Abstract
Metabolomic profiling of different parts (leaves, flowers and pods) of Acacia species (Acacia nilotica, Acacia seyal and Acacia laeta) was evaluated. The multivariate data analyses such as principal component analysis (PCA) and partial least square-discriminant analysis (PLS-DA) were used to differentiate the distribution of plant metabolites among different species or different organs of the same species. A.nilotica was characterized with a high content of saponins and A.seyal was characterized with high contents of proteins, phenolics, flavonoids and anthocyanins. A.laeta had a higher content of carbohydrates than A. nilotica and A. seyal. On the basis of these results, total antioxidant capacity, DPPH free radical scavenging activity and reducing power of the methanolic extracts of studied parts were evaluated. A.nilotica and A.seyal extracts showed less inhibitory concentration 50 (IC50) compared to A.laeta extracts which means that these two species have the strongest radical scavenging activity whereas A. laeta extracts have the lowest radical scavenging activity. A positive correlation between saponins and flavonoids with total antioxidant capacity and DPPH radical scavenging activity was observed. Based on these results, the potentiality of these plants as antioxidants was discussed.
Keywords: Acacia seyal, Antioxidant capacity, DPPH radical scavenging activity, Flavonoids, Metabolome, Multivariate data analysis, Saponins
1. Introduction
Acacia has a wide range of ecological amplitudes and is distributed in many regions all over the world. The genus includes more than 1350 species (Seigler, 2003). In spite of the huge number of Acacia species, there are very few researches regarding the phytochemistry of these plants. Acacia nilotica is described as a multipurpose medicinal and pharmaceutical plant (Ali et al., 2012). In traditional medicine, A. nilotica is used for the treatment of many diseases including tuberculosis, pneumonia, gonorrhea and small pox. A. nilotica showed a strong antimicrobial activity against both bacteria and fungi (Saini et al., 2008). Methanolic extract of A. nilotica leaves and ethanolic extract of stem bark were investigated against Gram positive and Gram negative bacteria. The results indicated that the extracts revealed antimicrobial activity against both types of bacteria (Mahesh and Satish, 2008; Banso, 2009). The ethanolic extract of A. nilotica leaves showed antimicrobial activity against Campylobacter coli isolated from goats (Solomon-Wisdom and Shittu, 2010). Saini et al. (2008) studied the antimicrobial activity of five species of Acacia and the results indicated that A. nilotica had the highest antifungal activity against Aspergillus niger and Candida albicans. Methanolic leaf extract of A. nilotica revealed a high antifungal activity against Aspergillus flavus, Drechslera turcicaand Fusarium verticillioides (Mahesh and Satish, 2008).A. nilotica bark extract prevents hepatic malondialdehyde formation and reduces liver injury (Singh et al., 2009). A. nilotica pods have been evaluated for the antihypertensive and antispasmodic activity. Methanolic extract of A. nilotica inhibited the spontaneous contraction of rabbit jejunum (Gilani et al., 1999).
Regarding Acacia laeta, there are a few studies relating to this species. Many species of Acacia are distributed in both Nile valley and desert regions in Egypt. The most common species in Egypt are: A. nilotica, A. laeta, Acacia seyal, Acacia raddiana, Acacia ehrenbergiana and Acacia tortilis. Although there are many studies that have been published regarding the phytochemical composition of A. nilotica, there are very few studies dealing with the phytochemistry and antioxidant activity of other species of Acacia.
In recent years, metabolomics which is defined as monitoring of metabolite concentration in a cell, tissue, organ or a whole plant (Ott et al., 2003) has become prominent as a part of systems biology. Moreover, metabolomics is of interest in chemical classification of plants for chemotaxonomy. Differentiation between different species of Acacia based on their metabolomic profiling has not been carried out yet.
In this study, a spectrophotometric method coupled with different multivariate data analyses such as PCA and PLS-DA was applied to Acacia metabolome aiming to investigate the metabolomic variation among different species of Acacia and among different organs of the same species and to evaluate these species as antioxidant potentialities.
2. Materials and methods
2.1. Collection of plant materials
Leaves, flowers and pods of three Acacia species (A. nilotica, A. seyal and A. laeta) were collected from the desert garden, Aswan University, 15 km south west of Aswan city in May 2012. Plant parts were dried in the shade at room temperature. The dried materials were powdered using an electrical grinder.
2.2. Spectrophotometrical analysis
2.2.1. Proteins, carbohydrates and anthocyanins determination
Powdered plant materials were used directly for the determination of carbohydrates, proteins and anthocyanins. Carbohydrates were determined using anthrone reagent (Morris, 1948) and protein was determined using Folin Ciocalteu reagent. The anthocyanin content of the plant was determined according to the modified method of Padmavati et al. (1997). The anthocyanin content in the supernatant was measured spectrophotometrically at 530 and 657 nm. The absorbance values were indicated as A530 and A657. The concentration was calculated using the following equation:
2.2.2. Phenolics, flavonoids, saponins, total antioxidant capacity (TAC), reducing power and DPPH free radical scavenging activity determination
Powdered plant materials (100 mg) were dissolved in 4 ml 80% methanol and the mixtures were vortexed for 1 min before placing in a water bath for 1 h at 60 °C. The mixtures were centrifuged at 800 rpm for 10 min and the supernatants were used for the determination of saponins, flavonoids, phenolics and total antioxidant capacity. The total concentration of phenolics in the extracts was determined according to the Folin–Ciocalteu method (Singelton et al., 1999) with gallic acid as a standard and expressed (mg) as gallic acid equivalents per gram of extract. Total flavonoid content was determined using the aluminum chloride colorimetric method according to Zhishen et al. (1999). Quercetin was used to make the calibration curve and the results were expressed as mg quercetin equivalents per gram of extract. Saponin content was determined using vanillin solution according to Ebrahimzadeh and Niknam (1998). The absorbance of the samples was measured at 473 nm and saponin content in samples was calculated from a standard curve constructed with purified saponins. Total antioxidant capacity (TAC) was determined using the phosphomolybdenum method according to Prieto and Pineda Aguilar (1999). The antioxidant capacity was expressed as ascorbic acid equivalent. Different concentrations of crude extracts (after evaporation of methanol) were used for evaluating the DPPH radical scavenging activity and reducing power. Free radical scavenging activity of the sample extracts was determined spectrophotometrically using the method of Blois (1958). The reaction mixture consisting of 0.5 M acetic acid buffer solution at pH 5.5, 0.2 mM DPPH in ethanol, and 50% (v/v) ethanol aqueous solution, was shaken vigorously with the extracts. After incubation at room temperature for 30 min in dark, the amount of DPPH remaining was determined by measuring the absorbance at 517 nm. Control without extract in the presence and absence of DPPH was used. The concentration of ascorbic acid was measured at the same wavelength. The scavenging activity on the DPPH radical was expressed as inhibition percentage using the following equation:
where Ac = Absorbance of negative control at 517 nm and As = Absorbance of sample at 517 nm (Wang and Mazza, 2002). The reducing power of the extracts was determined using the potassium ferricyanide reduction method (Oyaizu, 1986).
2.3. Data analysis
Principal component analysis (PCA) and partial least square-discriminant analysis (PLS-DA) were performed with the SIMCA-P software (v. 11.0, Umetrics, Umeå, Sweden) with Unit variance scaling methods for spectrophotometrical data. An Analysis of variance (ANOVA) using Minitab (v. 12.21) was used to evaluate the significant difference of the metabolite content among different species and among the same organs of the studied species. Pearson’s correlation was used to assess the correlation between the determined metabolites and both total antioxidant capacity and DPPH radical scavenging activity.
3. Results and discussion
3.1. Variation of metabolites among different Acacia species
The obtained data from spectrometric analysis of different Acacia species (A. nilotica, A. seyal and A. laeta) were subjected to PCA or PLS-DA. The most important information obtained from this analysis is the correlation between data sets which corresponds, in this study with the metabolome of the three species of Acacia. The differences or similarity among the samples can be detected by visualization of the score scatter plot while the metabolites responsible for the differences or similarities can be identified by the score loading plot. The partial least square-discriminant analysis (PLS-DA) was used for the spectrophotometer data of three organs (leaves, flowers and pods) of three species of Acacia (A. nilotica, A. seyal and A. laeta). The PLS-components 1 and 2 explain about 65.5% of the variation among data. Score scatter plot of PLS-DA shows three discriminated groups: the first group is A. nilotica, the second is A. seyal and the third is A. laeta (Fig. 1A). The score loading plot shows the compounds responsible for this discrimination. A. nilotica shows high contents of saponins, proteins and TAC. A. seyal has high contents of flavonoids, phenolics and anthocyanins, whereas A. laeta shows a high content of carbohydrates. A. nilotica and A. seyal together are characterized with higher contents of phenolics, anthocyanins, flavonoids, saponins, proteins and TAC than A. laeta (Fig. 1B). There are many morphological differences among those Acacia species but the results of this study may confirm the difference among them from the point of chemotaxonomy. Multivariate data analysis combined with different spectroscopic techniques was used to differentiate between different species belonging to a particular genus (Choi et al., 2005) or varieties of a particular species (Abdel-Farid et al., 2007) indicating that the chemometrics (multivariate data analysis) combined with the spectroscopic data are valuable tools in chemotaxonomy of species, varieties and cultivars.
Figure 1.
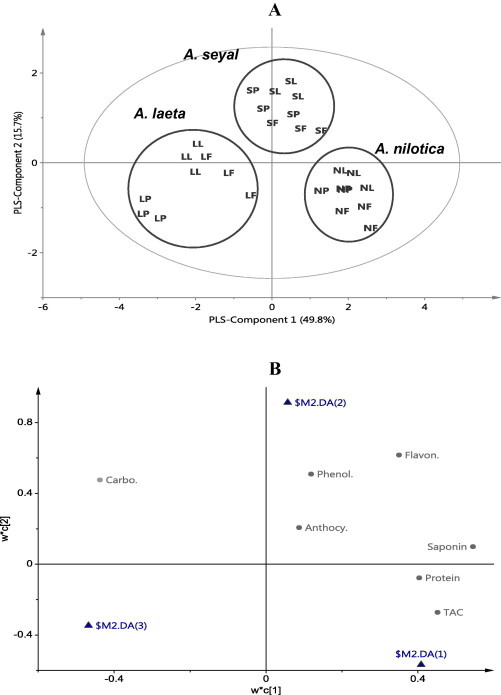
Score scatter plot (A) and score loading plot (B) of PLS-DA of three groups of Acacia species. NL = A. nilotica leaves, NF = A. nilotica flowers, NP = A. nilotica pods, SL = A. seyal leaves, SF = A. seyal flowers, SP = A. seyal pods, LL = A. laeta leaves, LF = A. laeta flowers, LP = A. laeta pods.
3.2. Variation of metabolites among different organs
PLS-components 1 and 2 interpret around 74.6% of the variance among the samples. The score scatter plot of PLS-component 1 versus 2 (Fig. 2A) shows three discriminated groups: leaves, flowers and pods. The score loading plot (Fig. 2B) reveals that leaves of the three species are characterized with high contents of flavonoids and phenolics. Flowers have high contents of saponins, proteins and TAC. Pods of the three species have high contents of anthocyanins and carbohydrates (Fig. 2B). It is expected for a plant to show variation of metabolites among different organs. High contents of flavonoids and phenolics in the leaves may reflect the contribution of leaves actively to plant fitness so they are expected to contain a higher content of compounds associated to defense mechanisms. These findings are in accordance with previous reports with different plants and different secondary metabolites such as glucosinolates in Arabidopsis (Brown et al., 2003) and phenylpropanoids and glucosinolates in Brassica rapa (Abdel-Farid et al., 2007).
Figure 2.
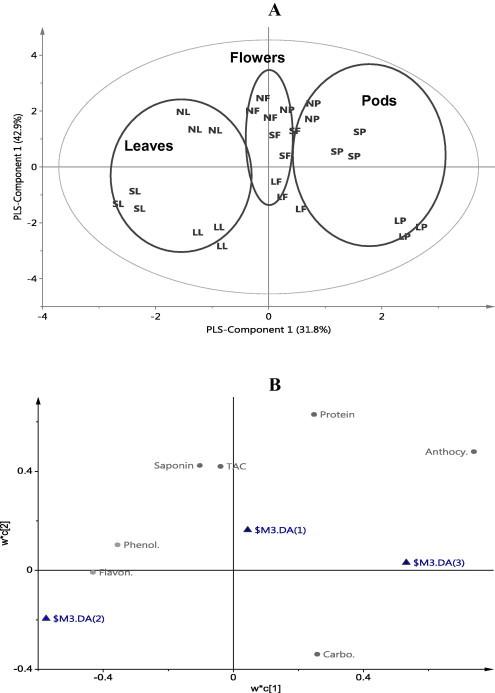
Score scatter plot (A) and score loading plot (B) of PLS-DA of three groups of organs (leaves, flowers and pods) of Acacia species. Labeling of each group is the same as Fig. 1.
3.3. Variation of metabolites among the same organ of different species
3.3.1. Variation of metabolites among leaves of different species
PC1 and PC2 of principal component analysis (PCA) explain 88.1% of the variation among samples. Score scatter plot of PC1 versus PC2 reveals three distinct groups. The first group is A. nilotica leaves, the second A. seyal and the third A. laeta (Fig. 3A). The score loading plot shows that A. nilotica leaves have high contents of saponins, anthocyanins, proteins and TAC (Fig. 3B). A. seyal leaves have high contents of phenolics and flavonoids, whereas A. laeta leaves have a high content of carbohydrates (Fig. 3B). One way analysis of variance (ANOVA) was performed to evaluate the significant difference in metabolite content among the organs of different species (Table 1). Leaves of different species and even cultivars or varieties of the same species have different metabolomic profiling (Abdel-Farid et al., 2007).
Figure 3.
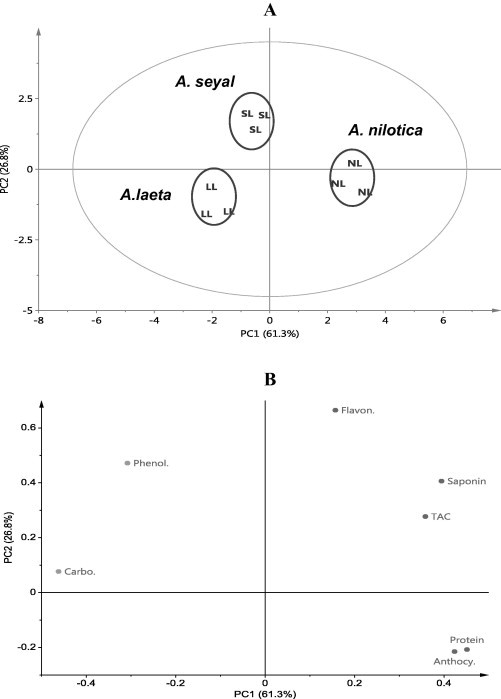
Score scatter plot (A) and score loading plot (B) of PCA of three groups of the leaves of Acacia species. NL = A. nilotica leaves, SL = A. seyal leaves, LL = A. laeta leaves.
Table 1.
Phytochemical analysis, total antioxidant capacity, DPPH radical scavenging activity and reducing power of the three organs (leaves, flowers and pods) of three species of Acacia (A. nilotica, A. seyal and A. laeta).
| Plant species and organs → | Leaves |
Flowers |
Pods |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolites ↓ | A. nilotica (NL) | A. seyal (SL) | A. laeta (LL) | A. nilotica (NF) | A. seyal (SF) | A. laeta (LF) | A. nilotica (NP) | A. seyal (SP) | A. laeta (LP) |
| Carbohydrates (mg/g) | 18.17 ± 0.328a | 30.90 ± 2.608b | 33.40 ± 0.771b | 18.82 ± 2.180a | 32.71 ± 0.323b | 29.83 ± 2.738b | 23.62 ± 1.573a | 35.20 ± 0.715b | 42.69 ± 0.708c |
| Proteins (mg/g) | 75.42 ± 2.409a | 14.57 ± 3.272b | 18.11 ± 1.739b | 77.41 ± 0.805a | 77.67 ± 1.555a | 37.05 ± 4.060b | 61.87 ± 3.909a | 62.80 ± 7.477a | 34.04 ± 3.785b |
| Saponins (mg saponin equivalent/g extract) | 60.78 ± 0.235a | 57.38 ± 0.489b | 43.14 ± 0.671b | 61.88 ± 0.136a | 57.18 ± 0.359b | 45.55 ± 0.151c | 61.49 ± 0.00a | 51.86 ± 2.475b | 40.02 ± 2.837c |
| Phenolics (mg gallic acid equivalent/g extract) | 9.51 ± 0.144a | 10.24 ± 0.104b | 9.92 ± 0.435ab | 8.40 ± 0.023a | 9.79 ± 0.011b | 9.78 ± 0.057b | 9.39 ± 0.013a | 10.11 ± 0.016b | 6.21 ± 0.306c |
| Flavonoids (mg quercetin equivalent/g extract) | 35.71 ± 0.427a | 48.60 ± 1.495b | 19.68 ± 0.796cb | 29.69 ± 1.719a | 32.62 ± 1.451a | 15.26 ± 0.057b | 23.01 ± 0.924a | 28.25 ± 0.398b | 7.36 ± 0.848c |
| Anthocyanins (μmol/g extract) | 1.60 ± 0.820a | UD | UD | 5.16 ± 0.238a | 6.42 ± 0.774a | 4.54 ± 0.792ac | 8.29 ± 0.654a | 11.63 ± 0.611b | 6.98 ± 0.827ac |
| TAC (ascorbic acid equivalent/g extract) | 1043 ± 294a | 827 ± 236a | 525 ± 25ab | 1257 ± 2a | 790 ± 283b | 599 ± 296b | 1251 ± 123a | 608 ± 238b | 387 ± 52b |
| % DPPH (scavenging at 100 μg/ml) | 65.86 ± 0.00a | 66.27 ± 0.00a | 39.16 ± 5.396a | 61.85 ± 43.54a | ND | ND | 63.86 ± 1.14a | 66.67 ± 2.27b | 42.17 ± 21.58c |
| Reducing power (O.D.) at 100 μg | 1.27 ± 0.06a | 0.93 ± 0.09b | 0.31 ± 0.15bc | 1.17 ± 1.70a | 0.51 ± 0.01b | 0.08 ± 0.06ab | 2.01 ± 0.05a | 0.50 ± 0.02b | 0.07 ± 0.03c |
Different letters in each group (leaves, flowers and pods) show significant difference at p < 0.05, UD = under detectable level, ND = not detected and TAC = total antioxidant capacity.
3.3.2. Variation of metabolites among flowers of different species
Score scatter plot of PC1 versus PC2 explains 93.5% of variation among data and reveals three distinct groups. A. nilotica, A. seyal and A. laeta (Fig. 4A). The score loading plot shows that A. nilotica flowers have high contents of saponins and TAC, A. seyal flowers have high contents of carbohydrates, proteins, anthocyanins, phenolics and flavonoids, whereas A. laeta leaves have less contents of the previous detected metabolites (Fig. 4B).
Figure 4.
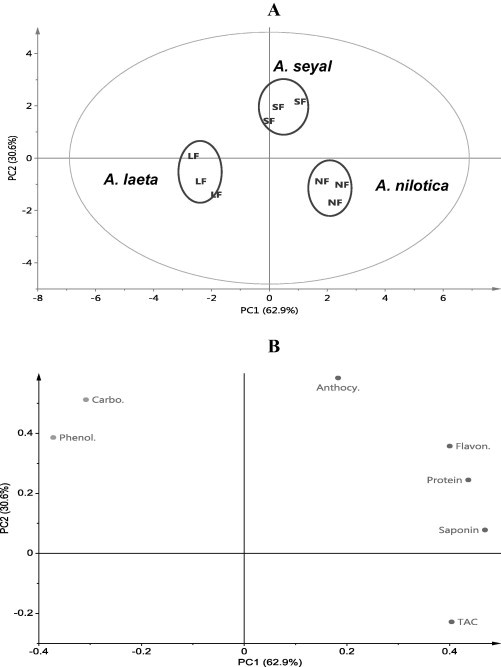
Score scatter plot (A) and score loading plot (B) of PCA of three groups of flowers of Acacia species. NF = A. nilotica flowers, SF = A. seyal flowers, LF = A. laeta flowers.
3.3.3. Variation of metabolites among pods of different species
PLS-1 versus 2 of PLS-DA explains 96.4% of the variation among samples and reveals three groups: A. nilotica, A. seyal and A. laeta (Fig. 5A). The score loading plot shows that A. nilotica pods have high contents of saponins and TAC, A. seyal pods have high contents of protein, flavonoids, phenolics, and anthocyanins whereas A. laeta pods have a high content of carbohydrates and a less content of other detected metabolites (Fig. 5B). In general A. nilotica is characterized with a higher saponin content and TAC, whereas A. seyal have a higher content of phenolics, flavonoids and anthocyanins and A. laeta has a higher content of carbohydrates.
Figure 5.
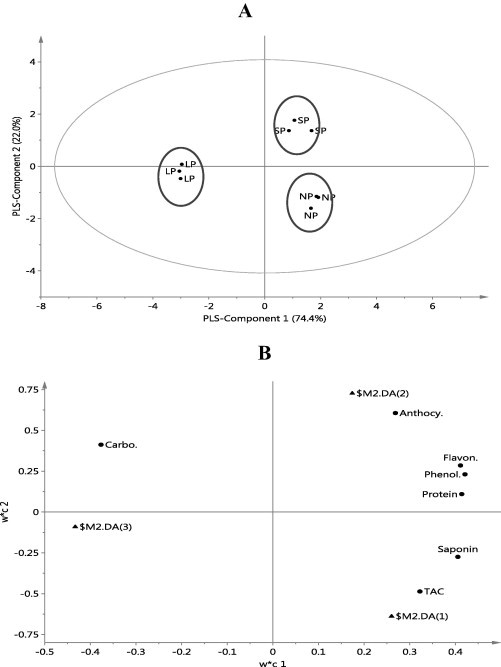
Score scatter plot (A) and score loading plot (B) of PLS-DA of three groups of pods of Acacia species. NP = A. nilotica pods, SP = A. seyal pods, LP = A. laeta pods.
3.4. TAC, DPPH free radical scavenging activity, IC50 and reducing power
Total antioxidant capacity, DPPH free radical scavenging activity and reducing power of methanol extracts of the three parts of the three species of Acacia are shown in Table 1. Total antioxidant capacity was high in A. nilotica and A. seyal extracts. At 100 μg/ml, A. seyal pods, leaves, A. nilotica leaf and pod extracts show the highest DPPH radical scavenging activity of 66.67%, 66.27%, 65.86% and 63.86%, respectively (Table 1). From the dose-dependent, A. nilotica pods, A. seyal leaves and pods and A. nilotica leaf extracts showed the lowest IC50 76.14, 76.98, 77.13 and 78.18 μg/ml, respectively (Table 2) whereas A. laeta leaves and pods showed the highest IC50 130.59 and 145.18 μg/ml, respectively (Table 2). A. nilotica pods, A. seyal leaves and pods and A. nilotica leaves have the lowest IC50 which means that of all extracts they have the strongest radical scavenging activity whereas A. laeta leaf and pod extracts have the lowest radical scavenging activity. The same extracts having the lowest IC50 have the lowest values of reducing power (Tables 1 and 2). A. laeta leaves and pods showed the lowest reducing power (Table 2). The highest content of secondary metabolites detected in both A. nilotica and A. seyal such as saponins, phenolics, anthocyanins and flavonoids may be the reason for increasing the potentialities of these extracts as antioxidants. The antioxidant activity of many plant extracts is attributed to the presence of saponins (Vu et al., 2013), flavonoids (Hamouz et al., 2011), anthocyanins (Sutharut and Sudarat, 2012) and phenolics (Basar et al., 2013).
Table 2.
IC50 values of methanol extracts of Acacia species potential as antioxidant (ND = not detected).
| Species name | Organ’s name | Inhibitory concentration (IC50) (μg/ml) |
|---|---|---|
| A. nilotica | Leaves | 78.18 |
| Flowers | 78.27 | |
| Pods | 76.14 | |
| A. seyal | Leaves | 76.98 |
| Flowers | ND | |
| Pods | 77.13 | |
| A. laeta | Leaves | 130.59 |
| Flowers | ND | |
| Pods | 145.18 | |
| Ascorbic acid (AA) | 5.99 |
3.4.1. Relation between antioxidant capacity and determined metabolites
Pearson’s correlation was carried out to test the correlation between total antioxidant capacity and the determined metabolites in all samples (three species and three organs). Total antioxidant capacity was found to be positively correlated with total carbohydrates, proteins, saponins and flavonoids (Table 3). The correlation of TAC with flavonoids is in accordance with many reports (Hajimahmoodi et al., 2008; Hamouz et al., 2011). Saponin content of eleven extracts of traditional Chinese antidiabetic plants (Xi et al., 2008), Soy bean saponin content (Lee et al., 2011) and shallot saponin content (Vu et al., 2013) were evaluated for their antioxidant capacity and a positive correlation between antioxidant activity and saponins was observed. Protein has a vital role in the prevention of lipid oxidation through inactivation reactive oxygen species and scavenging free radicals (Elias et al., 2008). In this study, carbohydrate was determined as total carbohydrates and this correlation may be attributed to the presence of glycosides in Acacia extracts. Acacia species is a rich source of some glycosides such as cyanogenic glycosides and these types of compounds may have a strong antioxidant activity. The results indicated that saponins and flavonoids showed a positive correlation with DPPH radical scavenging activity (Table 3) which confirm the importance of these compounds in antioxidant activity. Some species of Acacia such as A. nilotica have cytotoxic activity and reduce liver injury (Kaur et al., 2005; Singh et al., 2009). This potentiality may be attributed to its high contents from saponins, flavonoids and glycosides.
Table 3.
Correlation coefficient (r) and probability (p) between the detected metabolites and both total antioxidant capacity (TAC) and DPPH radical scavenging activity (ns = non significant).
| Metabolites | Carbohydrates | Proteins | Saponins | Phenolics | Flavonoids | Anthocyanins |
|---|---|---|---|---|---|---|
| Total antioxidant capacity (TAC) | 0.544 (p = 0.002) | 0.451 (p = 0.012) | 0.751 (p = 0.00) | ns | 0.394 (p = 0.031) | ns |
| DPPH radical scavenging activity | ns | ns | 0.828 (p = 0.006) | ns | 0.716 (p = 0.030) | ns |
4. Conclusion
Metabolomic analysis combined with multivariate data analyses such as PCA and PLS-DA is an effective tool in the differentiation between different species of Acacia and also different organs. This study reveals that A. seyal and A. nilotica have higher contents of the detected metabolites than A. laeta. The metabolites contributing to differentiation between species and organs are saponins, flavonoids, proteins and carbohydrates. Also the antioxidant activity of A. seyal and A. nilotica extracts is higher than that of A. laeta and this may be attributed to the higher content of saponins and flavonoids in A. seyal and A. nilotica than A. laeta. Saponins and flavonoids were positively correlated with both total antioxidant capacity and DPPH radical scavenging activity. Some species of Acacia have been studied extensively, not only due to their wide range of ecological amplitude, but also due to their nutritional and beneficial effects. One of these species is A. nilotica which has been studied extensively from the point of its allelopathic potential against seeds germination and seedlings growth and also its antimicrobial activity. Having a high content of phenolics, flavonoids and anthocyanins in some parts of A. seyal may open the door for an extensive study of that species regarding its allelopathic and antimicrobial potentialities of its extracts comparing to A. nilotica particularly as this species is growing surrounding the crop fields.
Acknowledgement
The authors thank Dr. Magdi A. El-Sayed for his valuable discussions and providing valuable comments and suggestions.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Farid I.B., Kim H.K., Choi Y.H., Verpoorte R. Metabolic characterization of Brassica rapa leaves by NMR spectroscopy. J. Agric. Food Chem. 2007;55:7936–7943. doi: 10.1021/jf071294b. [DOI] [PubMed] [Google Scholar]
- Ali A., Akhtar N., Khan B.A., Khan M.S., Rasul A., UZ-Zaman S., Khalid N., Waseem K., Mahmood T., Ali L. Acacia nilotica: a plant of multipurpose medicinal uses. J. Med. Plants Res. 2012;6:1492–1496. [Google Scholar]
- Banso A. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J. Med. Plants Res. 2009;3:82–85. [Google Scholar]
- Basar M.H., Hossain S.J., Sadhu S.K., Rahman M.H. A comparative study of antioxidant potential of commonly used antidiabetic plants in Bangladesh. Orient. Pharm. Exp. Med. 2013;13:21–28. [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- Brown P.D., Tokuhisa J.G., Reichelt M., Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Choi Y.H., Sertic S., Kim H.K., Wilson E.G., Michopoulos F., Lefeber A.W.M., Verpoorte R. Classification of Illex species based on metabolomic fingerprinting using nuclear magnetic resonance and multivariate data analysis. J. Agric. Food Chem. 2005;53:1237–1245. doi: 10.1021/jf0486141. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh H., Niknam V. A revised spectrophotometric method for determination of triterpenoid saponins. Ind. Drugs. 1998;35:379–381. [Google Scholar]
- Elias R.J., Kellerby S.S., Decker E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Gilani A.H., Shaheen F., Zaman M. Studies on Antihypertensive and antispasmodic activities of methanol extract of Acacia nilotica Pods. Phytother. Res. 1999;13:665–669. doi: 10.1002/(sici)1099-1573(199912)13:8<665::aid-ptr563>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Hajimahmoodi M., Hanifeh M., Oveisi M.R., Sadeghi N., Jannat B. Determination of total antioxidant capacity of green teas by the ferric reducing/antioxidant power assay. Iran J. Environ. Health Sci. Eng. 2008;5:167–172. [Google Scholar]
- Hamouz K., Lachman J., Pazderů K., Tomášek J., Hejtmánková K., Pivec V. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh color. Plant Soil Environ. 2011;57:478–485. [Google Scholar]
- Kaur K., Michael H., Arora S., Härkonen P., Kumar S. In vitro bioactivity-guided fractionation and characterization of polyphenolic inhibitory fractions from Acacia nilotica (L.) Wild. ex Del. J. Ethnopharmacol. 2005;99:353–360. doi: 10.1016/j.jep.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Jeon J.K., Kim S.G., Kim S.H., Chun T., Imm J.-Y. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Inter. J. Food Sci. Technol. 2011;46:2513–2519. [Google Scholar]
- Mahesh B., Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J. Agric. Sci. 2008;4:839–843. [Google Scholar]
- Morris D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science. 1948;107:254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Ott K.H., Aranibar N., Singh B., Stockton G.W. Metabonomics classifies pathways affected by bioactive compounds. Artificial neural network classification of NMR spectra of plant extracts. Phytochemistry. 2003;62:971–985. doi: 10.1016/s0031-9422(02)00717-3. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Padmavati M., Sakthivel N., Thara T.V., Reddy A.R. Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry. 1997;46:449–502. [Google Scholar]
- Prieto P., Pineda Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamine E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Saini M.L., Saini R., Roy S., Kumar A. Comparative pharmacognostical and antimicrobial studies of Acacia species (Mimosaceae) J. Med. Plants Res. 2008;2:378–386. [Google Scholar]
- Seigler D.S. Phytochemistry of Acacia-sensu lato. Biochem. Syst. Ecol. 2003;31:845–873. [Google Scholar]
- Singelton V.R., Orthifer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Singh B.N., Singh B.R., Singh R.L. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem. Toxicol. 2009;47:778–786. doi: 10.1016/j.fct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Solomon-Wisdom G.O., Shittu G.A. In vitro antimicrobial and phytochemical activities of Acacia nilotica leaf extract. J. Med. Plants Res. 2010;4:1232–1234. [Google Scholar]
- Sutharut J., Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Inter. Food Res. J. 2012;19:215–221. [Google Scholar]
- Vu O.H., Hang T.T.M., Yaguchi S., Ono Y., Pham T.M.P., Yamauchi N., Shigyo M. Assessment of biochemical and antioxidant diversities in a shallot germplasm collection from Vietnam and its surrounding countries. Gen. Res. Crop. Evol. 2013;60:297–1312. [Google Scholar]
- Wang J., Mazza G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor-in LPS/IFN-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002;50:4183–4189. doi: 10.1021/jf011613d. [DOI] [PubMed] [Google Scholar]
- Xi M., Hai C., Tang H., Chen M., Fang K., Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes Mellitus. Phyto. Res. 2008;22:228–237. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]


