Abstract
In this study our objective was to evaluate the antioxidant and antimicrobial activity of methanolic extracts of leaves and roots of Gentiana kurroo. The antioxidant activities of the extracts were examined using different biochemical assays namely diphenylpicrylhydrazyl (DPPH), nitroblue tetrazolium (NBT) and ferric reducing power (FRAP). In all the assays, root extract exhibited stronger antioxidant activity than that of leaves. The antibacterial activity of the extracts was also evaluated and MIC values were calculated by broth dilution method. Although, the extracts prevented the growth of both Gram positive and Gram negative bacteria, the MIC values of methanolic extract of the leaves were higher than those of the root extract. The antibacterial and antioxidant activity of the extracts was found to be positively associated with the total phenolic and flavonoid content of the extracts.
Keywords: Antibacterial, Antioxidant, Gentiana kurroo, Gentiopicrin, Gentianin, Gentiomarin
1. Introduction
Plants have to adapt to the changing environmental conditions for their sustenance. The oxidative environment presents a range of free radicals including superoxide, hydroxyl radical, nitric oxide and peroxynitrite, for living organisms to deal with. There are a number of concrete evidences about the role of free radicals in the development of various diseases including Cancer, neurodegeneration and some inflammatory diseases (Halliwell, 2006, 2007; Ferguson, 2010). Antioxidants have therefore gained importance for their capacity to neutralize free radicals. In this context, the antibacterial and antioxidant properties of various medicinal plants are being investigated throughout the world because of the toxicological concerns associated with the synthetic antioxidants and preservatives (Peschel et al., 2006).Gentiana kurroo is one of the critically endangered and endemic medicinal plants of the northwestern Himalayas. It belongs to family Gentianaceae, a cosmopolitan family comprising of more than 1600 species (Struwe and Albert, 2002; Daniel and Sabins, 2002). G. kurroo is a perennial herb with stem as modified rhizome (Behera and Raina, 2012). The dried roots and rhizomes of the plant contain some important bitter glycosides (gentiopicrin, gentianin) and alkaloids (gentiomarin) that have a wide range of pharmaceutical and medicinal utilities. The root stock and other parts of the plant are used as bitter tonic, expectorant, anthelmintic, stomachic and carminative (Kirtikar and Basu, 1935). In folklore medicine, the leaf powder of the plant is mixed with oil and applied on ulcers and fungal infections (Unial and Shiva, 2005). In the Amchi system of medicine the plant is used to treat fever, cough and hepatic ailments (Sharma et al., 2006). Although a number pharmacological activities have been attributed to different parts of G. kurroo, only anti-inflammatory and analgesic properties have been scientifically validated (Behera and Raina, 2012). Therefore, the aim of the present study was to determine the total phenolic and flavonoid content and to evaluate the antioxidant and antibacterial activity of methanolic extracts of leaves and roots of G. kurroo.
2. Materials and methods
2.1. Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), potassium ferricyanide, trichloroacetic acid, gallic acid (GA), rutin (RU), nitroblue tetrazolium (NBT), and Folin–Ciocalteu’s reagent, were purchased from Sigma–Aldrich (St. Louis, MO, USA). Aluminium chloride, Sodium carbonate, Mueller Hinton media were purchased from Himedia (Mumbai, India). All other chemical reagents used were of analytical grade.
2.2. Collection of plant material
The fresh leaves and roots of G. kurroo were collected from lower reaches of the Pir-Panjal range of Kashmir Himalaya. The taxonomic identification of plant was confirmed by Akhtar H. Malik at the Centre of Plant Taxonomy and Biodiversity, University of Kashmir (India).
2.3. Preparation of extracts
Leaves and roots of the plant were collected and dried under shade at room temperature. The plant material was then chopped and ground to fine powder using a mechanical blender. Dried root powder was packed in a Soxhlet apparatus and extracted with methanol at 60–65 °C for 3–4 h. The extract was filtered through a Whatman filter paper No. 1 and the filtrate collected was concentrated under reduced pressure at 40 °C. The extract was dried and stored at 4 °C in storage vials for experimental use.
2.4. Determination of total phenolic content
Total phenolic content of methanolic extracts root and leaf of G. kurroo was measured using the Folin–Ciocalteu reagent method as described earlier (Kaur and Kapoor, 2002). Briefly, from the stock solution of (1 mg/ml methanol) 200 μl of both of the crude extracts were made up to 3 ml with distilled water then mixed thoroughly with 0.5 ml of Folin–Ciocalteu reagent for 3 min, followed by the addition of 2 ml of 20% (w/v) sodium carbonate. The mixture was allowed to stand for a further 60 min in the dark and absorbance of the reaction mixtures was measured at 650 nm. Quantification was done on the basis of the standard curve of Gallic acid concentration range from 50 to 500 mg/ml (r2 = 0.998). Total phenolic content calculated from the calibration curve was expressed as mg of gallic acid equivalent (GAE)/g″dry weight.
2.5. Determination of total flavonoid content
Total flavonoid content of both crude extracts was determined using the aluminium chloride colorimetric method as described earlier (Chang et al., 2002). In brief, from the stock solution of 1 mg/ml crude extracts, 50 μl of each extract was made up to 1 ml with methanol, mixed with 4 ml of distilled water and subsequently with 0.3 ml of 5% NaNO2 solution. 0.3 ml of 10% AlCl3 solution was added after 5 min of incubation and then allowed to stand for 6 min. This was followed by the addition of 2 ml of 1 M NaOH solution to the mixture and final volume of the mixture was brought to 10 ml by the addition of double distilled water. The mixture was allowed to stand for 15 min and absorbance was measured at 510 nm. Quantification was done on the basis of the standard curve of rutin concentration ranging from 50 to 500 mg/ml (r2 = 0.999). Total flavonoid content calculated from a calibration curve was expressed as mg of rutin equivalent (RU)/g of dry weight.
2.6. DPPH assay
The antioxidant activity was determined by DPPH assay as described earlier with some modifications (Villano et al., 2007). From the stock solution different concentrations of extract (100 μg–600 μg/ml) were prepared. 200 μl of each concentration was mixed with 3.8 ml DPPH solution and incubated in the dark at room temperature for 60 min. Absorbance of the mixture was then measured at 517 nm control and Vitamin E was used as a positive. Scavenging ability of the sample to DPPH radical was determined according to the following equation:
2.7. NBT assay
Superoxide anion scavenging activity was performed as described earlier (Vyas and Kumar, 2005). From the stock solution (1 mg/ml) different concentrations of extract (100 μg–500 μg/ml) were prepared. The reaction was performed in 50 mM phosphate buffer (pH 7.8) containing extracts of various concentrations (100–600 μg/ml), 1.5 mM riboflavin, 50 mM NBT, 10 mM dl-methionine, and 0.025% v/v Triton X-100. The reaction was initiated by illuminating the reaction mixture and absorbance of formazan was recorded at 560 nm and percentage scavenging activity was described as inverse of the produced formazan.
2.8. Ferric reducing power assay
Ferric reducing/antioxidant power (FRAP) was determined following the method as described earlier (Zhao et al., 2008). Briefly, 100 μl of each concentration of the extracts (100–500 μg/ml) was mixed with 2.5 ml of 200 mM phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide and incubated at 50 °C for 20 min. After this, 2.5 ml of 10% trichloroacetic acid was added and the tubes were centrifuged at 10,000 rpm for 10 min. Five milliliters of the upper layer of the solution was mixed with 5.0 ml of distilled water and 1 ml of 0.1% ferric chloride and the absorbance of the reaction mixtures was measured at 700 nm. The final results were expressed as mg ascorbic acid equivalent/g of dry weight.
2.9. Antibacterial assay
The antimicrobial activity was tested against both Gram-negative (Escherichia coli, Salmonella enteritidis, Enterobacter cloacae) and Gram-positive bacteria (Streptococcus faecalis, Micrococcus luteus, Proteus mirabilis) obtained from the Department of Microbiology, RTM Nagpur University, Nagpur, India. The bacterial strains were grown on Mueller Hinton agars plates and suspended in MH broth. The minimum inhibitory concentration (MIC) values were determined using the broth microdilution method as described earlier (Ericcson and Sherris, 1971). Serial dilutions of the stock solutions of the crude extracts in broth medium were prepared in a microtiter plate and the microbial suspensions were added in the microwells at the concentration of 5 × 105 organisms/ml. The MIC values were determined as the lowest concentrations preventing visible growth. Streptomycin was used as a positive control. Each assay was repeated three times.
2.10. Statistical analysis
Data were expressed as Mean ± SD. Statistical analysis was performed by SPSS 11.5. One-way analysis of variance (ANOVA) was utilized to evaluate differences.
3. Results
3.1. Total phenolic content
The total phenolic content of the leaf and root methanolic extracts of G. kurroo was determined by the method described above. The total phenolic content for the root extract was found to be 68 ± 2.4 (GAE)/g DW and for the leaf extract 34 ± 1.8 (GAE)/g DW (Table 1).
Table 1.
Total phenolic and flavonoid content of root and leaf extracts of G. kurroo.
| Extract | Total phenolic contenta | Total flavonoid contentb |
|---|---|---|
| Leaf extract | 34 ± 1.8 | 20 ± 1.5 |
| Root extract | 68 ± 2.4 | 41 ± 2.2 |
Each value is a mean of three biological replicas.
mg gallic acid equivalent (GAE)/g DW.
mg rutin equivalent/g DW.
3.2. Total flavonoid content
The total flavonoid content of G. kurroo root and leaf extracts is given in Table 1. The total flavonoid content for the root extract was found to be higher (41 ± 2.2 rutin equivalent/g DW) than the leaf extract (20 ± 1.5 rutin equivalent/g DW).
3.3. Antioxidant activity
Plants rich in secondary metabolites including phenolics, flavonoids and carotenoids exhibit antioxidant activities which are due to their redox properties and chemical structures. The antioxidant property of the crude extracts was investigated and compared by various biochemical assays like, DPPH, NBT and FRAP assays. The methanolic extract of root demonstrated comparatively stronger antioxidant activity as compared to the leaf extract. The DPPH scavenging activity was found to be 53%, 72% and 91% at 600 μg/ml for leaf extract, root extract and Vitamin C respectively (Fig. 1). Superoxide scavenging activity determined by NBT assay was found to be 51%, 63% and 91.7% at 600 μg/ml for leaf extract, root extract and ascorbic acid respectively (Fig. 2). Presence of antioxidant substances or reductants in the plant extracts leads to the reduction of Fe3+ ferricyanide complex to the ferrous form (Fe2+). We also evaluated the reducing power of the crude extracts and significant changes were observed with the increase in the concentration of the extract (100–500 mg/ml). For root extract absorbance values ranged from 0.28 to 0.64 and for leaf extract the values were between 0.24 and 0.59 (Fig. 3). Ascorbic acid was used as a positive control.
Figure 1.
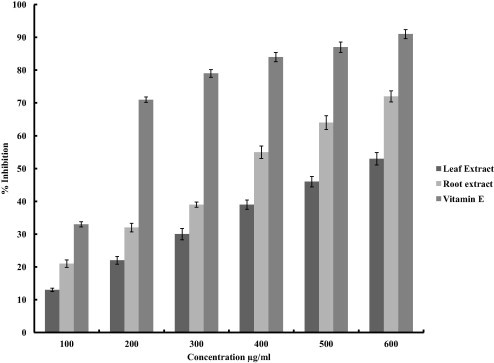
Free radical scavenging activity of methanolic extracts of leaves and roots of G. kurroo Vitamin E was included as a positive control. Activity was measured by the scavenging of DPPH radicals and each value is expressed as the mean ± standard deviation.
Figure 2.
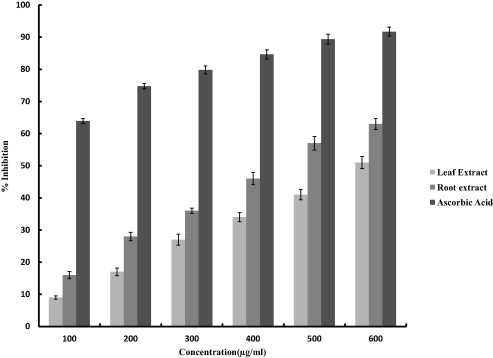
Superoxide scavenging activity of methnolic extracts of leaves and roots of G. kurroo. Ascorbic acid was included as a positive control. Activity was measured using NBT assay and each value is expressed as the mean ± standard deviation.
Figure 3.
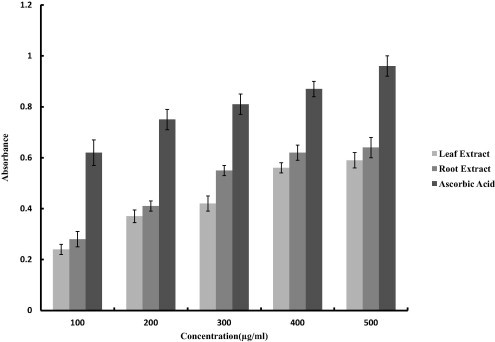
Determination of ferrous reducing capacity of methnolic extracts of leaves and roots of G. kurroo. Ascorbic acid was taken as a positive control. Each value is expressed as the mean ± standard deviation.
3.4. Antibacterial activity
The in vitro antibacterial properties of methanolic extracts of roots and leaves of G. kurroo are presented in Table 2. The tested extracts of G. kurroo roots and leaves possessed antibacterial activity against both Gram positive and Gram negative bacteria. The antibacterial activity of root extract was found to be comparatively higher than that of leaf extract. The MIC value of the root extract ranged from 0.15 ± 0.04 to 0.75 ± 0.05 mg/ml and that of leaf from 0.22 ± 0.08 to 0.90 ± 0.02 mg/ml. The root extract exhibited highest antibacterial activity against M. luteus (0.15 mg/ml) and lowest activity against S. enteritidis (0.75 ± 0.05). A similar trend was exhibited by the leaf extract although the MIC values were higher than those of the root extract.
Table 2.
Antimicrobial activity of G. kurroo extracts (MIC value expressed in mg/ml).
| Microorganism | Leaf extract | Root extract | Streptomycin |
|---|---|---|---|
| Proteus mirabilis | 0.27 ± 0.01 | 0.24 ± 0.04 | 0.055 ± 0.002 |
| Streptococcus faecalis | 0.29 ± 0.02 | 0.22 ± 0.04 | 0.025 ± 0.002 |
| Escherichia coli | 0.75 ± 0.01 | 0.67 ± 0.06 | 0.055 ± 0.001 |
| Salmonella enteritidis | 0.90 ± 0.02 | 0.75 ± 0.05 | 0.020 ± 0.003 |
| Micrococcus luteus | 0.22 ± 0.08 | 0.15 ± 0.04 | 0.020 ± 0.004 |
| Enterobacter cloacae | 0.60 ± 0.04 | 0.55 ± 0.03 | 0.015 ± 0.001 |
Each value is a mean of three biological replicas.
4. Discussion
In our study we determined the total phenolic and flavonoid content of methanolic extracts of leaves and roots of G. kurroo and both of the extracts showed high phenolic and flavonoid content. Antioxidant and antibacterial activity of these crude extracts may be attributed to the high phenolic and flavonoid content. Our results are consistent with research carried out previously (Wani et al., 2013). Phenolic compounds are important plant constituents because of their free radical scavenging ability facilitated by their hydroxyl groups and the total phenolic concentration could be used as a basis for rapid screening of antioxidant activity (Yi et al., 2007). Phenolic compounds are also involved in conferring plants with oxidative stress tolerance. Flavonoids are highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various other free radicals implicated in several diseases (Bravo, 1998). Flavonoids, on the other hand, suppress reactive oxygen formation, chelate trace elements involved in free-radical production, scavenge reactive species, and up-regulate and protect antioxidant defenses (Agati et al., 2012). Crude extracts of fruits, herbs, vegetables, cereals, and other plant materials rich in phenolics and flavonoids, are increasingly being used in the food industry for their antioxidative properties and health benefits. In our study the methanolic extracts of root showed comparatively higher antioxidant activity than the methanolic extract of leaves of G. kurroo, which is in accordance with the total phenolic and flavonoid content of the two extracts.
The tested extracts of G. kurroo roots and leaves possessed relatively higher antibacterial activity against Gram positive than Gram negative bacteria. The antibacterial activity of root extract was found to be comparatively higher than that of leaf extract. The reason for higher sensitivity of the Gram-positive bacteria than Gram negative bacteria could be attributed to their differences in cell membrane constituents. Gram-positive bacteria contain an outer peptidoglycan layer, which is an ineffective permeability barrier. Similar results were obtained from a research carried on strawberry tree leaves (Orak et al., 2011). Orak et al., 2011, determined that the Strawberry tree (Arbutus unedo L.) leaf extracts inhibited only Gram-positive bacteria but showed no activity against Gram-negative bacteria. These results suggest that G. kurroo may be a potential source of broad spectrum of antibacterial agents. The antibacterial activity of the extracts could be attributed to the high content of flavonoids which have been reported to be involved in the inhibition of nucleic acid biosynthesis and metabolic processes (Cushnie and Lamb, 2005).
5. Conclusion
The results suggest that G. kurroo is a potential source of antibacterial and antioxidant molecules. The leaves and roots of the plant can be used as natural antioxidants and preservatives in food and non-food systems. However, further phytochemical analysis is required for the isolation of bioactive molecules from the plant that may show a broad spectrum of pharmacological activities.
Acknowledgments
We acknowledge the Department of botany and the Department of Chemistry for providing necessary chemicals. We also acknowledge the Centre of Plant Taxonomy and Biodiversity, University of Kashmir for the identification of plant material. Shahid A. Malik acknowledges UGC, New Delhi for providing fellowship.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shoib A. Baba, Email: sahmadb11@rediffmail.com.
Shahid A. Malik, Email: shahidmalik004@gmail.com.
References
- Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Behera M.C., Raina R. Gentiana kurroo – a critically endangered bitter herb. Int. J. Med. Arom. Plants. 2012;2:22–29. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Chang C., Yang M., Wen H., Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M., Sabins S.D. Chemical systematics of family gentianaceae. Curr. Sci. 2002;47:109–111. [Google Scholar]
- Ericcson H.M., Sherris J.C. Antibiotic sensitivity testing: report of an international collaborative study. Acta Pathol. Microbiol. Scand. 1971;217:1. [PubMed] [Google Scholar]
- Ferguson L.R. Chronic inflammation and mutagenesis. Mutat. Res. Fund. Mol. M. 2010;690:3–11. doi: 10.1016/j.mrfmmm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration; where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Kaur C., Kapoor H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002;37:153–161. [Google Scholar]
- Kirtikar K.R., Basu B.D. second ed. Bishen Singh Mahendra Pal Singh, Periodicals Expert; Dehradun: 1935. Indian Medicinal Plants. [Google Scholar]
- Orak H.H., Yagar H., Isbilir S.S., Demirci A.S., Gümüş T., Ekinci N. Evaluation of antioxidant and antimicrobial potential of strawberry tree (Arbutus Unedo L.) leaf. Food Sci. Biotechnol. 2011;20:1249–1256. [Google Scholar]
- Peschel W., Sanchez-Rabaneda F., Dieckmann W., Plescher A., Gartzia I. An industrial approach in the search of natural antioxidants from vegetable and fruits wastes. Food Chem. 2006;97:137–150. [Google Scholar]
- Sharma P.K., Sethi G.S., Sharma S.K., Sharma T.K. Ethnomedicinal observations among the inhabitants of cold desert area of Himachal Pradesh. Ind. J. Trad. Know. 2006;5:358–361. [Google Scholar]
- Struwe L., Albert A. Cambridge University Press; U.K.: 2002. Gentianaceae Systematics and Natural History. [Google Scholar]
- Unial B., Shiva V. Traditional knowledge on medicinal plants among rural women of Garhwal Himalayas, Uttaranchal. Ind. J. Trad. Know. 2005;4:259–266. [Google Scholar]
- Villano, D., Fernandez-Pachon, M.S., Moya, M.L., Troncoso, A.M., Garcıa-Parrilla, M.C., 2007. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 71, 230–235. [DOI] [PubMed]
- Vyas D., Kumar S. Purification and partial characterization of a low temperature responsive Mn-SOD from tea (Camellia sinensis (L.) O. Kuntze.) Biochem. Biophys. Res. Commun. 2005;329:831–838. doi: 10.1016/j.bbrc.2005.02.051. [DOI] [PubMed] [Google Scholar]
- Wani B.A., Ramamoorthy D., Rather M.A., Arumugam N., Qazi A.K., Majeed R., Hamid A., Ganie S.A., Ganai B.A., Anand R., Gupta A.P. Induction of apoptosis in human pancreatic MiaPaCa-2 cells through the loss of mitochondrial membrane potential by Gentiana kurroo root extract and LC–ESI-MS analysis of its principal constituents. Phytomedicine. 2013;20:723–733. doi: 10.1016/j.phymed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Yi O., Jovel E.M., Towers N.G.H., Wahbe T.R., Cho D. Antioxidant and antimicrobial activities of native Rosa sp. from British Columbia, Canada. Int. J. Food Sci. Nutr. 2007;58:178–189. doi: 10.1080/09637480601121318. [DOI] [PubMed] [Google Scholar]
- Zhao H., Fan W., Dong J., Lu J., Chen J. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008;107:296–304. [Google Scholar]


