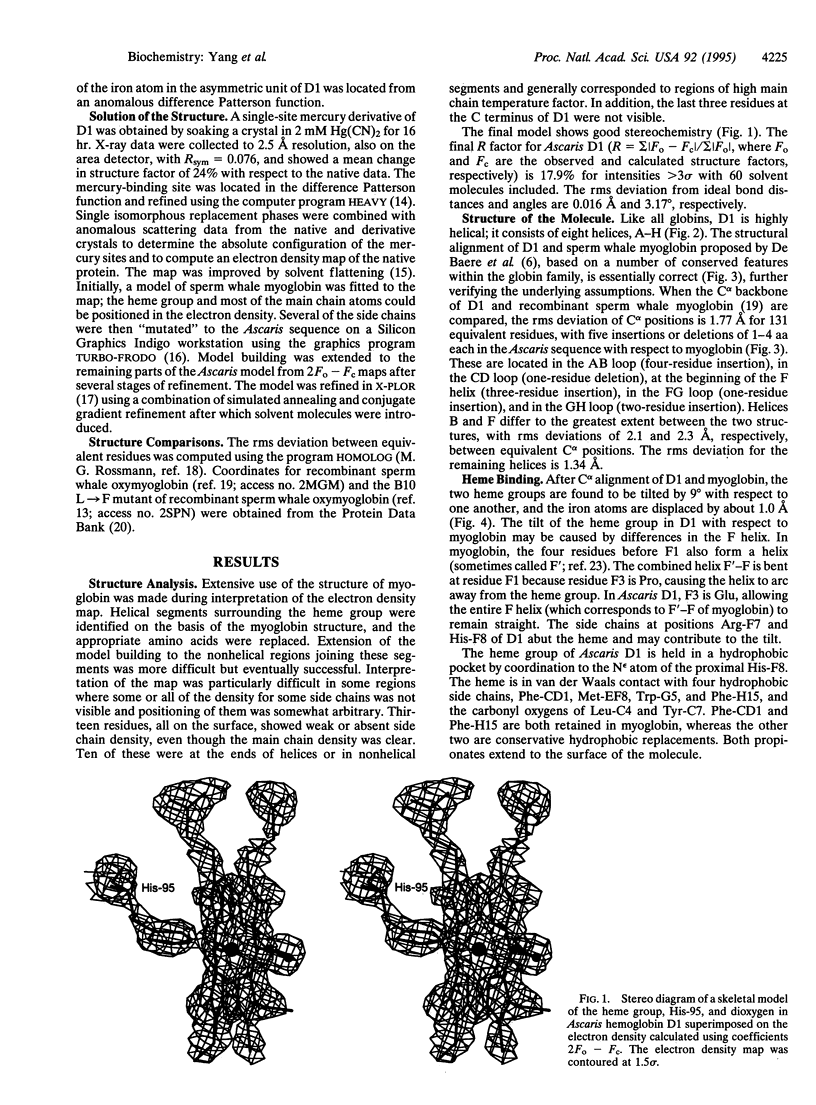
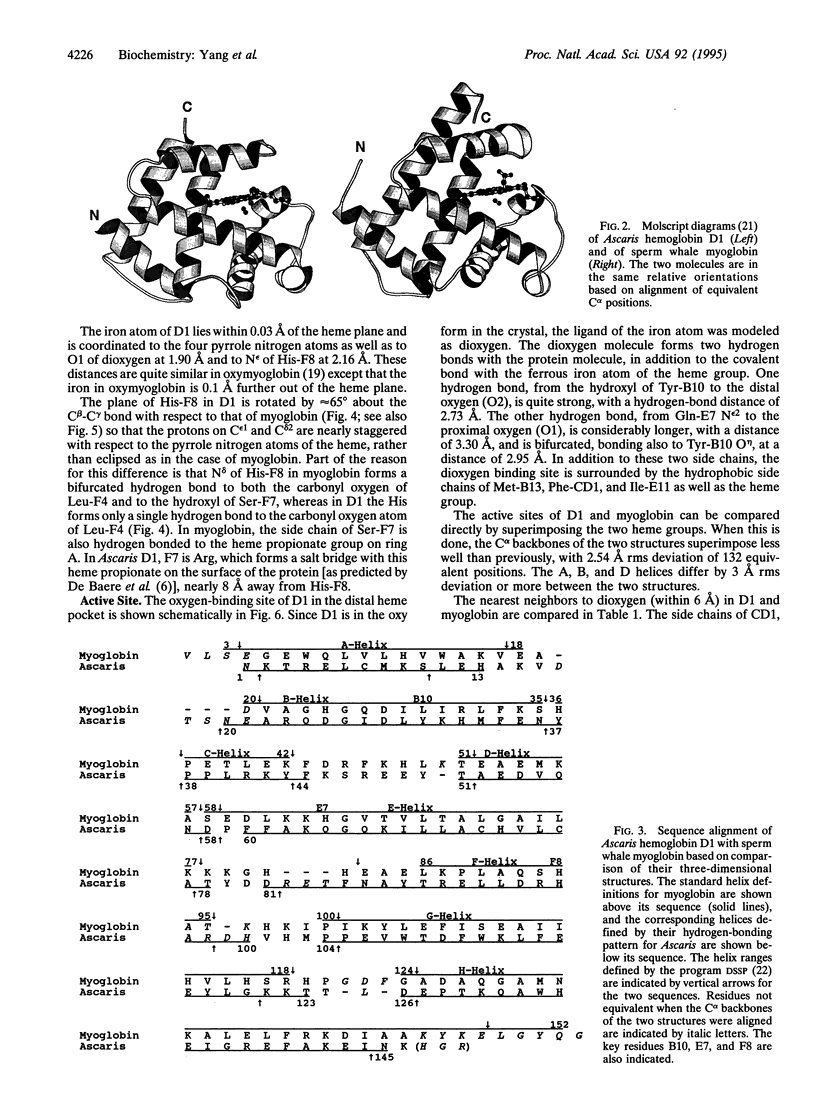
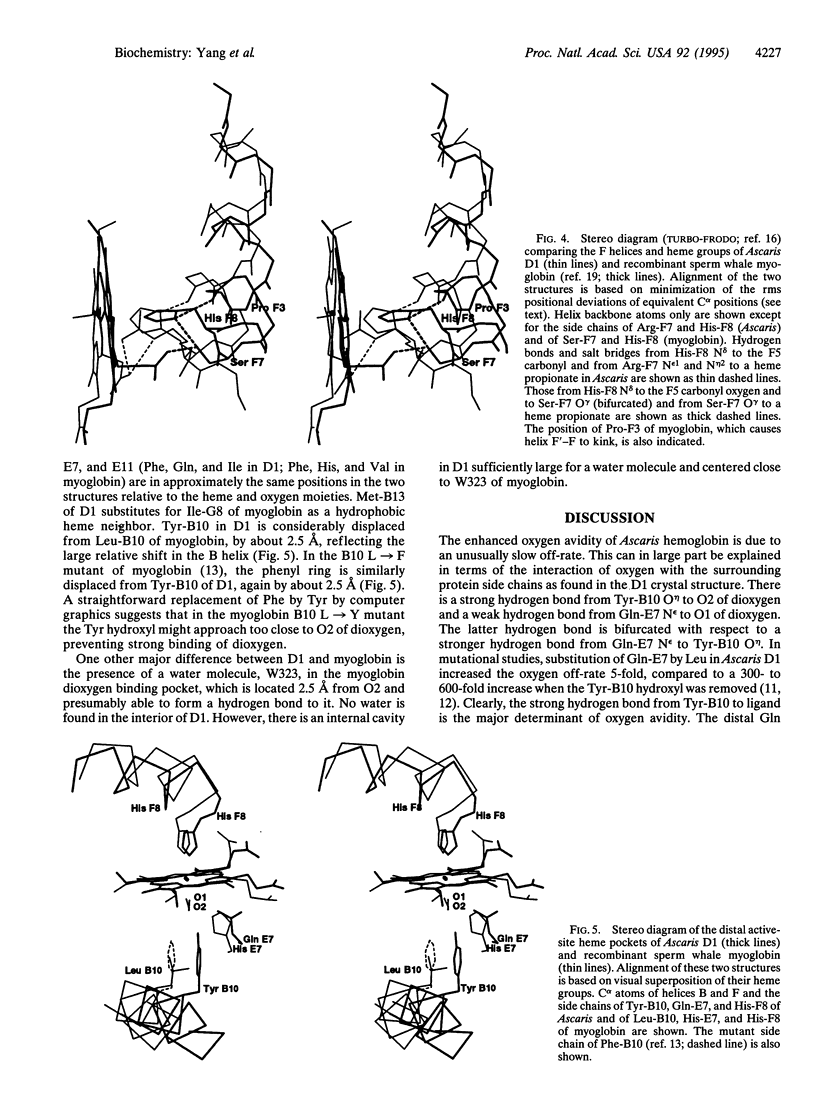
Abstract
The perienteric hemoglobin of the parasitic nematode Ascaris has an exceptionally high affinity for oxygen. It is an octameric protein containing two similar heme-binding domains per subunit, but recombinant constructs expressing a single, monomeric heme-binding domain (domain 1; D1) retain full oxygen avidity. We have solved the crystal structure of D1 at 2.2 A resolution. Analysis of the structure reveals a characteristic globin fold and illuminates molecular features involved in oxygen avidity of Ascaris perienteric hemoglobin. A strong hydrogen bond between tyrosine at position 10 in the B helix (tyrosine-B10) and the distal oxygen of the ligand, combined with a weak hydrogen bond between glutamine-E7 and the proximal oxygen, grips the ligand in the binding pocket. A third hydrogen bond between these two amino acids appears to stabilize the structure. The B helix of D1 is displaced laterally by 2.5 A when compared with sperm whale myoglobin. This shifts the tyrosine-B10 hydroxyl far enough from liganded oxygen to form a strong hydrogen bond without steric hindrance. Changes in the F helix compared with myoglobin contribute to a tilted heme that may also be important for oxygen affinity.
Full text
PDF
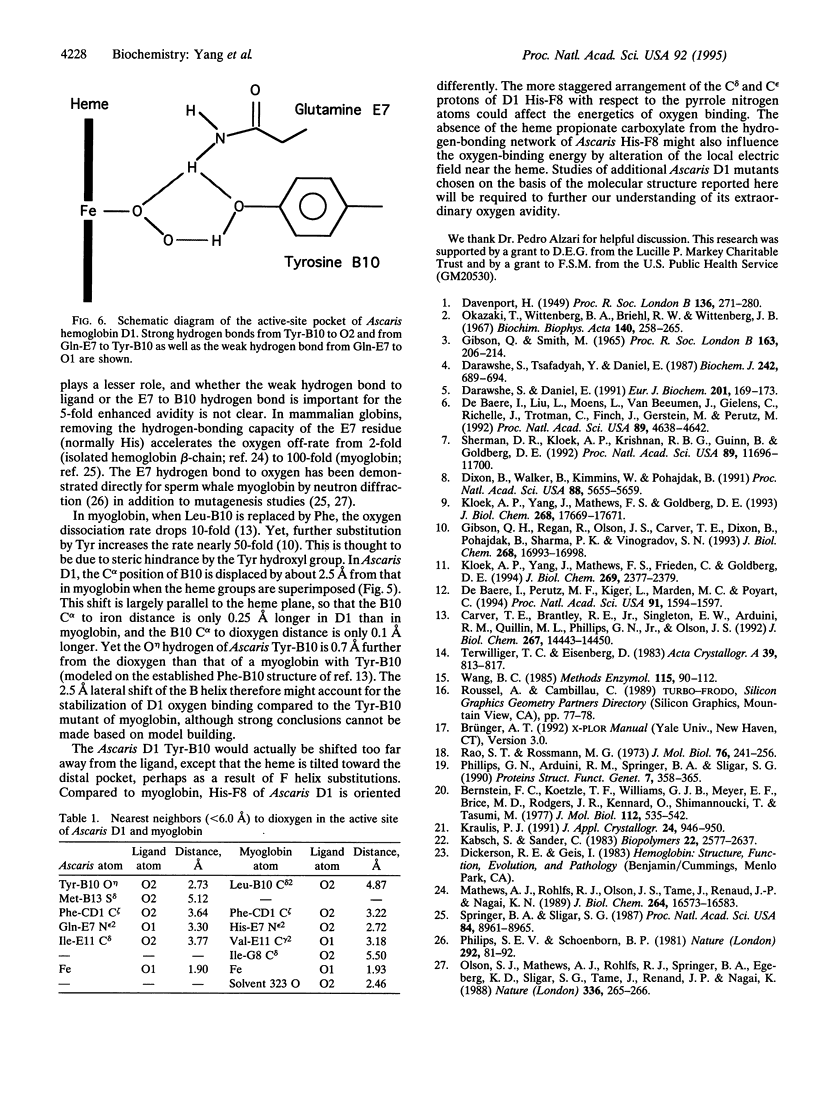
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Carver T. E., Brantley R. E., Jr, Singleton E. W., Arduini R. M., Quillin M. L., Phillips G. N., Jr, Olson J. S. A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J Biol Chem. 1992 Jul 15;267(20):14443–14450. [PubMed] [Google Scholar]
- Darawshe S., Daniel E. Molecular symmetry and arrangement of subunits in extracellular hemoglobin from the nematode Ascaris suum. Eur J Biochem. 1991 Oct 1;201(1):169–173. doi: 10.1111/j.1432-1033.1991.tb16270.x. [DOI] [PubMed] [Google Scholar]
- Darawshe S., Tsafadyah Y., Daniel E. Quaternary structure of erythrocruorin from the nematode Ascaris suum. Evidence for unsaturated haem-binding sites. Biochem J. 1987 Mar 15;242(3):689–694. doi: 10.1042/bj2420689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baere I., Liu L., Moens L., Van Beeumen J., Gielens C., Richelle J., Trotman C., Finch J., Gerstein M., Perutz M. Polar zipper sequence in the high-affinity hemoglobin of Ascaris suum: amino acid sequence and structural interpretation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4638–4642. doi: 10.1073/pnas.89.10.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baere I., Perutz M. F., Kiger L., Marden M. C., Poyart C. Formation of two hydrogen bonds from the globin to the heme-linked oxygen molecule in Ascaris hemoglobin. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1594–1597. doi: 10.1073/pnas.91.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B., Walker B., Kimmins W., Pohajdak B. Isolation and sequencing of a cDNA for an unusual hemoglobin from the parasitic nematode Pseudoterranova decipiens. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5655–5659. doi: 10.1073/pnas.88.13.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Regan R., Olson J. S., Carver T. E., Dixon B., Pohajdak B., Sharma P. K., Vinogradov S. N. Kinetics of ligand binding to Pseudoterranova decipiens and Ascaris suum hemoglobins and to Leu-29-->Tyr sperm whale myoglobin mutant. J Biol Chem. 1993 Aug 15;268(23):16993–16998. [PubMed] [Google Scholar]
- Gibson Q. H., Smith M. H. Rates of reaction of Ascaris haemoglobins with ligands. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):206–214. doi: 10.1098/rspb.1965.0067. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kloek A. P., Yang J., Mathews F. S., Frieden C., Goldberg D. E. The tyrosine B10 hydroxyl is crucial for oxygen avidity of Ascaris hemoglobin. J Biol Chem. 1994 Jan 28;269(4):2377–2379. [PubMed] [Google Scholar]
- Kloek A. P., Yang J., Mathews F. S., Goldberg D. E. Expression, characterization, and crystallization of oxygen-avid Ascaris hemoglobin domains. J Biol Chem. 1993 Aug 25;268(24):17669–17671. [PubMed] [Google Scholar]
- Mathews A. J., Rohlfs R. J., Olson J. S., Tame J., Renaud J. P., Nagai K. The effects of E7 and E11 mutations on the kinetics of ligand binding to R state human hemoglobin. J Biol Chem. 1989 Oct 5;264(28):16573–16583. [PubMed] [Google Scholar]
- Okazaki T., Wittenberg B. A., Briehl R. W., Wittenberg J. B. The hemoglobin of Ascaris body walls. Biochim Biophys Acta. 1967 Jun 27;140(2):258–265. doi: 10.1016/0005-2795(67)90466-7. [DOI] [PubMed] [Google Scholar]
- Olson J. S., Mathews A. J., Rohlfs R. J., Springer B. A., Egeberg K. D., Sligar S. G., Tame J., Renaud J. P., Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988 Nov 17;336(6196):265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Arduini R. M., Springer B. A., Sligar S. G. Crystal structure of myoglobin from a synthetic gene. Proteins. 1990;7(4):358–365. doi: 10.1002/prot.340070407. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Schoenborn B. P. Neutron diffraction reveals oxygen-histidine hydrogen bond in oxymyoglobin. Nature. 1981 Jul 2;292(5818):81–82. doi: 10.1038/292081a0. [DOI] [PubMed] [Google Scholar]
- Rao S. T., Rossmann M. G. Comparison of super-secondary structures in proteins. J Mol Biol. 1973 May 15;76(2):241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- Sherman D. R., Kloek A. P., Krishnan B. R., Guinn B., Goldberg D. E. Ascaris hemoglobin gene: plant-like structure reflects the ancestral globin gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11696–11700. doi: 10.1073/pnas.89.24.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B. A., Sligar S. G. High-level expression of sperm whale myoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8961–8965. doi: 10.1073/pnas.84.24.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]