Abstract
Cell cultures of Psoralea corylifolia L. were established from the leaf disk derived callus. The effect of different biotic elicitors prepared from the fungal extract (Aspergillus niger and Penicillium notatum), yeast extract and chitosan with different concentrations was studied. The increased synthesis of psoralen in 16-day old cell cultures under 16 h of light and 8 h of dark period was studied. Elicitation of psoralen in A. niger elicitor treated cells was found 9-fold higher over control cells. Treating the cells with P. notatum, yeast extract and chitosan elicitors lead to four to seven-fold higher psoralen accumulation over control cells. The extract of A. niger at 1.0% v/v increased the significant accumulation of psoralen (9850 μg/g DCW) in the cultured cells. Our study clearly shows that all the elicitors had the potential to increase the accumulation of psoralen but the A. niger elicitor at 1.0% v/v induced maximum accumulation.
Keywords: Elicitation, Psoralen, Psoralea corylifolia L., Suspension cultures
1. Introduction
Plants are the prime source of medicinally important compounds. Many plant products are used as pharmaceuticals, pigments, herbicides, etc. Plant cell culture has been used for the production of various valuable phytochemicals. Psoralea corylifolia L. is an important medicinal plant found in the tropical and subtropical regions of the world. It synthesizes diverse phenylpropanoids such as furanocoumarins, isoflavonoids etc. (Boardley et al., 1986). Psoralen is the furanocoumarin and commercially important for having broad range of pharmacological activities such as photosensitizing, photobiological and phototherapeutic properties (Frank et al., 1998). Psoralen has been used for the photochemotherapy of vitiligo and skin diseases such as psoriasis, mycosis fungoides and eczema (Khushboo et al., 2010; Ozkan et al., 2012). It also shows antitumor (Szliszka et al., 2011), antibacterial (Chanda et al., 2011) and antifungal properties (Srinivasan and Sarada, 2012). Plant cell culture offers uniform secondary product synthesis by overcoming the effect of unforeseen climatic conditions and diseases in field grown plants. Elicitors are one of the important factors that can act as a switch for increasing the yield of secondary metabolites of useful bioactive compounds in plant cell cultures (Gaid et al., 2011; Wiktorowska et al., 2010 and Karwasara et al., 2010). Most of the elicitors used in earlier studies originated from fungal (Karwasara et al., 2011 and Taha et al., 2012), bacterial (Seung-Mi Kang et al., 2009) and yeast cell extracts (Kundu et al., 2012 and Sun et al., 2012) or their purified fraction. In addition some abiotic stress agents like heavy metals, UV light, osmotic stress and pH are also known to increase secondary metabolite accumulation in cultured cells. However, there is no way to predict that an elicitor will be effective in a specific cell system on metabolite accumulation. The main aim of the present work was to study the effect of different elicitors prepared from fungi (Aspergillus niger and Penicillium notatum), yeast and chitosan on psoralen accumulation in cultured cells of P. corylifolia.
2. Materials and methods
2.1. Plant material
P. corylifolia L., plants were grown in the greenhouse of botanical garden of Yeshwant Mahavidyalaya, Nanded and were used for experimental purpose. Young leaves from 3 month-old plants were washed thoroughly under running tap water for 10 min and surface sterilized with 0.1 % (w/v) aqueous HgCl2 solution for 2 min. Finally the leaves were washed 4–5 times with sterile distilled water to remove any traces of HgCl2.
2.2. Culture media
The explants were cut into circles of 1 cm diameter, then were transferred on ready-made (Murashige and Skoog, 1962) basal medium (Himedia, Mumbai) supplemented with 1 mg 2,4-dichloro phenoxy acetic acid (2,4-D) l−1, 0.5 mg naphthalene acetic acid (NAA) l−1, 1.5 mg benzylaminopurine (BAP) l−1 (Sigma–Aldrich, USA), and 3% w/v sucrose. The medium was solidified with 0.8% agar (Himedia, Mumbai) and the pH of media was adjusted to 5.8 using 0.1 N NaOH or HCl before autoclaving. Twenty ml media was dispensed in each 1.25 × 15 cm test tubes (Borosil) plugged with nonabsorbent cotton wrapped with muslin cloth. The test tubes containing medium were autoclaved at 1.06 kg cm−2 at 121 °C under 15 lbs/sq. ft. pressure for about 20 min.
2.3. Maintenance of culture
The cultures were maintained under fluorescent light with 16 h photoperiod (40 μE m−2 S−1) by cool daylight fluorescent incandescent tubes (40 W, Philips, Kolkata) at 25 ± 2 °C. Callus cultures thus raised were transferred to fresh medium once in every 3 weeks.
2.4. Cell suspension cultures
Suspension cultures were initiated using 6 g callus as inoculum in a 250 ml conical flask containing 100 ml of the modified MS medium without agar. The cultures were shaken on a rotary shaker at 120 rpm. The pH of the medium was adjusted to 5.8 by 0.1 N NaOH/HCl before autoclaving. Culture medium was autoclaved at 121 °C under 15 lbs/sq. ft. pressure for 20 min.
The cells were collected on the 16th day by filtration and blotted with filter paper to remove excess water and fresh weight was determined. Dry weight (with ∼10% moisture) was determined after drying the cells at 60 °C until a constant weight was obtained. All experiments were carried out in 250 ml Erlenmeyer flasks containing 100 ml growth medium inoculated with 10 ml cell suspension (16-day old cultures).
2.5. Fungal elicitor preparation
Elicitors were prepared from cultures of A. niger (NCIM No.621) and P. notatatum (NCIM No.757) received from the National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory, Pune, India. The fungal filaments were grown in 1000 ml conical flasks containing 250 ml of potato dextrose broth for 10 days at room temperature. Fully grown mycelia with spores were homogenized and centrifuged at 4000 rpm and the supernatants were autoclaved for 20 min at 121 °C and used as elicitors (Kim et al., 2011).
2.6. Yeast elicitor
Ten grams of yeast extract was dissolved in 100 ml of double distilled water and ethanol was added up to 80% (v/v) and was kept at 4 °C for 3 days for precipitation. The supernatant was decanted and the precipitate was redissolved in 100 ml double distilled water then autoclaved and was used as elicitor (Diwan and Malpathak, 2011).
2.7. Chitosan
Chitosan, a well-known elicitor for plant cell cultures (Santamaria et al., 2011) was also used to study psoralen accumulation. The stock solution was prepared by dissolving 1 g of crab shell chitosan (Sigma Chemical Company, USA) in 2 ml of glacial acetic acid by adding it drop wise. The final volume was made up to 100 ml by adding double distilled water. The pH of the solution was adjusted to 5.7 with NaOH or HCl before autoclaving (Malerba et al., 2012).
2.8. Elicitation method
The cell cultures of P. corylifolia L. were grown in 250 ml Erlenmeyer flasks having 100 ml of growth medium. Different concentrations of elicitor viz. extract of A. niger, P. notatum, yeast (0.5–3 % v/v) and chitosan (25–300 mg/l) were added on the 16th day to the cultures. Since the stationary phase was found from 16th to 24th day of growth. The elicitors were added during the stationary phase of growth and the cultures were incubated. The optimization of higher psoralen accumulation in cultures varied from 12 h to 96 h incubation and standardized their concentration for maximum metabolite accumulation. After harvesting the cultures, media and cells were separated and the fresh and dry weight was determined after blotting the cells with filter paper to remove excess of water. Dry weight was determined after drying the cells at 60 °C in a hot air oven until a constant weight was obtained. Psoralen was extracted from both spent media and cells separately and analyzed by HPLC. The biomass was expressed in grams Dry Cell Weight per liter (g DCW/l) and the product in microgram/gram Dry Cell Weight (μg/g DCW) (Kim et al., 2011).
2.9. Extraction of psoralen from cells grown in suspension cultures
Extraction and determination of the content of psoralen from cells grown in suspension cultures was carried out. All the solvents used were of HPLC grade and the steps involved in the extraction procedure were as follows:
Cells grown in suspension cultures were separated from the liquid nutrient medium after every treatment from identical replicate flasks, by centrifuging them at 7000–8000 rpm (Eppendorf Centifuge 5804R), at 16 °C. The cells were blotted dry on filter papers and dried in oven at 60 °C, till constant weight was obtained. The dried tissues were ground with liquid nitrogen in a pre-cooled mortar and pestle. Then the tissue was extracted overnight in methanol (10 times w/v) on a rotary shaker at 25 °C and 100 rpm. The procedure was repeated three times and the methanolic extracts were pooled together. The extracts were filtered through the Whatman No. 1 filter paper and dried in the Rotavapor R210/R215 (Buchi, Switzerland). The residue was redissolved in chloroform:water (1:1 v/v) and then centrifuged at 7000–8000 rpm, at 16 °C to separate the layers of chloroform and water. The lower chloroform layer was collected and the upper layer of water was discarded. The layer at the interphase was re-extracted with chloroform and again the chloroform layer was collected. The chloroform fractions from both the separations were combined and dried in the Rotavapor R210/R215 (Buchi, Switzerland). The residue was redissolved in 1 ml of methanol and was filtered through 0.2–0.4 μM nylon filters before analysis by HPLC. Twenty microliter of extract suspended in methanol, from each cell line, was analyzed by HPLC. The standards were also dissolved in methanol at strength of 0.5 mg/l and 20 μl was used for HPLC analysis (Catapan et al., 2009).
2.10. Extraction of spent medium (without cells) from suspension cultures
All the solvents used during extraction were of HPLC grade and the extraction procedure involved the following steps:
Cells grown in suspension cultures were separated from the liquid nutrient medium from identical replicate flasks. The cells were separated on 16th day at 8000 rpm at 16 °C. The spent medium was extracted overnight with equal volumes (v/v) of chloroform on a rotary shaker at 100 rpm and 25 °C. Two layers of chloroform and water from the medium were formed. The extraction was repeated for 3 days and the chloroform extract fractions were combined. The layers were separated by centrifuging them at 8000 rpm at 16 °C. The chloroform fraction was dried in the Rotavapor R210/R215 (Buchi, Switzerland) as described earlier. The residue was resuspended in 1 ml of methanol, filtered through 0.2–0.4 μM nylon filters before analysis by HPLC. Twenty microliter of extract from each type of medium was analyzed by HPLC (Catapan et al., 2009).
2.11. HPLC analysis
Psoralen content in independent samples was determined by using HPLC two Agilent1120 compact LC with single binary pump; variable wavelength detector (G1313A) and the column used was Eclipse C18 (250 × 4.6 mm, 5 μm). The flow rate was 0.5 ml/min and the elution was monitored with a detection wavelength of 250 nm with the Ezchrome elite compact compliance software for data processing. The mobile phase was methanol:water (80:20) with 0.1% triflouro acetic acid and the peak area was calculated by comparing with a standard samples purchased from the Sigma Aldrich, USA (Fig. 1).
Figure 1.
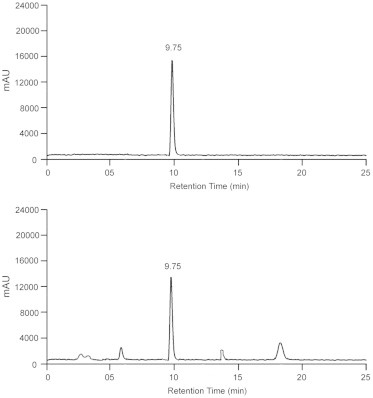
HPLC chromatogram showing standard psoralen (top figure) with the chromatogram showing the presence of psoralen (bottom figure) in cell culture extract of Psoralea corylifolia L.
2.12. Sampling and statistical analyses
All the experiments were conducted in a completely randomized design with four replicates. The data was subjected to statistical analyses following standard procedures.
3. Results
Cell suspension culture has been effectively utilized commercially for production of secondary metabolites. In recent years, use of plant cell cultures is viewed as an alternative to traditional agriculture for industrial production of secondary plant metabolites. In the present study, the work was undertaken to explore relationship among cell elicitor concentration, time for induction of secondary metabolite and age of cell culture for optimization of secondary metabolite accumulation.
3.1. Elicitor dose
All the elicitors were screened for various dose concentrations (0.5–3.0% v/v) to optimize for high quantity of psoralen accumulation. From Figs. 2 and 3, it is clear that all the elicitors prepared from fungal cell extract, yeast extract and chitosan showed significant increase in the psoralen accumulation over the control cultures (945 μg/g DCW). The best dose of the elicitors with maximum accumulation of psoralen was used in further experiments. Addition of A. niger elicitor to the cultured cells of P. corylifolia L. increased the psoralen accumulation up to 1.0% v/v concentration. The psoralen production decreased beyond this concentration level of elicitor. Maximum accumulation of psoralen (8634 μg/g DCW) was observed at 1.0% v/v dose with an eight-fold increase over control cells (945 μg/g DCW). The elicitor prepared from P. notatum showed the maximum psoralen accumulation (7124 μg/g DCW) with increase in concentration up to 1.5% v/v. The increase in concentration of yeast extract elicitor up to 1.5% v/v increased the psoralen accumulation (3870 μg/g DCW). The increase in elicitor dose level up to 3.0% v/v led to decline in the psoralen accumulation. The cells treated with different concentrations of chitosan (Fig. 3) (25–300 mg/l) showed maximum (7982 μg/g DCW) psoralen accumulation at 125 mg/l. All the elicitors used in the present study induced higher quantity of psoralen in the culture cells over control. The highest quantity of psoralen was recorded with elicitor of A. niger.
Figure 2.
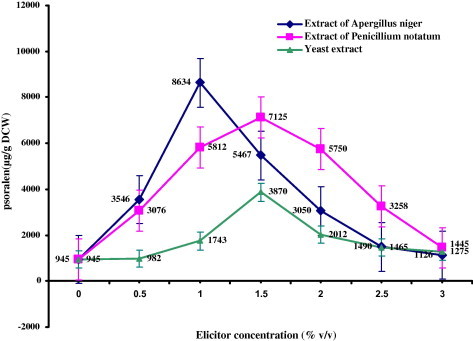
Effect of different elicitors added to 16 day old cell suspension culture of Psoralea corylifolia L. incubated for 48 h under 16 h of light and 8 h of dark period on the production psoralen (μg/g DCW – Dry Cell Weight).
Figure 3.
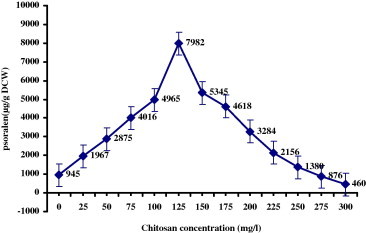
Effect of chitosan added to 16 day old cell suspension cultures of Psoralea corylifolia L. incubated for 48 h under 16 h of light and 8 h of dark period on the production of psoralen (μg/g DCW – Dry Cell Weight).
3.2. Culture age
The dose of the elicitors optimized for maximum accumulation of psoralen was used in further experiments. Fig. 4 shows the relation between culture age and the effect of elicitation on psoralen accumulation. The cell cultures of different age were treated with all the elicitors used separately in the study. The addition of A. niger elicitor to the cultured cells of P. corylifolia L. increased the psoralen accumulation. The maximum increase in psoralen accumulation was recorded in 20 days old culture and it decreased with increase in age of the culture thereafter. The accumulation of psoralen increased with increase in age of cell culture up to 16 days and thereafter the increase was inconsistent. Similar results were recorded with all the three elicitors used in this study. The cultures in the early stationary phase of growth responded with higher amount of accumulation. The optimum age of the culture for elicitation was 16–24 day and quantity of psoralen in the cells declined thereafter.
Figure 4.
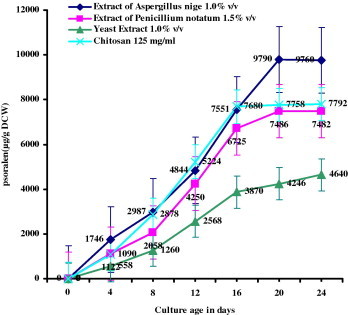
Effect of culture age on the production of psoralen (μg/g DCW – Dry Cell Weight) with optimum concentrations of the individual elicitors added to 16 day old cell suspension cultures of Psoralea corylifolia L. incubated for 48 h under 16 h of light and 8 h of dark period.
3.3. Incubation time
The effect of incubation time with elicitors is shown in the Fig. 5. The effect of accumulation time of psoralen in P. corylifolia L. cultures varied from 12 to 96 h. The optimum incubation time for psoralen induction with A. niger and yeast was found to be 72 h. and there was a steep decline in quantity of psoralen after 72 h. Chitosan and P. notatum treated cells showed the maximum psoralen accumulation in 48 h and decreased thereafter.
Figure 5.
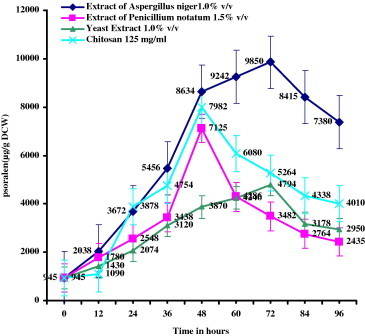
Effect of incubation period on the production of psoralen (μg/g DCW – Dry Cell Weight) with optimum concentrations of the individual elicitors added to 16 day old cell suspension cultures of Psoralea corylifolia L. incubated for 48 h under 16 h of light and 8 h of dark period.
In this study, the highest amount of psoralen was obtained (9850 μg/g DCW) in the cells treated with A. niger elicitor. The optimum age of cell culture following elicitation for maximum accumulation of psoralen was 16–24 days. The optimum incubation time for psoralen accumulation with all the elicitors was between 48 and 72 h.
4. Discussion
Plant cell culture offers an alternative way for the production of various phytochemicals in vitro. In the present study, it is observed that the secondary metabolite production is highly influenced with the addition of biotic elicitors. Fungal elicitation has been a prominent inducer of secondary metabolism.
Our experimental results have shown that the accumulation of psoralen in the suspension cultures can be stimulated by biotic elicitors. Therefore, elicitation offers an attractive strategy for increasing the metabolite production in vitro culture system significantly (Goel et al., 2011). The stimulation of psoralen accumulation by biotic elicitors such as A. niger, P. notatum, yeast extract and chitosan has also been observed in the cell cultures of plant species viz. Calendula officinalis (Wiktorowska et al., 2010), Sorbus aucuparia (Gaid et al., 2011) and Abrus precatorius (Karwasara et al., 2010, 2011). The cell wall extract preparation of A. niger possessed an oligosaccharide elicitor that induced high level of shikonin (Wen and Ri-qiang, 1996.) Another significant effect of the elicitors observed in the experiments was the rapid increase in psoralen accumulation with elicitor dosage, further increase led to decrease in the psoralen content in cell suspension cultures (Seung-Mi et al., 2009 and Kundu et al., 2012). Thus, the accumulation of psoralen is a dose elicitor dependent response of P. corylifolia L. cell cultures. Similar results were reported in taxol and ajmalicine production (Yari Khosroushahi et al., 2006 and Namdeo et al., 2002). The growth and accumulation of secondary metabolites were influenced by the type and mode of elicitor preparation (Karwasara et al., 2011) and this was observed in the present study also.
The physiological state of the cells at the moment of transfer from growth to production medium is crucial (Schaltmann et al., 1995). The optimum age of the culture for elicitation is different in different plant cell systems (Seung-Mi et al., 2009). In most of the cases, response of the cells was maximum if they were challenged at the end of the growth phase or at the onset of the stationary phase (Yari Khosroushahi et al., 2006). On the contrary, taxol and baccatin were produced during the initial stationary phase of growth in Taxus baccata cell cultures (Barradas-Dermitz et al., 2010). The response to elicitation is dependent on growth phase of the culture that affects the quantitative response as well as the product pattern (Vázquez-Flota et al., 2009). The amount of metabolite production varied with duration time of incubation with elicitors (Karwasara et al., 2010).
The optimum time of elicitor treatment is very essential to produce the secondary metabolites on a large scale. Therefore, the time required for triggering the elicitation in 16 days older culture in all the elicitors was studied and was up to 96 h. The results show that the amount of metabolite production varied with the duration of incubation time with elicitors. This result indicates that the duration of cell cultured with elicitors is much important for the maximum psoralen accumulation. An important factor for enhancing the psoralen production was the stage of cell growth for elicitor treatment (Catapan et al., 2009). It is believed that the stronger stimulation of secondary metabolites by biotic elicitor usually occurred in the late exponential growth stage of plant cell culture (Namdeo et al., 2002 and Schaltmann et al., 1995). However, the resveratrol and viniferins production in Vitis vinifera cells were treated with elicitor during the early stage of growth (Santamaria et al., 2011). The high availability of the nutrient in the medium or cells can be used for secondary metabolites biosynthesis (Sun et al., 2012). Thus, it is best to produce large amounts of biomass under rapid growth conditions and then transfer the accumulated biomass to the second stage of culture to promote secondary metabolite production (Schaltmann et al., 1995).
5. Conclusion
The results of this study show that the biotic elicitors influenced the accumulation of psoralen in the cultured cells. The present result exhibited that the cell cultures treated with all the elicitors showed maximum accumulation of psoralen over the control cell. However, the extract of A. niger (1.0% v/v) was found to be the best for maximum metabolite elicitation. Therefore, these results may contribute to the enhancement of psoralen production by using different biotic elicitors in cell suspension cultures.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Syed Abrar Ahmed, Email: sdabrar645@yahoo.com.
Mirza Mushtaq Vaseem Baig, Email: mmvbaig@gmail.com.
References
- Barradas-Dermitz D.M., Hayward-Jones P.M., Mata-Rosas M., Palmeros-Sánchez B., Platas-Barradas O.B., Velásquez-Toledo R.F. Taxus globosa S. cell lines: initiation, selection and characterization in terms of growth, and of baccatin III and paclitaxel production. Biocell. 2010;34(1):1–6. [PubMed] [Google Scholar]
- Boardley M., Stirton C.H., Harborne J.B. A chemotaxonomic survey of the tribe Psoralea in Africa. Biochem. Syst. Ecol. 1986;14(6):603–613. [Google Scholar]
- Catapan E., Moreno F.N., Luís Busi da Silva M., Otuki M.F., Niero R., Filho V.C., Augusto Protocols for in vitro culture and phytochemical analysis of Phyllanthus species (euphorbiaceae) Methods Mol. Biol. 2009;547:167–177. doi: 10.1007/978-1-60327-287-2_14. [DOI] [PubMed] [Google Scholar]
- Chanda S., Kaneria M., Nair R. Antibacterial activity of Psoralea corylifolia L. seed and aerial parts with various extraction methods. Res. J. Microbiol. 2011;60(2):124–131. [Google Scholar]
- Diwan R., Malpathak N. Bioprocess optimization of furanocoumarin elicitation by medium renewal and re-elicitation: a perfusion-based approach. Appl. Biochem. Biotechnol. 2011;163(6):756–764. doi: 10.1007/s12010-010-9080-3. [DOI] [PubMed] [Google Scholar]
- Frank S., Caffieri S., Raffaelli A., Vedaldi D., Dall’Acqua F. Characterization of psoralen-oleic acid cyclo adducts and their possible involvement in membrane photo damage. J. photochem. Photobiol. B. 1998;44(1):39–44. doi: 10.1016/S1011-1344(98)00103-1. [DOI] [PubMed] [Google Scholar]
- Gaid M.M., Scharnhop H., Ramadan H., Beuerle T., Beerhues L. 4-Coumarate: CoA ligase family members from elicitor-treated Sorbus aucuparia cell cultures. J. Plant Physiol. 2011;168(9):944–951. doi: 10.1016/j.jplph.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Goel M.K., Mehrotra S., Kukreja A.K. Elicitor-induced cellular and molecular events are responsible for productivity enhancement in hairy root cultures: an insight study. Appl. Biochem. Biotechnol. 2011;165(5–6):1342–1355. doi: 10.1007/s12010-011-9351-7. [DOI] [PubMed] [Google Scholar]
- Karwasara V.S., Jain R., Tomar P., Dixit V.K. Genetic transformation and elicitation as yield enhancement strategy for glycyrrhizin production by cell cultures of Abrus precatorius L. in vitro. Cell. Dev. Biol. Plant. 2010;46:354–362. [Google Scholar]
- Karwasara V.S., Tomar P., Dixit V.K. Influence of fungal elicitation on glycyrrhizin production in transformed cell cultures of Abrus precatorius L. Pharmacogn Mag. 2011;7(28):307–313. doi: 10.4103/0973-1296.90411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khushboo P.S., Jadhav V.M., Kadam V.J., Sathe N.S. Psoralea corylifolia Linn.-“Kushtanashini”. Pharmacogn Rev. 2010;4(7):69–76. doi: 10.4103/0973-7847.65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Sung M.K., Kim J.S. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm Res. 2011;60(10):909–917. doi: 10.1007/s00011-011-0351-4. [DOI] [PubMed] [Google Scholar]
- Kundu A., Jawali N., Mitra A. Shikimate pathway modulates the elicitor-stimulated accumulation of fragrant 2-hydroxy-4-methoxybenzaldehyde in Hemidesmus indicus roots. Plant Physiol. Biochem. 2012;56:104–108. doi: 10.1016/j.plaphy.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Malerba M., Crosti P., Cerana R. Defense/stress responses activated by chitosan in sycamore cultured cells. Protoplasma. 2012;249(1):89–98. doi: 10.1007/s00709-011-0264-7. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962;15(3):473–497. [Google Scholar]
- Namdeo A.G., Patil S., Fulzele D.P. Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol. Prog. 2002;18(1):159–162. doi: 10.1021/bp0101280. [DOI] [PubMed] [Google Scholar]
- Ozkan I., Köse O., Ozmen I., Arca E. Efficacy and safety of non-laser, targeted UVB phototherapy alone and in combination with psoralen gel or calcipotriol ointment in the treatment of localized, chronic, plaque-type psoriasis. Int. J. Dermatol. 2012;51(5):609–613. doi: 10.1111/j.1365-4632.2011.05257.x. [DOI] [PubMed] [Google Scholar]
- Santamaria A.R., Mulinacci N., Valletta A., Innocenti M., Pasqua G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011;59(17):9094–9101. doi: 10.1021/jf201181n. [DOI] [PubMed] [Google Scholar]
- Schaltmann J.E., Moreno P.R.H., Selles M., Vinke J.L., ten Hoopen H.J.G., Verpoorte R. Two-stage batch process for the production of ajmalicine by Catharanthus roseus: the link between growth and production stage. Biotechnol. Bioeng. 1995;47(1):53–59. doi: 10.1002/bit.260470107. [DOI] [PubMed] [Google Scholar]
- Seung.-Mi Kang, Ji-Yun Min., Yong.-Duck Kim, Karigar C.S., Seon.-Won Kim, Gwan.-Hyo Goo Effect of biotic elicitors on the accumulation of bilobalide and ginkgolides in Ginkgo biloba cell cultures. J. Biotechnol. 2009;139(1):84–88. doi: 10.1016/j.jbiotec.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Sarada D.V. Antifungal activity of phenyl derivative of Pyranocoumarin from Psoralea corylifolia L. Seeds by inhibition of acetylation activity of trichothecene 3-O-acetyltransferase (Tri101) J. Biomed. Biotechnol. 2012;2012:310850. doi: 10.1155/2012/310850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xiao J., Wang X., Yuan X., Zhao B. Improved cardenolide production in Calotropis gigantea hairy roots using mechanical wounding and elicitation. Biotechnol Lett. 2012;34(3):563–569. doi: 10.1007/s10529-011-0804-4. [DOI] [PubMed] [Google Scholar]
- Szliszka E., Czuba Z.P., Sędek Ł., Paradysz A., Król W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011;63(1):139–148. doi: 10.1016/s1734-1140(11)70408-x. [DOI] [PubMed] [Google Scholar]
- Taha H.S., Abd El-kawy A.M., El-Kareem Abd., Fathalla M. A new approach for achievement of inulin accumulation in suspension cultures of Jerusalem artichoke (Helianthus tuberosus) using biotic elicitors. J. Genet. Eng. Biotechnol. 2012;10(1):33–38. [Google Scholar]
- Vázquez-Flota F., Hernández-Domínguez E., de Lourdes Miranda-Ham M., Monforte-González M. A differential response to chemical elicitors in Catharanthus roseus in vitro cultures. Biotechnol Lett. 2009;31(4):591–595. doi: 10.1007/s10529-008-9881-4. [DOI] [PubMed] [Google Scholar]
- Wen N., Ri-qiang C. Fractionation and biological activity of Aspergillus oryzae elicitor promoting biosynthesis of shikonin derivatives. Acta Botanica Sinica. 1996;38(5):367–374. [Google Scholar]
- Wiktorowska E., Długosz M., Janiszowska W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb. Technol. 2010;46(1):14–20. [Google Scholar]
- Yari Khosroushahi A., Valizadeh M., Ghasempour A., Khosrowshahli M., Naghdibadi H., Dadpour M. Improved Taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol. Intl. 2006;30(3):262–269. doi: 10.1016/j.cellbi.2005.11.004. [DOI] [PubMed] [Google Scholar]


