parameters and left atrium peak systolic global longitudinal strain values (p>0.05).
Conclusions
Nighttime systolic blood pressure and nighttime diastolic blood pressure were higher among the patients with tinnitus. In light of these results, we can conclude that both clinical blood pressure measurement and ambulatory blood pressure monitoring are important for patients with tinnitus.
MeSH Keywords: Arterial Pressure, Echocardiography, Tinnitus
Background
Tinnitus affects nearly 10% of the population and may be caused by many factors. It can be divided into 2 types: subjective types with underlying causes in the inner ear (increased damage in the ear with divided stapedial tendons) or auditory pathway; and objective types with underlying vascular and muscular disorders, osseous diseases, or neoplasia. Subjective tinnitus is often associated with sensorineural hearing loss [1,2]. Tinnitus prevalence increases with old age due to autonomous nervous system dysfunction. When tinnitus is severe, it may negatively affect concentration, work performance, and social life. Studies have reported that 71% of the patients developed depression due to tinnitus [3,4]. Psychological conditions such as depression and anxiety may lead to changes in nighttime blood pressure [5]. Thus, diurnal blood pressure changes and some autonomous nervous system dysfunctions occur [6]. Autonomous nerve system dysfunctions exacerbate tinnitus and cause a vicious cycle. Thus far, no study has investigated the correlation between autonomous nerve system dysfunctions and changes in diurnal blood pressure and heart rate variability. Two-dimensional speckle tracking echocardiography, which has recently become widely used, is very important in discovering myocardial deformation, practically assessing heart beat variability, investigating cardiac effects of diurnal blood pressure, and uncovering the correlation between these cardiac effects with tinnitus. We hypothesized that nighttime blood pressure and heart rate variability play a key role in the etiology of tinnitus among those who have clinically normal blood pressure. In the present study, we aimed to examine the correlation among heart rate variability, left atrium (LA) global strain, and nighttime blood pressure among patients with tinnitus.
Material and Methods
The study was conducted in our hospital, according to the provisions of the Helsinki Declaration, and with the ethical approval of our hospital ethics committee. All of the patients were informed prior to the study and their informed consents were obtained.
Study population
Eighty patients with tinnitus complaints and 80 healthy individuals were included in the study. Tinnitus was diagnosed by an otorhinolaryngologist. Patients with hypertension, otologic disorders, neurologic disorders, metabolic disorders, pharmacologic causes, psychological diseases, or vascular pathologies were excluded from the study because these conditions cause tinnitus.
Study design
The study included 2 groups: those with tinnitus (Group 1, n=80) and the control group (Group 2, n=80). Tinnitus was diagnosed by an otorhinolaryngologist. Patients were examined by a neurology specialist and psychiatry specialist in terms of secondary tinnitus causes. Conventional echocardiography images and 2-dimensional speckle tracking echocardiography images of the patients were taken, and ambulatory blood pressure monitoring and Holter electrocardiography monitoring were performed. Echocardiographic measurements were performed by another observer blind to the study.
Assessments
Clinical blood pressure measurement
Blood pressure measurements of the patients were performed using a sphygmomanometer following the rules of standard blood pressure measurements, in the sitting position after resting at least for 5 minutes. Those patients in whom the mean of 3 subsequent blood pressure measurements was ≤140/90 mm Hg in the first clinic visit were included in the study.
Assessment of ambulatory blood pressure
Following casual systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements, 24-hour ambulatory blood pressure monitoring (ABPM) was obtained automatically in the nondominant arm by an oscillometric portable monitor (SpaceLabs, Medical Inc., Model: 92512, Redmond, WA, USA) every 20 minutes from 07.00 to 22.00 hours and every 30 minutes from 22.00 to 07.00 hours. Daytime was defined as the time interval between 07.00 to 22.00 hours and nighttime as the time interval between 22.00 to 07.00 hours. Cuff size was selected in accordance with the arm circumference of the subjects. All the patients and controls were advised to maintain their daily activities and avoid vigorous exercise during ABPM monitoring. The recordings of the monitor were downloaded to a computer and the ABPM data were analyzed for: (i) SBP and DBP during awake and sleep times, and (ii) percentage decline in nocturnal SBP and DBP calculated using the formula [(mean daytime BP – mean night-time BP)/mean daytime BP]×100, with normal values being ≥10% [7].
Determination of heart rate variability
A 24-hour Holter electrocardiography monitoring was performed. During the monitoring period, ‘Holter WIN-PV plus’ software was used. The Holter registries of all of the cases were manually evaluated in order to leave artefacts out of the assessment, and then ‘time-domain’ heart rate variability parameters were automatically established. For the assessments, the following parameters were selected: 24-hour SDNN (standard deviation of all normal R-R intervals), SDNN-i (the mean of the standard deviation of normal 5-minute RR intervals), SDANN (standard deviation of sequential 5-minute R-R interval means in registries), pNN50 (consecutive R-R intervals differ by more than 50 msec), RMSSD (root mean square of the successive differences), and NN (RR interval between 2 adjacent QRS). The patients were asked not to take drugs before 48 hours prior to Holter electrocardiography monitoring and not to drink caffeinated beverages.
Determination of Body Mass Index was formulated as follows:
Conventional echocardiographic assessment
Transthoracic echocardiographic examinations were performed with a Vivid 7 Dimension® (GE Vingmed Ultrasound AS N-3190 Horten, Norway) echocardiography instrument and 2.5 MHz transducer. Patients were assessed in a left side decubitus position after a 5-minute rest. We first assessed pericardia, valve morphologies, and wall movements using M mod and 2-dimensional echocardiography. LV ejection fraction (LVEF) was measured using parasternal long axis. In the apical 4-chamber view with Pulsed-wave (PW) Doppler echocardiography in relation with LV inflow, Doppler sample volume was measured parallel to the long axis of the LV and mitral annulus plane and means were obtained. For the assessments, early diastolic flow velocity (E), late diastolic flow velocity (A), and deceleration time (DT) were registered. In the apical 4-chamber view, 5-mm PW Doppler sample volume was performed in the junction of posterior wall and mitral annulus. With sample volume parallel to the wall axis, peak early (E1) diastolic flow rate was registered. All of the measurements were repeated during the subsequent 3 heart beats and means were taken. All of the measurements were performed based on American Echocardiography Association standards [8].
Two-dimensional speckle tracking echocardiographic assessment
Apical 4-chamber view and apical 2-chamber view were recorded with grey-scale. Later on, the records were processed using acoustic tracking software (EchoPAC version 7.0, GE Vingmed). Means of the global strain 15 atrial segment measurements were calculated. Frame frequency was 50–90 frames per second. LAGLSs was measured during LV systole (Figure 1).
Figure 1.
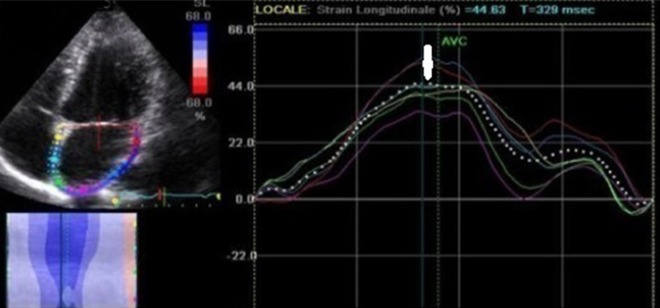
Measurements of LAGLSs on an image obtained from apical four-chamber view (LAGLSs: peak left atrial strain during left ventricular systole, white narrow: LAGLSs).
Statistical analyses
Results are reported as the mean ±SD and statistical analysis of clinical data between 2 groups consisted of unpaired t-tests for parametric data and Mann-Whitney U test analysis for nonparametric data, and analysis of variance for repeated measures for parametric data. To analyze the correlation between variables, Pearson correlation coefficient was used. Statistical analysis was performed using PASW 18 (SPSS/IBM, Chicago, IL, USA) and the level of significance was established at the 0.05 (2-sided).
Reproducibility
Intra- and inter-observer reproducibility for the LAGLSs were evaluated. For intra-observer evaluation, views from 30 randomly selected patients were reassessed after a week. The Bland-Altman analysis and the intra-class correlation coefficient (ICC) demonstrated good inter- and intra-observer agreement; for interobserver agreement for LAGLSs, the mean difference was 1.9 (−1.9, 4.5) and ICC 0.88; and for intraobserver agreement, the mean difference was 1.4 (−2.1, 3.2) and ICC 0.92.
Results
Comparison of demographic, clinical, and blood pressure characteristics of the groups
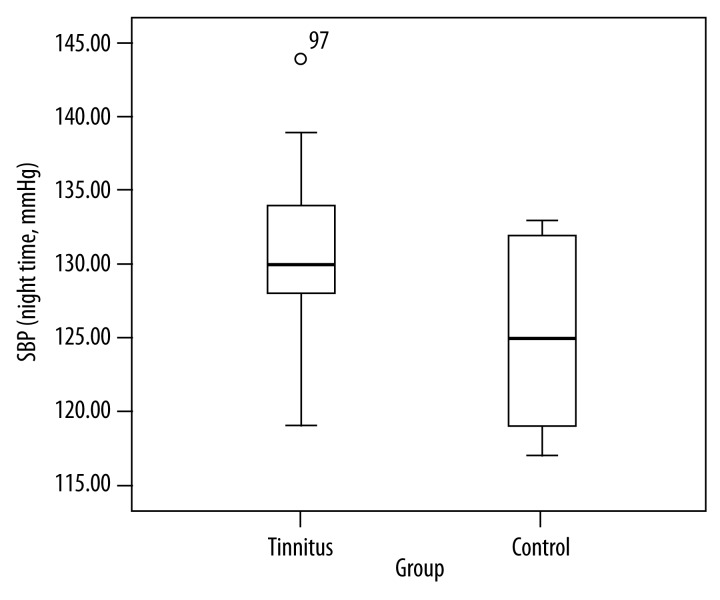
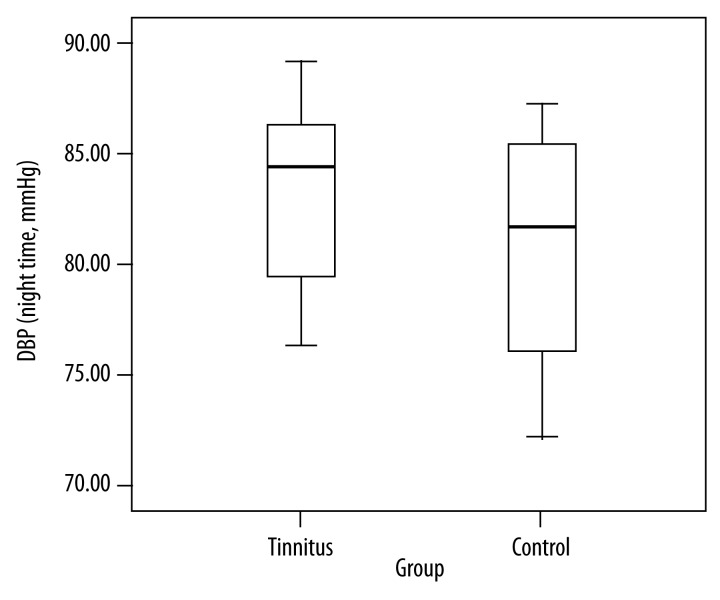
Table 1 includes comparisons of the groups in terms of demographic, clinical, and blood pressure characteristics. Eighty cases with tinnitus (Group 1) and 80 healthy individuals (Group 2) were included in the study. Group 1 included 38 men and 42 women and Group 2 included 40 men and 40 women. The groups were similar in terms of sex. Mean age was 65.1±11.8 years in Group 1 and 56.1±10.1 years in Group 2. There were 25.9% of the participants in Group 1 and 24.2% of the participants in Group 2 who actively smoked cigarettes. There was no significant difference between the groups in terms of BMI. Mean heart rate in Group 1 (66.3±8.1) was significantly lower than in Group 2 (77.5±12.1) (p<0.05). There was no statistically significant difference between the groups in terms of cholesterol and keratin values. Mean duration of tinnitus was 3.4 months. There was no statistically significant difference between the groups in terms of clinical BP, 24-hour BP, or daytime BP (p>0.05). Nighttime SBP in Group 1 was significantly higher than in Group 2 (p<0.0001). DBP measured in Group 1 was considerably higher than in Group 2 (p<0.05).
Table 1.
Demographic, clinical and blood pressure characteristics of groups.
| Parameters | Group 1 (n: 80) | Group 2 (n: 80) | P value |
|---|---|---|---|
| Age (years) | 65.1±11.8 | 56.1±10.1 | 0.0001 |
| Gender (male/female) | 38/42 | 40/40 | NS |
| Body mass index (kg/m2) | 24.3±1.1 | 24.9±0.5 | NS |
| Current smokers (%) | 25.9 | 24.2 | NS |
| Heart rate (beat/min) | 66.3±8.1 | 77.5±12.1 | 0.0001 |
| SBP (mmHg) | 125.1±5.6 | 120.8±2.70 | NS |
| DBP (mmHg) | 81.5±4.0 | 78.2±2.7 | NS |
| 24-h SBP (mmHg) | 121.7±5.1 | 119.6±4.6 | NS |
| 24-h DBP (mmHg) | 81.8±2.9 | 78.2±3.4 | NS |
| Daytime SBP (mmHg) | 124.1±2.9 | 122.1±3.1 | NS |
| Daytime DBP (mmHg) | 80.3±3.7 | 79.0±2.3 | NS |
| Nighttime SBP (mmHg) | 130.3±5.4 | 125.1±5.4 | 0.0001 |
| Nighttime DBP (mmHg) | 82.8±3.9 | 80.7±4.7 | 0.01 |
| Serum cholesterol (mmol/l) | 5.73±0.93 | 5.84±0.96 | NS |
| Serum creatinine (μmol/l) | 88.1±16.2 | 89.2±15.6 | NS |
Data are means ±SD; NS – not significant; SBP – systolic blood pressure; DBP – diastolic blood pressure.
Comparison of heart rate variability, left atrium global longitudinal strain, and echocardiographic characteristics of the groups
Table 2 shows characteristics of the groups in terms of HRV, LAGLSs, and echocardiographic parameters. There was no significant difference between Group 1 and Group 2 in terms of LAGLSs values. There was no significant difference between Group 1 and Group 2 in terms of LVSD, LVDD, LVEF, DT, E, A, or E/E1 (p>0.05).
Table 2.
Heart rate variability and echocardiographic characteristics of groups.
| Parameters | Group 1 (n: 80) | Group 2 (n: 80) | P value |
|---|---|---|---|
| NN (ms) | 564.7±10.9 | 573.4±3.5 | NS |
| SDNN (ms) | 88.8±5.3 | 94.6±8.0 | NS |
| SDANN (ms) | 75.4±3.0 | 80.3±2.2 | NS |
| SDNN-i (ms) | 63.9±3.4 | 68.2±2.3 | NS |
| RMSSD (ms) | 57.9±4.1 | 59.3±3.0 | NS |
| pNN50 (%) | 12.8±1.26 | 14.5±0.7 | NS |
| LVSD (mm) | 29.1±2.6 | 29.3±2.06 | NS |
| LVDD (mm) | 45.4±3.3 | 47.0±2.46 | NS |
| LVEF (%) | 64.0±5.0 | 65.4±3.45 | NS |
| E (m/s) | .81±0.07 | .8±0.07 | NS |
| A (m/s) | .84±0.05 | .76±0.09 | NS |
| E/E1 | 9.37±0.7 | 8.8±0.39 | NS |
| DT (ms) | 188.8±14.7 | 180.7±9.3 | NS |
| LAGLSs (%) | 32.5±1.9 | 35.0±2.03 | NS |
NN – number of pairs of adjacent NN intervals differing by more than 50 ms in the entire recording, SDNN: Standard deviation of all NN intervals; SDANN – standard deviation of the averages of NN intervals in all 5 min segments of the entire recording; SDNN-i – mean of the standard deviations of all NN intervals for all 5 min segments of the entire recording; pNN50 – NN50 count divided by the total number of all NN intervals; RMSSD – the square root of the mean of the sum of the squares of differences between adjacent NN intervals; LVSD – left ventricle systolic diamater; LVDD – left ventricle diastolic diamater; LAGLSs – left atrium global longitudinal peak systolic strain; DT – deceleration time; LVEF – left ventricular ejection fraction, peak E – peak mitral velocity of early diastolic filling from transmitral flow, Peak A indicates peak mitral inflow contraction velocity; E1 – early diastolic filling using DTI; DTI – Doppler tissue imaging.
Correlation analyses
In Group 1, there was: a positive and strong correlation between nighttime SBP and nighttime DBP (p<0.0001, r: 0.77); a negative and weak correlation between nighttime SBP and NN (p: 0006, r: −0.27); a negative and weak correlation between nighttime SBP and SDNN-i (p: 0.002, r: −0.31); a negative and moderate correlation between nighttime DBP and NN (p: 0.0001, r: −0.45); a negative and weak correlation between nighttime DBP and SDNN-i (p: 0.0001, r: −0.38); a positive and weak correlation between SDNN-i and LAGLSs; and a positive and strong correlation between SDNN-i and LAGLSs (p: 0.009, r: 0.26). In Group 2, there was a positive and strong correlation between nighttime SBP and nighttime DBP (p: 0.0001, r: 0.78); a negative and weak correlation between nighttime SBP and LAGLSs (p: 0.0001, r: −0.25); and a negative and weak correlation between nighttime DBP and LAGLSs (p: 0.0001, r: −0.27).
Discussion
This was the first study to scrutinize the correlation among nighttime blood pressure, heart rate variability, and left atrium global longitudinal strain among patients with subjective tinnitus. We found nighttime SBP and nighttime DBP were higher among the patients with tinnitus (Figures 2 and 3). We found lower heart rate in the group of the patients with tinnitus than the control group. Mean age was considerably higher in the tinnitus group than in the control group. However, there was no statistically significant difference between the groups in terms of LAGLSs values or heart rate variability parameters. It becomes important to discover the degree of subjective tinnitus and to treat tinnitus because of its high prevalence in society and significant effects upon quality of life. Abnormality during the decreases in nighttime blood pressure occurs among 30–50% of essential hypertensive patients and this rate goes up with old age, high BP, diabetes, and renal dysfunctions [10–14]. In our study, there were no subjects with diabetes or renal dysfunctions. Mean age in the tinnitus group was considerably higher than in the control group. Autonomic nervous system dysfunction is often seen among the elderly people.
Figure 2.
Comparison of nighttime systolic blood pressure in the groups (SBP: Systolic blood pressure).
Figure 3.
Comparison of nighttime diastolic blood pressure in the groups (DBP: diastolic blood pressure).
Elderly people often have of autonomic nervous system dysfunction. Autonomic nervous system effects upon the cardiovascular system have long been known. That blood pressure and heart rate show daily circadian rhythm is proof of autonomic activity [15,16]. It is possible that blood pressure and heart rhythm may correspondingly decrease during nighttime, when autonomic activity is at the lowest level. In our study, that mean age in the tinnitus group was higher, which explains why nighttime blood pressure was high as compared with the control group but current blood pressure level is not sufficient to make a hypertension diagnosis. We found that heart rate was lower among the patients with tinnitus, which may have been because tinnitus occurs more among the elderly and may be related to dysfunction of the autonomic nervous system. Piroddaa et al. [17] reported that hemodynamic imbalance caused internal ear damage among the group with abnormal vasomotor function, which resulted in tinnitus. In this study, it was noted that bradycardia was prevalent among the patient group with tinnitus. In our study, too, the fact that mean age was high and heart rate was low may have been correlated with abnormal vasomotor function. In light of these results; we suggest that tinnitus may be controlled by increasing heart rate and regulating nighttime blood pressure. Besides, heart rate variability may change among the elderly [18,19], but in our study there was no significant difference between the groups in terms of heart rate variability. Although mean age in the tinnitus group was higher than in the control group, no significant difference occurred in terms of heart rate variability that may have been correlated with the duration of tinnitus. Duration of tinnitus in our study was 3.4 months. There was no significant difference between the groups in terms of LAGLSs values, which may be because high nighttime blood pressure was not high enough to cause myocardial deformation. We left objective tinnitus causes out of our study. But, there may be momentary changes in emotional state. Sympathetic discharge increases occur in heart under the control of emotional stress and parasympathetic nerve system [20,21], which may lead to changes in nighttime blood pressure and tinnitus complaints. The etiopathogenesis has not been clearly understood in subjective tinnitus described by the patient in who secondary causes are excluded.
Limitation of study
Group 1 was older than group 2, but this is not an important result.
Conclusions
Our study will help understand the etiology of tinnitus. Measuring only clinic blood pressure may cause wrong diagnoses among patients with tinnitus. Therefore, we recommend that ambulatory blood pressure be followed in order to assess nighttime blood pressure.
Abbreviations
- E1
early diastolic filling using DTI
- DBP
diastolic blood pressure
- DT
deceleration time
- DTI
Doppler tissue imaging
- NN
number of pairs of adjacent NN intervals differing by more than 50 msec in the entire recording
- SDNN
standard deviation of all NN intervals
- SDANN
standard deviation of the averages of NN intervals in all 5-min segments of the entire recording
- SDNN-i
mean of the standard deviations of all NN intervals for all 5-min segments of the entire recording
- pNN50
NN50 count divided by the total number of all NN intervals
- RMSSD
the square root of the mean of the sum of the squares of differences between adjacent NN intervals
- LVSD
left ventricle systolic diameter
- LVDD
left ventricle diastolic diameter
- LAGLSs
left atrium global longitudinal peak systolic strain
- LVEF
left ventricular ejection fraction
- Peak E
peak mitral velocity of early diastolic filling from transmitral flow
- Peak A
peak mitral inflow contraction velocity
- SBP
systolic blood pressure
Footnotes
Source of support: Departmental sources
References
- 1.Ciuman RR. Inner ear symptoms and disease: Pathophysiological understanding and therapeutic options. Med Sci Monit. 2013;19:1195–210. doi: 10.12659/MSM.889815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocalan R, Akin FC, Yilmaz YF, et al. Division of the stapedial tendon results in noise-induced damage to the inner ear. Med Sci Monit. 2014;20:742–46. doi: 10.12659/MSM.890158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician. 2004;69:120–26. [PubMed] [Google Scholar]
- 4.Marsot-Dupuch K. Pulsatile and nonpulsatile tinnitus: a systemic approach. Semin Ultrasound. 2001;22:250–70. doi: 10.1016/s0887-2171(01)90010-1. [DOI] [PubMed] [Google Scholar]
- 5.Voss M. Tinnitus. In: Jafek B, editor. ENT Secrets’de. Philadelphia, Pa: Hanley & Belfus; 1996. pp. 58–61. [Google Scholar]
- 6.Sweetow R. Tinnitus. In: Northern J, editor. Hearing Disorders’da. Needham Heights, Mass: Allyn and Bacon; 1996. pp. 299–308. [Google Scholar]
- 7.Kario K, Matsuo T, Kobayashi H, et al. Relation between nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensives: advanced silent cerebrovascular damage in extreme-dippers. Hypertension. 1996;27:130–35. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Andersson G, Eriksson J, Lundth LG, Lyttkens L. Tinnitus and cognitive interference: a Stroop paradigm study. J Speech Lang Her Res. 2000;43(5):1168–73. doi: 10.1044/jslhr.4305.1168. [DOI] [PubMed] [Google Scholar]
- 10.Takeda A, Toda T, Fujii T, Matsui N. Bedtime administration of long-acting antihypertensive drugs restores normal nocturnal blood pressure fall in nondippers with essential hypertension. Clin Exp Nephrol. 2009;13(5):467–72. doi: 10.1007/s10157-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Ortiz L, Gomez Marcos MA, Martin-Moreiras J, et al. Pulse pressure and nocturnal fall in blood pressure are predictors of vascular, cardiac and renal target organ damage in hypertensive patients (LOD-RISK study) Blood Press Monit. 2009;14(4):145–51. doi: 10.1097/MBP.0b013e32832e062f. [DOI] [PubMed] [Google Scholar]
- 12.Takakuwa H, Ise T, Kato T, et al. Diurnal variation of hemodynamic indices in nondipper hypertensive patients. Hypertens Res. 2001;24(3):195–201. doi: 10.1291/hypres.24.195. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Staessen JA, Lu L, et al. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50(2):333–39. doi: 10.1161/HYPERTENSIONAHA.107.087767. [DOI] [PubMed] [Google Scholar]
- 14.Covic A, Goldsmith DJ. Ambulatory blood pressure measurement in the renal patient. Curr Hypertens Rep. 2002;4:369–76. doi: 10.1007/s11906-002-0066-6. [DOI] [PubMed] [Google Scholar]
- 15.Pieper SJ, Hammill SC. Heart rate variability: technique and investigational applications in cardiovascular medicine. Mayo Clin Proc. 1995;70:955–64. doi: 10.4065/70.10.955. [DOI] [PubMed] [Google Scholar]
- 16.Malik M. Heart rate variability Standarts of Measurement, Physiological Interpretation and Clinical Use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 17.Piroddaa A, Brandolinia C, Ferria GG, et al. Possible influence on heart rate on tinnitus. Hypertension. 2009;72(1):45–46. doi: 10.1016/j.mehy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Kayıkçıoğlu M, Payzın S. Heart Rate Variability. Turkish Cardiology Assosciation Research. 2001;29:238–45. [Google Scholar]
- 19.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 20.Meric C, Gartner M, Collet L, Chéry-Croze S. Psychopathological profile of tinnitus sufferers: evidence concerning the relationship between tinnitus features and impact on life. Audiol Neurotol. 1998;3(4):240–52. doi: 10.1159/000013796. [DOI] [PubMed] [Google Scholar]
- 21.Russo J, Katon W, Sullivan M, et al. Severity of somatisation and its relationship to psychiatric disorders and personality. Psychosomatics. 1994;35(6):546–56. doi: 10.1016/S0033-3182(94)71723-0. [DOI] [PubMed] [Google Scholar]